Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Analyses of protein corona on bare and silica-coated gold nanorods against four mammalian cells
Received 21 October 2014
Accepted for publication 7 December 2014
Published 20 February 2015 Volume 2015:10(1) Pages 1521—1545
DOI https://doi.org/10.2147/IJN.S76187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Minakshi Das,1 Dong Kee Yi,2 Seong Soo A An1
1Department of Bionanotechnology, Gachon University, Seongnam, Republic of Korea; 2Department of Chemistry, Myongji University, Yongin, Republic of Korea
Abstract: The purpose of this study was to investigate the mechanisms responsible for the toxic effects of gold nanorods (AuNRs). Here, a comprehensive study was performed by examining the effects of bare (uncoated) AuNRs and AuNRs functionalized with silica (SiO2-AuNRs) against various mammalian cell lines, including cervical cancer cells, fibroblast cells, human umbilical vein endothelial cells, and neuroblastoma cells. The interactions between AuNRs and mammalian cells were investigated with cell viability and mortality assays. Dihydrorhodamine-123 assay was carried out for evaluating reactive oxygen species (ROS) generation, along with mass spectroscopy analysis for determining the composition of the protein corona. Our results suggest that even the lowest concentrations of AuNRs (0.7 µg/mL) induced ROS production leading to cell mortality. On the other hand, cellular viability and ROS production were maintained even at a higher concentration of SiO2-coated AuNRs (12 µg/mL). The increased production of ROS by AuNRs seemed to cause the toxicity observed in all four mammalian cell types. The protein corona on the bare AuNRs did not appear to reduce ROS generation; however, different compositions of the protein corona on bare and SiO2-coated AuNRs may affect cellular behavior differently. Therefore, it was determined that SiO2-coated AuNRs would be more advantageous than bare AuNRs for cellular applications.
Keywords: gold nanorods, silica coating, oxidative stress, mammalian cells, cell toxicity, protein corona
Introduction
Nanoparticles are comparable in size with many organic entities and subcellular compounds; thus, nanoparticles may interact with a range of biological systems, depending on composition and specific application. Such interactions may be helpful for treating various diseases; however, the use of these nanoparticles could also have adverse effects leading to toxicity and cellular death. Numerous studies have described the interactions of gold nanorods (AuNRs) with various mammalian cells.1–3 AuNRs have been described as not entering the nucleus but remaining entrapped in the vesicles. However, the exact pathway for AuNRs is not known. In general, larger particles move through cells via phagocytosis, whereas receptor-mediated endocytosis is considered the most important working mechanism.4
While it is essential to understand the potential benefits of AuNRs on cellular behavior, it is equally important to address any toxicity concerns. Thus, another key challenge is determining the exact mechanism responsible for cellular toxicity. Several biochemical tests have been applied to determine the levels of nanomaterials that were toxic to cell lines, using viability, reactive oxygen species (ROS), and genotoxicity assays.5 Two major components were deemed to be responsible for the toxic effects of AuNRs covered with surface ligands, which were identified as cetyltrimethylammonium bromide (CTAB) bilayers and residual or desorbed reagent-free CTAB molecules. This surfactant was shown to be poisonous to cells, even at low concentrations.1 In addition, ROS formation and cellular oxidative stress were proposed as possible mechanisms for nanoparticle toxicity.6–8 It was reported that oxidative stress by nanoparticles was correlated with increased ROS.9 Furthermore, the interaction of nanomaterials with the biological fluids creating protein layers on the surface of the nanomaterials, termed the “protein corona”, has garnered much attention. Therefore, in the present study, we investigated the interaction of nanomaterials and mammalian cells to determine the cytotoxicity mechanism induced by AuNRs and AuNRs functionalized with a silica coating (SiO2-AuNRs) in four different cell lines: cervical cancer cells (HeLa), fibroblast cells (FY-11), human umbilical vein endothelial cells (HUVECs), and neuroblastoma cells (SH-SY5Y). Cytotoxicity was analyzed using several cell viability assays, including the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay and a CellTiter-Glo® assay. For this evaluation, we focused on the oxidative effects induced by nanomaterials, which resulted in decreased cellular viability and increased cellular death. In addition, mass spectroscopy (MS) analysis indicated involvement of the protein corona layer formed on the AuNRs and SiO2-AuNRs in inducing free-radical production inside the cells.
