Back to Journals » Infection and Drug Resistance » Volume 14
Analysis of Antibiotic Resistance and Virulence Traits (Genetic and Phenotypic) in Klebsiella pneumoniae Clinical Isolates from Pakistan: Identification of Significant Levels of Carbapenem and Colistin Resistance
Authors Imtiaz W, Syed Z, Rafaque Z , Andrews SC, Dasti JI
Received 21 November 2020
Accepted for publication 1 January 2021
Published 25 January 2021 Volume 2021:14 Pages 227—236
DOI https://doi.org/10.2147/IDR.S293290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Wajiha Imtiaz,1,2,* Zainab Syed,1,* Zara Rafaque,3 Simon Colin Andrews,2 Javid Iqbal Dasti1
1Department of Microbiology, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad 45320, Pakistan; 2School of Biological Sciences, Whiteknights, University of Reading, Reading RG6 6AJ, UK; 3Department of Microbiology, Hazara University, Mansehra, Pakistan
*These authors contributed equally to this work
Correspondence: Javid Iqbal Dasti
Department of Microbiology, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan
Tel +92-51-90644175
Email [email protected]
Background: The emergence of carbapenem-resistant and hypervirulent hypermucoviscous Klebsiella pneumoniae strains poses a significant public health challenge. We determined the MDR profiles, antibiotic resistance factors, virulence gene complement, and hypermucoviscous features of 200 clinical K. pneumoniae isolates from two major tertiary care hospitals in Islamabad and Rawalpindi, Pakistan.
Methods: Susceptibility profiling and phenotypic analysis were performed according to the CLSI guidelines. Genetic determinants of antibiotic resistance and virulence were detected by PCR. Biofilm formation analysis was performed by microtiter plate assay.
Results: The isolates displayed a high degree of antibiotic resistance: 36% MDR-CRKP; 38% carbapenem resistance; 55% gentamicin resistance; 53% ciprofloxacin resistance; and 59% aztreonam resistance. In particular, the level of resistance against fosfomycin (22%) and colistin (15%) is consistent with previous reports of increased resistance levels. Combined resistance to carbapenem and colistin was 7%. Genetic factors associated with colistin resistance (mcr-1 and mcr-2 genes) were detected in 12 and 9% of the isolates, respectively. Significant differences in resistance to gentamicin and levofloxacin were observed between the 200 isolates. Many of the isolates harbored genes specifying extended-spectrum and/or carbapenem-resistant β-lactamases: blaCTX-M-15 (46%), blaNDM-1 (39%), and blaOXA-48 (24%). The prevalence of the hypermucoviscous phenotype was 22% and 13% of the MDR isolates carried the rmpA gene (regulator for mucoid phenotype). Key virulence factor genes detected include those encoding: porins (ompK35 and ompK36; at 56 and 55% prevalence, respectively); adhesins (fimH, mrkD, and ycfM; at 19, 18, and 22% prevalence, respectively); and the polysaccharide regulator, bss, at 16% prevalence.
Conclusion: This report highlights carbapenem-resistant K. pneumoniae (CRKP) prevalence, emerging resistance to fosfomycin, and the presence of mcr-1 and mcr-2 in colistin-resistant isolates. Further, the detection of rmpA signifies the prevalence of the hypermucoviscous trait in CRKP clinical isolates from Pakistan.