Materials and methods
Materials
Gold (III)chloride trihydrate (HAuCl4), sodium borohydride (NaBH4), CTAB, ascorbic acid (AA), and silver nitrate (AgNO3) were purchased from Sigma-Aldrich (St Louis, MO, USA). 3-Mercaptopropyltrimethoxy silane (MPS) and ammonium hydroxide (NH4OH) were purchased from Aldrich (Milwaukee, WI, USA). Ultrapure deionized water was used for preparing all solutions and subsequent experiments.
Phosphate-buffered saline (PBS, pH 7.4), MTT assay reagent, and dihydrorhodamine-123 (DHR) were purchased from Sigma-Aldrich. Dulbecco’s Modified Eagle’s Medium (DMEM) and DMEM/F12 were purchased from Gibco-Invitrogen (Grand Island, NY, USA). Endothelial Cell Basal Medium-2 (EBM-2) was purchased from Lonza (Walkersville, MD, USA). Heat-inactivated fetal bovine serum (FBS), penicillin and streptomycin, and other tissue culture reagents were purchased from Thermo Scientific (Waltham, MA, USA). The CellTiter-Glo® assay kit was purchased from Promega (Madison, WI, USA).
Synthesis of AuNRs
AuNRs were synthesized according to previously described methods.10 Briefly, 0.25 mL of aqueous 0.01 M HAuCl4·3H2O solution and 7.5 mL of 0.10 M CTAB were mixed in a conical flask, after which 0.6 mL of 0.01 M ice-cold NaBH4 solution was added to the flask. Following the addition of NaBH4, the clear white solution turned to a brick-brown color, indicating the formation of Au nanoparticles. This solution was aged for 2.5 hours at 25°C–28°C to form the seed solution.
Meanwhile, in another beaker, 9.5 mL of 0.1 M CTAB, 0.4 mL of 0.01 M HAuCl4·3H2O, and 0.03 mL of 0.01 M AgNO3 were mixed. A volume of 0.064 mL of 0.1 M ascorbic acid was added to the mixture, which immediately turned the solution from orange–yellow to colorless. Finally, 0.010 mL of the seed solution was added, the solution was gently mixed for 10 seconds, and then it was left undisturbed for 24 hours.
Synthesis of SiO2-AuNRs
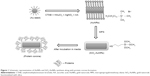
SiO2-AuNRs were synthesized according to a previously published method.11–14 A volume of 3 mL of the AuNRs solution was centrifuged to remove excess CTAB and was redispersed in distilled water. A solution containing 5.58 μL of MPS in 20 mL of ethanol was prepared, and 80 μL of the solution was added to the AuNRs solution under vigorous magnetic stirring for 30 minutes to achieve a silica coating of intermediate thickness (around 3 nm). A volume of 20 μL of NH4OH (pH 9) was then added, and the solution was vigorously stirred for 1 hour. The solution was washed three times with ethanol by centrifugation and was treated with 70% ethanol to eliminate any bacteria. A schematic representation of the AuNRs and SiO2-AuNR synthesis process is shown in Figure 1.
Characterizations
Transmission electron microscopy (TEM) was performed using a JEM-3010 microscope (JEOL, Tokyo, Japan) operating at 300 kV. TEM samples were prepared by depositing 10 μL of the SiO2-AuNRs suspension on carbon-coated copper grids, which was followed by the removal of excess solution and vacuum drying in an oven for 24 hours. Ultraviolet-visible (UV-Vis) spectra of the SiO2-AuNRs were measured using an Optizen 2120 UV spectrophotometer (Mecasys, Daejeon, Korea) from 400 nm to 1,000 nm in 1 cm cuvettes. Zeta potential measurements were conducted using a Zetasizer Nano ZS system (Malvern Instruments, Malvern, UK).
Cell culture and treatment with AuNRs/SiO2-AuNRs
FY-11 cells were cultured in DMEM containing 10% (v/v) FBS and 1% (w/v) penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2 for 24 hours. Then, cells were seeded at a density of 2×104 cells/well in flat-bottom 96-well plates (SPL Life Sciences, Seoul, Korea) and maintained at the same temperature and atmospheric conditions for up to 24 hours to allow cells to attach to the bottom of the plate. The cells were then washed with PBS and treated with AuNRs and SiO2-AuNRs in a serum-free medium at concentrations ranging from 0.7 μg/mL to 12 μg/mL for 24 hours. After 24 hours, the medium was removed and the cells were washed twice with PBS to remove excess NRs. Cells cultured with NR-free medium served as control samples.
Cell viability analysis using MTT assay
The effect of AuNRs and SiO2-AuNRs on FY-11 cell viability was evaluated using an MTT colorimetric assay, as described in previous studies.15 MTT solution (approximately 0.5 mg/mL) was added to wells containing fresh medium and previously cultured cells. The cultures were incubated at 37°C for 2 hours. Formazan crystals were then dissolved in dimethyl sulfoxide by discarding the medium. UV absorbance was measured using a microplate reader, and the data were interpreted as the percentage of viable cells relative to the control. The same procedure was repeated for HeLa cells, but for the SH-SY5Y and HUVEC cells, DMEM/F12 and EBM-2 medium, respectively, was used for culturing the cells.
CellTiter-Glo® assay of cell viability
The CellTiter-Glo® assay was performed according to the manufacturer’s protocol. The previously described culture procedures were repeated for the HeLa cells. DMEM/F12 and EBM-2 medium was used for culturing the SH-SY5Y and HUVEC cells, respectively.
Cell mortality assay
Cell mortality was assessed as previously described6 using a Trypan blue assay (Sigma). The FY-11 cells were plated in 12-well plates, with each well containing 2×104 cells/well. The cells were treated with various concentrations of AuNRs and SiO2-AuNRs (0.7, 1.5, 3, 6, and 12 μg/mL) that were added to the culture medium. Cells cultured in nanoparticle-free medium were used as the control. After 24 hours, the supernatant was collected, and the cells were detached with 300 μL of trypsin–ethylenediaminetetraacetic acid solution. The mixture, consisting of the supernatant and detached cells, was centrifuged at 1,200 rpm for 5 minutes. The obtained pellet was then dispersed in 500 μL of Trypan blue. After staining for 5 minutes, the cells were counted using a Countess Automated Cell Counter (Invitrogen, Grand Island, NY, USA). Cell mortality (%) was expressed in terms of dead cell number/total cell number. This procedure was repeated for the HeLa, SH-SY5Y, and HUVEC cell lines.
Measurement of intracellular ROS
ROS generation was determined using DHR-123, as described previously.7 Cells were plated into 96-well plates. After 24 hours of incubation, the medium was discarded, and the cells were preincubated with 100 μL of 10 μM DHR-123 solution and the growth medium at 5% CO2, 95% air at 37°C for 30 minutes. Following the incubation period, the medium was removed and cells were washed three times with PBS. The cells were then exposed to AuNRs and SiO2-AuNRs at concentrations of 0.75, 1.5, 3, 6, and 12 μg/mL for 24 hours. The fluorescence intensity of each well was analyzed using a microplate reader (Victor 3; Perkin-Elmer, Waltham, MA, USA) with an excitation filter of 485 nm and an emission filter of 535 nm. This procedure was repeated for the HeLa, SH-SY5Y, and HUVEC cell lines.
Identification of the protein corona using MS
AuNRs and SiO2-AuNRs were incubated in DMEM and Roswell Park Memorial Institute (RPMI) medium for 1 hour at 37°C with rotation. After 1 hour, the samples were centrifuged at 18,000× g for 30 minutes, and the supernatant was discarded. PBS was then added to resuspend the AuNRs and SiO2-AuNRs. This washing procedure was repeated three times, and the samples were then sent for MS determination at Diatech (Korea) to confirm the formation of the protein corona.
Statistical analysis
Statistical analysis performed was based on three replicates of each experiment. The significant differences were examined using Student’s t-test. Significance was analyzed at P<0.05.