Keywords: Klebsiella pneumoniae, multidrug resistance, carbapenemases, colistin resistance, hypermucoviscous K. pneumoniae
Introduction
Klebsiella pneumoniae (KP) is often associated with hospital and community acquired infections including pneumonia, septicemia, and urinary tract infections (UTIs). Emergence of multidrug-resistant (MDR) KP reduces treatment options and is associated with higher mortality rates (up to 40–50%), particularly in organ transplant patients.1 In the last decade, increased resistance against third-generation cephalosporins resulted in the greater use of carbapenems which subsequently contributed to the emergence of carbapenem-resistant Enterobacteriaceae (CRE) strains.2 Resistance against carbapenems is mainly conferred by plasmids and transposons encoding carbapenemases although other factors such as AmpC β-lactamase over-production, porin defects, and efflux pumps also contribute significantly to resistance.3 In Europe, the high frequency of sporadic CRE infections has resulted in CRE emergence being considered an epidemic.4 Carbapenem-resistant K. pneumoniae (CRKP) is a prominent member of CRE strain set and it has become a substantial threat in both hospital and community health settings. Its combined resistance to almost all frontline antibiotics, including penicillins, β-lactams, carbapenems, fluoroquinolones, and aminoglycosides poses an unprecedented challenge. CRKP strains carry class A K. pneumoniae carbapenemase (KPC), and class B New-Delhi (NDM-1) and Verona integron-encoded metallo-β-lactamases (VIM).5 MDR-CRKP strains have been implicated in transfer of resistance to other closely related pathogens such as Enterobacter spp. and Escherichia coli. Because of severe and life-threatening infections in healthy hosts, the recent emergence of hyper-virulent (hypermucoviscous) K. pneumoniae (hvKP) in the Asian Pacific region has attracted much attention. Such strains cause critical pneumonia, endophthalmitis, liver abscesses, and meningitis, and they have the unusual and concerning characteristic of metastatic spread to different organs in otherwise healthy adults.6
The first reported emergence of CRKP from Pakistan was in 2010.7 Subsequently, the presence of blaNDM-1 (conferring carbapenem resistance) was confirmed in clinical strains.8 Recent developments suggest that CRKP strains display increased resistance to colistin and fosfomycin (some of the few antibiotics still effective against NDM-1 producing strains).9,10 The current study determines the MDR profile, antibiotic resistance genes, virulence profile, and hypermucoviscous genotypes of clinical K. pneumoniae strains in Pakistan.
Materials and Methods
Sample Collection and Antibiotic Susceptibility Profiling
Two hundred clinical isolates of K. pneumoniae were collected from two tertiary-care hospitals located in the twin cities of Rawalpindi and Islamabad. The study protocol was approved by the Institutional Review Board of the Armed Forces Institute of Pathology and informed consent of patients was acquired. Ethical approval was granted by Bio-Ethical Committee (BEC) of Quaid-i-Azam University Islamabad. Experimental work was conducted at Quaid-i-Azam University (Islamabad) in compliance with the recommendations of the Institutional Ethical Committee. The isolates were identified using Gram-staining, colony morphology, and biochemical testing using the API 10S kit (Biomerieux). Antibiotic susceptibility to ampicillin (30 μg), ceftriaxone (30 μg), aztreonam (30 μg), gentamicin (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), fosfomycin (50 μg), polymyxin B (300 U), colistin (10 μg), imipenem (10 μg), and meropenem (10 μg) was determined by disc diffusion assays. Data were interpreted according to the guidelines provided by the Clinical and Laboratory Standards Institute (CLSI, 2017). E. coli ATCC 25922 was used as a control strain throughout the experimental procedures.
Phenotypic Detection of ESBLs and Carbapenemases
The ESBL phenotypes were confirmed via double-disc synergy testing, using discs of amoxicillin-clavulanic acid (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), and aztreonam (30 μg). ESBL production was inferred by any distortion or ≥5 mm augmentation of an inhibition zone of a cephalosporin disc towards the amoxicillin-clavulanate disc.11 Carbapenemase production was assessed by the Modified Hodge test (MHT). E. coli ATCC 25922 and K. pneumoniae ATCC 700603 were used as control strains. The formation of cloverleaf indentations on a lawn of E. coli ATCC 25922 characterized the tested isolates as carbapenemase producers (CLSI M100, 2017).
Determination of MICs of K. pneumoniae Isolates
The broth micro-dilution method was used to quantify antibacterial resistance against gentamicin, levofloxacin, and colistin. Briefly, adjusted cultures (to give a final CFU/mL of ~5 x 105) of the test organisms were inoculated into Muller-Hinton broth (Oxoid, UK) in microtiter plates containing two-fold serial dilutions of an initial antibiotic stock solution giving final concentrations ranging from 0.125 to 128 μg/mL. The bacterial cultures were incubated for 18 h at 37°C before growth was assessed; the lowest concentrations of antibiotic where inhibition occurred were designated as the MIC, as per CLSI guidelines. The reference breakpoints for the interpretation of MICs against gentamicin, levofloxacin, and colistin were set as ≥16, and ≥2 μg/mL, respectively.12
DNA Extraction and Amplification of Genes
DNA was extracted by the phenol-chloroform method.13 PCR amplifications were performed for the genes encoding carbapenemases (blaNDM, blaOXA-48, and blaKPC), ESBLs (blaTEM, blaSHV, blaCTXM-14, blaCTXM-15, blaOXA), and efflux pump proteins (acrAB, tolC). Plasmid-encoded genes specifying colistin resistance (mcr-1, mcr-2), the virulence gene for hypermucoviscosity (rmpA), iron acquisition genes (iucA, kfu), biofilm regulator (bss), adhesion genes (fimH, mrkD, ycfM), the serum resistance factor gene (traT), and porins genes (ompK35, ompK36) were amplified as described elsewhere.14 Plasmid DNA was extracted using a Thermo Scientific Gene JET plasmid miniprep kit.