Results
Characterization of AuNRs and SiO2-AuNRs
The CTAB-stabilized AuNRs were encapsulated with a CTAB bilayer on their surface. For typical SiO2-AuNRs synthesis, removal of the unbound CTAB is essential; therefore, the washing step must be performed very carefully. Here, with the use of a silane-coupling agent, uniform layers of SiO2 were formed, with an aspect ratio of 3.0±0.2. A uniform silica coating over AuNRs can be seen in Figure 2.
  | Figure 2 Transmission electron microscope images of AuNRs (A) and intermediate SiO2-AuNRs, showing a silica shell thickness of around 3 nm (B and C). |
Characterization UV-Vis spectra
The UV-Vis spectra of the AuNRs before and after coating with SiO2 showed that the physiochemical properties of the AuNRs are altered (Figure 3). The prepared AuNRs have a weak transverse plasmon band at 522 nm and a strong longitudinal plasmon band at 630 nm, whereas for the SiO2-AuNRs, the longitudinal surface plasmon band was red-shifted by 5 nm. This shift is attributed to an increase in the local refractive index of the medium surrounding the AuNRs after the formation of SiO2 shell.
Characterization of zeta potential
The AuNR surface is positively charged due to the presence of polycations; thus, the zeta potential value was observed to be 66.2 mV, whereas after coating with SiO2, the surface becomes negatively charged with a value of −25.7 mV, as shown in Figure 4A and B. These zeta potential values confirm the stability and decreased aggregation of the AuNRs and SiO2-AuNRs, and therefore the zeta potential results confirm the coating of the AuNR surfaces with SiO2.
Cellular viability based on the CellTiter-Glo® assay
The mitochondrial function and cellular viability of the HeLa, FY-11, SH-SY5Y, and HUVEC cells, in the presence of AuNRs and SiO2-AuNRs, are shown in Figure 5A–D. AuNRs induced toxicity even at the lowest concentration, whereas SiO2-AuNRs maintained more than 80% of cellular viability for all concentrations. Similar viability was observed in the case of all four cell types.
Cellular viability based on MTT assay
The cytotoxicity of AuNRs incubated with the cells was shown to be quite high, decreasing metabolic activity by about 50%, whereas even at high SiO2-AuNRs concentrations, 80% viability was maintained, as shown in Figure 6A–D. As shown in Figure 6, the toxic effect of the AuNRs on mitochondrial activity increased with increasing concentrations.
Cellular mortality
In this study, cellular mortality was monitored using the Trypan blue assay, where dead cells were stained blue, while live cells remained unchanged. Mortality was expressed as the ratio of dead cells to total cells. Here, greater cell mortality (%) was observed in the presence of AuNRs, whereas cellular mortality was relatively low with the SiO2-AuNRs. As shown in Figure 7, the HeLa, FY-11, SH-SY5Y, and HUVEC cells had an average mortality percentage of around 0.4%, even at the highest concentration of 12 μg/mL SiO2-AuNRs, whereas mortality even at lower concentrations of AuNRs was quite high at around 0.3% for all cells. For the SiO2-AuNRs, cellular mortality remained almost similar to the control for all cells, while the AuNRs exhibited relatively high cell mortality.
NR-induced ROS generation
The formation of intracellular free-radical levels could induce oxidative damage to cellular components, ultimately resulting in necrosis. The potential of AuNRs and SiO2-AuNRs to induce oxidative stress was determined by measuring the ROS levels. Significant ROS elevation was observed for the HeLa, FY-11, SH-SY5Y, and HUVEC cells after 24 hours of exposure to the AuNRs and SiO2-AuNRs at concentrations including 0.7, 1.5, 3, 6, and 12 μg/mL. These results demonstrate that the formation of free radicals is significantly induced by AuNRs and SiO2-AuNRs in different cell lines. Based on these obtained results, for 12 hours, a negligible increase in the production of hydrogen peroxide (H2O2), hydroxyl radical (•OH), and superoxide anion (O2•−) was observed from the AuNRs, whereas the production from the SiO2-AuNRs was almost similar to the control. After an 18-hour incubation period, the AuNRs increased ROS production by an average of 20%. Cells containing SiO2-AuNRs exhibited a slight increase in ROS by around 5%, which was similar to the control. Finally, after 24 hours of incubation, it was observed that cells treated with AuNRs produced a higher percentage of H2O2, •OH, and O2•− than those treated with SiO2-AuNRs did, as shown in Figure 8, which led to cellular death. AuNRs produced almost 60% of free radicals in all cell lines, whereas the production induced by the SiO2-AuNRs remained around 20%, resulting in greater cellular viability.