Detection of Virulence Phenotypes in K. pneumoniae Isolates
Hypermucoviscous phenotypes were tested by using the string test through the formation of a ≥5 mm mucoviscous string from a single bacterial colony. Nigrosine staining was performed for visualization of K. pneumoniae capsule under light microscopy. The isolates were tested for haemagglutination, and for gelatinase and lipase production. The biofilm potential of the isolates was quantified using a microtiter plate assay by comparing the “cut-off” optical densities (ODc) with negative controls at 492 nm.11
Results
Sample Distributions and Antibiotic Resistance
Two hundred clinical K. pneumoniae isolates were collected from nine different sample sites (Table 1; see Table S1 for full data set). The overall resistance level for carbapenems (imipenem and meropenem) was 38%. The greatest frequency of resistant was to ampicillin (94%), followed by ceftriaxone (71%). The frequencies of resistance against gentamicin (55%), ciprofloxacin (53%), colistin (15%), aztreonam (59%), and fosfomycin (22%) were above the threshold levels at which these antibiotics should no longer be used in empirical treatment of K. pneumoniae infections15 (Table 2). Resistance against polymyxins B and E was 16 and 15%, respectively. The prevalence of MDR within the 200 isolates was 63% (125), of which 58% (72) were carbapenem-resistant (MDR-CRKP) (Table 1). Of the 72 MDR-CRKP strains, 28% (20) were ESBL producers. The prevalence of MDR-KP within K. pneumoniae isolates was similar in female and male patients, although there was a more than twofold higher prevalence of the combined MDR with HMV traits in isolates from males than from females. Also, a high proportion of the MDR (38%) and combined MDR/HMV (60%) strains were isolated from urine whereas much lower proportions were found in tissue, bile, swab, and catheter tip samples (Table 1).
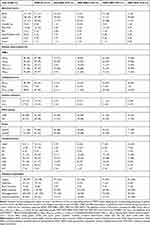 |
Table 1 Genetic and Phenotypic Traits of MDR Klebsiella pneumoniae Isolates |
 |
Table 2 Genotypic and Phenotypic Comparison Between Hypermucoviscous and Non-Hypermucoviscous K. pneumoniae Isolates |
MIC Values for Gentamicin, Levofloxacin and Colistin
MICs for gentamicin, levofloxacin, and colistin were determined for the 63 carbapenemase producers and 137 non-producers. For gentamicin, 38% of the carbapenemase producers exhibited MICs >256 µg/mL whereas only 9% of the non-producers showed resistance to such gentamicin concentrations. For levofloxacin, the MIC observed for the carbapenemase producers was twofold higher (128 µg/mL) than the non-producers (64 µg/mL). For the majority of the carbapenem-sensitive isolates, colistin MIC values were between 4 and 8 µg/mL. The differences in MICs for levofloxacin, gentamicin, and colistin between carbapenem-resistant and -sensitive isolates were statistically significant (p < 0.01).
Genetic Factors Specifying Antibiotic Resistance
The presence of genes encoding ESBL factors (blaTEM, blaSHV, blaCTXM, blaOXA), carbapenemases (blaKPC, blaNDM-1, blaOXA-48 type), efflux pump (acrAB, tolC) and colistin markers (mcr-1, mcr-2) was investigated. Among the carbapenemase genes, blaNDM-1 was the most predominant (39%) and its occurrence was significantly higher in comparison to blaKPC (5%). The frequency of occurrence for the carbapenemase-encoding blaOXA-48 gene was 24%, and the colistin-resistance mcr-1 and mcr-2 genes were detected in 12 and 9% of isolates, respectively. Carriage rates for blaTEM, blaSHV, blaCTXM-14, blaCTXM-15, and blaOXA were ~3 fold higher in MDR-ESBL strains than in MDR (non-ESBL) strains (Table 1).
Prevalence Virulence Factors and hvKP
For the fimbrial and non-fimbrial adhesins, the occurrence rates amongst the 200 isolates were as follows: fimH, 19%; mrkD, 18%; and ycfM, 22%. The polysaccharide regulator gene (bss) was detected in 16% of the isolates. Overall, 22% of the isolates displayed a mucoviscous phenotype (string-test positive), while a similar 24% of the MDR isolates displayed this phenotype (Table 1). The rmpA gene encoding the “regulator of mucoid phenotype” was found in 49% of the hypervirulent phenotypes, but was not found in any of the non-HMV strains (Table 2). Isolates demonstrating an HMV phenotype showed higher resistance against fosfomycin and colistin, but a lower resistance to polymyxin B (Table 2). The frequency of occurrence of the virulence determinants iucA, kfu, bss, fimH, and mrkD was higher in the hypermucoviscous MDR isolates (Table 1). The porin genes, ompK35 and ompK36, were found in 56 and 55% of isolates, respectively, and had slightly higher occurrence in the non-hypermucoviscous strains compared to the HMV strains (Table 2). Furthermore, haemagglutination (100%), capsule (99%), and biofilm formation (94%) were observed at high frequency in 200 K. pneumoniae isolates. Gelatinase production and blood hemolysis were observed in 12 and 8% of the isolates.
Discussion
Over the past decade, preferential use of carbapenems has generated significant selective pressure leading to the emergence of MDR-CRKP16 and such strains are increasingly associated with life-threatening infections.17 Likewise, emergence of hyper-virulent Klebsiella strains is causing an increasing challenge, particularly in countries lacking central surveillance systems for infectious diseases.18 However, little is known about the prevalence of hvKP strains in Pakistan. In addition, very limited data are available on colistin and fosfomycin resistant CRKP. In this study, we observed that 38% of the K. pneumoniae isolates collected from patients were CRKP, which indicates an alarming increase of CRKP frequency in Pakistan with respect to that reported in 2010.7 Similar increases in CRKP have been observed in India and China.19 The resistance levels recorded for aztreonam (59%), gentamicin (55%), ciprofloxacin (53%), and imipenem (38%) were higher than the recent antimicrobial surveillance study in South Korea reporting 13.5, 24.4, 24, and 3.1% resistance, respectively.20 Our findings align with the resistance pattern of multidrug-resistance in K. pneumoniae isolates from Iran with 36% aztreonam, 15.9% gentamicin, 19.4% levofloxacin, and 11.8% imipenem resistance.21 The highest acquired resistance observed here (after the broad-spectrum cephalosporin, ceftriaxone) was for aztreonam, indicating the frequent usage of this drug against K. pneumoniae infections within South Asian hospital settings. This is concerning as aztreonam is the only mono-cyclic β-lactam antibiotic (monobactam) that is effective against IMP-, VIM-, NDM–type carbapenemase-producing K. pneumoniae.22 The resistance levels recorded for colistin (15%), polymyxin B (16%), and fosfomycin (22%) were comparatively low with respect to other antibiotics tested, possibly because of their limited administration due to their associated toxicity and/or to their stringent dose administration which is based on the principle of “highest safe” rather than “minimum effective dosage”; such prescription practices would limit the emergence and spread of resistance.22
The 22% fosfomycin and 15% colistin resistance observed here highlight a significant increase in the resistance against these antibiotics in recent years. The resistance level recorded for colistin (15%) is approximately five times that reported in 2019 and 2020, i.e. 2% and 2.9%, respectively.23,24 The recent surge in colistin resistance is alarming25 since polymyxins, particularly colistin methanesulfonate (CMS), are routinely used to treat CRKP infection.26 Colistin resistance is mainly associated with genetic mutations in housekeeping genes such as mgr and phoAB; however, the plasmid-mediated mcr-1 and mcr-2 factors pose a threat for rapid global spread of such resistance.27 Plasmid-mediated resistance determinants elevate the MICs of antibiotics, resulting in higher antibiotic doses for effective treatment, which in turn leads to an increased probability that chromosomal mutations will arise that confer a further increase in resistance.28 Our data match previous findings29 demonstrating that horizontal transfer of mcr-1 contributes to an elevated MIC value against colistin and that some mcr positive isolates remain within the susceptible range (Table 3). For majority