Effects of the protein corona on NRs
To present a comprehensive characterization, MS analysis was performed to determine the biomolecular entities formed by dispersing AuNRs and SiO2-AuNRs into the cell culture medium. Table 1 reports the number of proteins attached to AuNRs and SiO2-AuNRs incubated in DMEM and RPMI.
This analysis showed that in both types of cell culture medium (DMEM and RPMI), the total number of proteins attached to the AuNR surface was less than that of the SiO2-AuNR surface. As previously shown,16 this nano–bio interface is due to three dynamically interacting components: 1) the surface of NRs whose characteristics are determined by their physiochemical composition, 2) the changes that occur following the solid–liquid interface when the particle interacts with components in the surrounding medium, and 3) the contact area of the solid–liquid interface with its biological substrates. Specific NR properties have been shown to greatly contribute to the NR interactions with medium.17 For example, certain NR properties may result in increased adsorption of ions, proteins, organic materials, and detergents, which permits double-layer formation and minimizes the free surface energy by surface modification. There are several factors affecting the protein corona formation, including protein–AuNRs interactions, protein–protein interactions, and protein–medium interactions.18
As shown in Figure 9, the DMEM and RPMI mediums encouraged the attachment of a comparable number of proteins. The major difference observed was with the surface charges, as the number of unique proteins attached in the AuNRs increases by approximately 50% more than that of the SiO2-AuNRs. The difference in surface charge encouraged the formation of different hybrid bionanostructures that, in turn, exerted different biological effects when interacting with cells. Furthermore, as shown in Figure 10, the unique proteins that got attached to the AuNRs were cell matrix adhesion proteins. These cell matrix adhesion proteins were present in DMEM- and RPMI-incubated AuNRs, whereas they were absent in DMEM- and RPMI-incubated SiO2-AuNRs.
The main species of cell matrix adhesion proteins that were adsorbed onto the metallic NR surfaces were identified as important proteins involved in key biological processes (Table 2), which included alpha actinin 1 (actin-binding proteins, several roles in several cells), fibronectin (extracellular matrix glycoprotein, binds to integrins and plays a role in cell adhesion, growth, migration, differentiation, wound healing, and embryonic growth), angiotensin (peptide hormone, causes vasoconstriction and increase in blood pressure), nidogen (basement membrane glycoprotein, plays a role in cell–extracellular matrix interactions), and vitronectin (glycoprotein found in serum and extracellular matrix, plays a role in cell adhesion and spreading).
Discussion
Interest in the use of AuNRs for biomedical applications has grown due to their unique physiochemical properties; however, their current use has been limited because of major concerns over their toxicity. AuNRs are toxic to cells due to the presence of CTAB, which is required for AuNR stabilization. Therefore, surface functionalization, such as with SiO2, has been used in an effort to reduce AuNR toxicity.
For AuNRs, the intensity of the longitudinal plasmon band corresponding to the long axis of the NRs has been shown to be much higher than that of the transverse plasmon band corresponding to the short axis of the NRs, because of the enhanced surface electric field due to surface plasmon excitation.15 Therefore, in the case of SiO2-AuNRs, the shift in the longitudinal plasmon band is larger than that of the transverse band, which is at a wavelength close to the characteristic band of Au nanoparticles of similar diameter.
Every particle in a mixture carries some charge, which is typically negative rather than positive. This is attributed to the presence of chemical groups on the surface of the particle, which are ionized to form a charged surface. Sometimes, ions with an opposite charge may be adsorbed to the surface, and at other times chemical compounds may be intentionally added to yield a specific charge. In this study, CTAB acts as a chemical compound that generates the charge for the AuNRs. Since the CTAB-stabilized AuNRs possess a highly positive surface charge, the wrapping of negative ions around the metal is strongly favored. Hence, a SiO2 coating over the AuNRs was formed, and zeta potential analysis was performed to confirm the coating of SiO2-AuNRs.
In the present study, a visible difference in cell viability was observed based on the results of the viability assays used, as different mechanisms were involved. For the MTT assay, water-soluble MTT was converted to an insoluble formazan crystal. The formazan was then solubilized by inorganic solvent, and the concentration was determined by determining the optical density at 570 nm. Alternatively, for the CellTiter-Glo® assay, a homogeneous method based on the amount of adenosine triphosphate (ATP) present, which indicates the presence of metabolically active cells, was used to determine the number of viable cells.