of the mcr-1 and mcr-2 positive isolates, the MIC for colistin ranged from 4 to 8 μg/mL and can thus be defined as colistin resistant.30 This is the first report from Pakistan that indicates an association of the plasmid-borne mcr genes with elevated colistin resistance as determined by the microbroth dilution method. Although a report in 2017 indicated colistin resistance in CRE stains from Pakistan, no attempt was made to determine the presence of the mcr genes.9 However, here we find a clear co-association of mcr and carbapenemase loci with 92% (23/25) of mcr isolates carrying at least one carbapenemase gene (Table 3). Although mcr-1 does not play a direct role in resistance to other antibiotics, the results reported here showing a coexistence of mcr-1 with ESBL and blaNDM determinants reflect a major development in the emergence of resistance in K. pneumoniae, where efficacy of carbapenems and colistin is threatened.31
 |
Table 3 Colistin Resistance in CRKP and HMV K. pneumoniae Strains |
In this study, the MICs determined for fluoroquinolone, aminoglycoside, and polymyxin were notably higher for the carbapenemase-producing K. pneumoniae. CRKP frequently harbors a combination of resistance mechanisms including carbapenemases, drug efflux, and loss of porin function32,33 leading to higher levels of resistance to antibiotics, as observed here in the carbapenemase-producing isolates. Molecular screening for the resistance markers in 200 K. pneumoniae isolates in this study confirmed blaNDM-1 as the predominant (39% occurrence) carbapenemase-encoding gene followed by blaOXA-48 (24%) and blaKPC,(5%). Very high occurrence of blaNDM-1 and blaOXA-48 carbapenemases suggests that they have a predominant role in the recent emergence of CRKP phenotypes in Pakistan.34,35
Hypervirulent K. pneumoniae strains are endemic in the Asian Pacific region,36 but data are extremely scarce on prevalence of such strains in Pakistan.37 In this study, 13% of the MDR isolates carried rmpA which is the first report for the presence of the rmpA gene in hvKP isolates, with resistance to frontline antibiotics, from Pakistan.37 Overall, 22% of the isolates exhibited a positive string test and 11% of the isolates carried the rmpA gene. Up to 100% rmpA prevalence in hypermucoviscous isolates has been reported previously.38 Another study showed 41% rmpA carriage in non-hypermucoviscous strains indicating additional underlying factors required for the hvKP phenotype.39 Contrary to other studies that observed lower antimicrobial resistance in hvKP isolates,6 here a higher resistance against cephalosporins, aminoglycosides, fluoroquinolones, and carbapenems were found in hvKP strains. Until 2015, hypervirulent and the multidrug-resistant K. pneumoniae isolates were reported to belong to distinct clones with no reports of MDR and virulence convergence.40 However, a recent combination of these traits is evident from a report of convergent multidrug-resistant hypervirulent strains from South and Southeast Asia in 2020.41
Conclusion
This study highlights the prevalence of CRKP clinical isolates with notable virulence potential. The results support previous studies indicating that the blaNDM-1- and blaOXA-48-encoded carbapenemases are the most prevalent K. pneumoniae carbapenemases in Pakistan. The rise in colistin and fosfomycin resistant strains globally points to significant treatment constraints in the near future. In general, the K. pneumoniae isolates studied here showed a high prevalence of resistance gene carriage but a relatively low virulence gene carriage; interestingly, the hypermucoviscous isolates exhibited increased resistance potential, a trait not frequently associated with the hypermucoviscous K. pneumoniae isolates.
Abbreviations
MDR, multidrug-resistant; ESBLs, extended-spectrum beta-lactamases; MICs, minimum inhibitory concentration; UTIs, urinary tract infections; ECDC, European Center for Disease Prevention and Control; AmpC, Ampicillinase C; CRE, carbapenem-resistant Enterobacteriaceae; CRKP, carbapenem-resistant Klebsiella pneumoniae; PCR, polymerase chain reaction; HMVKP, hypermucoviscous Klebsiella pneumoniae.
Data Sharing Statement
The data generated from the research work is presented in this article. Any additional information required can be requested from the corresponding author as per ethical guidelines.
Ethics Approval and Consent to Participate
The study was reviewed and granted approval by the Bio-Ethical Committee (BEC) of Quaid-i-Azam University, Islamabad; under the protocol number #BEC-FBS-QAU2019-148. The clinical samples were part of the routine hospital laboratory procedure.
Acknowledgments
We are thankful to Higher Education Commission of Pakistan for supporting doctoral work of Wajiha Imtiaz.
Author Contributions
All authors contributed significantly in conception, study design experimental work, data acquisition analysis and interpretation or in all aspects. Furthermore, all authors took part in drafting, revising and critically reviewing the article and gave final approval of the version being published and agreed on the journal to which article has been submitted and remain accountable for all aspects of the work reported.
Funding
Work of Wajiha Imtiaz was funded by HEC indigenous PhD scholarships covering monthly stipend University fee and lab reagents that helped us to execute this study (HEC-PIN no: 315-15481-2BS3-165). Higher Education Commission Pakistan played no direct role in collection, analysis, interpretation and publication of this work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
2. Center for Disease Dynamics E, Policy. State of the World’s Antibiotics, 2015. Washington, DC: CDDEP; 2015.
3. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80:629–661.
4. Brolund A, Lagerqvist N, Byfors S, et al. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Eurosurveillance. 2019;24(9):1900123. doi:10.2807/1560-7917.ES.2019.24.9.1900123
5. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–67. doi:10.1093/cid/cir202
6. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:107–118. doi:10.4161/viru.22718
7. Khan E, Ejaz M, Zafar A, et al. Increased isolation of ESBL producing Klebsiella pneumoniae with emergence of carbapenem resistant isolates in Pakistan: report from a tertiary care hospital. J Pak Med Assoc. 2010;60:186.
8. Kamarasamy K, Toleman M, Walsh T. Emergence of a new antibiotic resistance in India, Pakistan, and the UK: a prospective survey. Lancet Infect Dis. 2010;10:597–602. doi:10.1016/S1473-3099(10)70143-2
9. Qamar S, Shaheen N, Shakoor S, Farooqi J, Jabeen K, Hasan R. Frequency of colistin and fosfomycin resistance in carbapenem-resistant Enterobacteriaceae from a tertiary care hospital in Karachi. Infect Drug Resist. 2017;10:231. doi:10.2147/IDR.S136777
10. Aslam B, Chaudhry TH, Arshad MI, et al. The first bla KPC harboring Klebsiella pneumoniae ST258 strain isolated in Pakistan. Microb Drug Resist. 2020;26(7):783–786. doi:10.1089/mdr.2019.0420
11. Gharrah MM, Mostafa El-Mahdy A, Barwa RF. Association between virulence factors and extended spectrum beta-lactamase producing Klebsiella pneumoniae compared to nonproducing isolates. Interdiscip Perspect Infect Dis. 2017;2017:1–14. doi:10.1155/2017/7279830
12. Wayne P. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI Doc M100-S20. 2010.
13. McKiernan H, Danielson P. Molecular diagnostic applications in forensic science. In: Molecular Diagnostics.
14. El Fertas-Aissani R, Messai Y, Alouache S, Bakour R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol Biol. 2013;61(5):209–216. doi:10.1016/j.patbio.2012.10.004
15. England PH. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2018–2019. London, UK: PHE; 2019.
16. Hawser SP, Bouchillon SK, Lascols C, et al. Susceptibility of Klebsiella pneumoniae isolates from intra-abdominal infections and molecular characterization of ertapenem-resistant isolates. Antimicrob Agents Chemother. 2011;55(8):3917–3921. doi:10.1128/AAC.00070-11
17. Behera B, Mathur P, Das A, Kapil A. Ertapenem susceptibility of extended spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary care centre in India. Singapore Med J. 2009;50(6):628.
18. Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32(3):32. doi:10.1128/CMR.00001-19
19. Wang B, Pan F, Wang C, et al. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int J Infect Dis. 2020.
20. Kim D, Park BY, Choi MH, et al. Antimicrobial resistance and virulence factors of Klebsiella pneumoniae affecting 30 day mortality in patients with bloodstream infection. J Antimicrob Chemother. 2019;74(1):190–199. doi:10.1093/jac/dky397
21. Mansury D, Motamedifar M, Sarvari J, Shirazi B, Khaledi A. Antibiotic susceptibility pattern and identification of extended spectrum β-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae from Shiraz, Iran. Iran J Microbiol. 2016;8(1):55.
22. Livermore D. Advice on Carbapenemase Producers: Recognition, Infection Control and Treatment. London: Health Protection Agency; 2011.
23. Gautam V, Thakur A, Sharma M, et al. Molecular characterization of extended-spectrum β-lactamases among clinical isolates of Escherichia coli & Klebsiella pneumoniae: a multi-centric study from tertiary care hospitals in India. Indian J Med Res. 2019;149(2):208. doi:10.4103/ijmr.IJMR_172_18
24. Effah CY, Sun T, Liu S, Wu Y. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob. 2020;19(1):1–9. doi:10.1186/s12941-019-0343-8
25. Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–2143. doi:10.1093/jac/dkv086
26. Jayol A, Nordmann P, Brink A, Poirel L. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob Agents Chemother. 2015;AAC:05055.
27. Pragasam AK, Shankar C, Veeraraghavan B, et al. Molecular mechanisms of colistin resistance in Klebsiella pneumoniae causing bacteremia from India—a first report. Front Microbiol. 2017;7:2135. doi:10.3389/fmicb.2016.02135
28. Mitra S, Mukherjee S, Naha S, Chattopadhyay P, Dutta S, Basu S. Evaluation of co-transfer of plasmid-mediated fluoroquinolone resistance genes and blaNDM gene in Enterobacteriaceae causing neonatal septicaemia. Antimicrob Resist Infect Control. 2019;8(1):46. doi:10.1186/s13756-019-0477-7
29. Liu -Y-Y, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi:10.1016/S1473-3099(15)00424-7
30. Testing ECoAS. Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. Växjö, Sweden: European Committee on Antimicrobial Susceptibility Testing; 2016. Available from:: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf.
31. Xu Y, Lin J, Cui T, Srinivas S, Feng Y. Mechanistic insights into transferable polymyxin resistance among gut bacteria. J Biol Chem. 2018;jbc. RA117:000924.
32. Dalmolin TV, Bianchini BV, Rossi GG, et al. Detection and analysis of different interactions between resistance mechanisms and carbapenems in clinical isolates of Klebsiella pneumoniae. Braz J Microbiol. 2017;48(3):493–498. doi:10.1016/j.bjm.2017.01.003
33. Khan FA, Hellmark B, Ehricht R, Söderquist B, Jass J. Related carbapenemase-producing Klebsiella isolates detected in both a hospital and associated aquatic environment in Sweden. Eur J Clin Microbiol Infect Dis. 2018;37(12):2241–2251. doi:10.1007/s10096-018-3365-9
34. Sultan BA, Irfan S. Detection of metallo-beta lactamases (IMP, VIM, NDM) and KPC carbapenemases in carbapenem resistant Enterobacteriaceae: report from Clinical Laboratory Aga Khan University Karachi, Pakistan. Infect Dis J Pak. 2014;584.
35. Lomonaco S, Crawford MA, Lascols C, et al. Resistome of carbapenem- and colistin-resistant Klebsiella pneumoniae clinical isolates. PLoS One. 2018;13(6):e0198526. doi:10.1371/journal.pone.0198526
36. Siu LK, Yeh K-M, Lin J-C, Fung C-P, Chang F-Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi:10.1016/S1473-3099(12)70205-0
37. Sayeed MA, Latif N, Mahmood SF. Hypermucoviscous Klebsiella syndrome it’s in the community! J Pak Med Assoc. 2017;67:1930–1932.
38. Jung S, Chae H, Park Y, et al. Microbiological and clinical characteristics of bacteraemia caused by the hypermucoviscosity phenotype of Klebsiella pneumoniae in Korea. Epidemiol Infect. 2013;141(2):334–340. doi:10.1017/S0950268812000933
39. Qu -T-T, Zhou J-C, Jiang Y, et al. Clinical and microbiological characteristics of Klebsiella pneumoniae liver abscess in East China. BMC Infect Dis. 2015;15(1):161. doi:10.1186/s12879-015-0899-7
40. Hennequin C, Robin F. Correlation between antimicrobial resistance and virulence in Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 2016;35(3):333–341. doi:10.1007/s10096-015-2559-7
41. Wyres KL, Nguyen TNT, Lam MMC, et al. Genomic surveillance for hypervirulence and multi-drug resistance in invasive Klebsiella pneumoniae from South and Southeast Asia. Genome Med. 2020;12(1):1–16. doi:10.1186/s13073-019-0706-y
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