Typically, polycationic materials exhibit higher cytotoxicity. AuNRs stabilized with CTAB and washed once with water by centrifugation showed strong cytotoxicity due to free CTAB remaining in the solution. However, in the case of the SiO2-AuNRs, more than 70% cell viability was observed even at the highest concentrations, indicating that the removal of excess CTAB and modification with SiO2 contributed to a significant decrease in cytotoxicity. The SiO2-AuNRs exhibited lower toxicity, which is essential for biomedical applications using AuNRs. In addition, the absence of CTAB on the AuNR surfaces has been shown to affect biological processes inside the cells, while binding to the cell membrane.19
Since material properties affect the kinetics of cell death, the mechanisms of nanomaterial-mediated cell toxicity may vary depending on the composition of the material each cell type interacts with. ROS generation has been suggested as an initial cellular response to nanomaterial internalization and subsequent cell death. In this study, the nanomaterial-mediated cell responses prior to cell death, specifically the production of intracellular ROS, were measured at 6 hours via the DHR assay. Our results showed that AuNRs increased the production of ROS in all cells by 60%, depending on the time, whereas ROS production for the SiO2-AuNRs was negligible. These results indicated that SiO2 scavenged the production of the ROS. The mechanism for the production of free radicals and its relation to toxicity are described as follows.
Typically, ROS generated by cells within an enclosed environment may easily turn into a source of cell and tissue injury. O2 is essential for human survival, and aerobic energy metabolism depends upon oxidative phosphorylation, which plays a vital role through which the oxidoreduction energy of mitochondrial electron transport is eventually converted to the high-energy phosphate bond of ATP. Aerobic organisms use O2 as the final electron acceptor for mitochondrial cytochrome c oxidase, which is able to catalyze the four electron reduction of O2, leading to H2O formation (Equation 1). During mitochondrial oxidative phosphorylation, and other electron transfer reactions, including those of the superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH) can be formed within cells (Equation 2). These reactive O2 metabolites are usually collectively referred to as ROS, and their generation in a biological environment exposes most living organisms to the so-called “oxygen paradox”. O2 is essential for life, but it is also potentially hazardous, since ROS may become a source of cell and tissue injury.
Equation 1
In a human body, four electron reduction reactions occur, leading from O2 to H2O production:
- O2 + e− → O2•− (+H+ ↔ HO2•)
- O2•− + e− → (O22− + 2H+) →H2O2
- H2O2 + e− → •OH + OH−
- OH + e− → +H+ → H2O.
Equation 2
When the human body is exposed to a foreign material (metal nanoparticles), then carbon-centered free radicals are generated by interaction with ROS:
- R–H + •OH → R• + H2O (organic radical)
- R• + O2 → ROO• (peroxyl radical)
- ROO• + R–H → ROOH + R• (organic peroxide)
- ROOH + nanoparticle → RO• + −OH + nanoparticle (alkoxy radical).
Thus, the free radicals produced may be very dangerous, leading to cell death. In this study, the results obtained were different for each cell type; however, the production of intracellular ROS increased due to the presence of AuNRs, while SiO2-AuNRs showed scavenging properties.
Based on the principles involved in ROS production, the question arises of what induces increased free-radical production within cells. Recently, many researchers have addressed the relationship between nanomaterials and biological fluid interaction. A layer formed over the nanomaterial has been defined as the “corona”.20 When NRs enter a biological fluid (medium), they are coated with proteins that may undergo conformational changes, leading to the exposure of new epitopes, altered function, and/or avidity effects.18 The concept of the NR–protein corona is important for tuning the surface properties, charges, resistance to aggregation, and toxicity of NRs. Notably, in this study, we showed that the interactive NR surface may be prebound to chemical substances that reflect its prior history and could subsequently influence its protein adsorption kinetics.
Conclusion
The results of this study demonstrate that the AuNRs due to the presence of CTAB molecules are the source of toxicity. Providing a coating over the CTAB-coated AuNRs with alkoxysilane is one way to prevent toxicity, which explains why the overcoated rods examined in this work were far more biocompatible.
In addition, we analyzed the protein corona formed over AuNRs and SiO2-AuNRs to determine any significant differences and to identify the cause of toxicity observed on the two types of NRs. Based on our hypothesis, we confirmed with MS that two different groups of protein corona were formed on the AuNRs and SiO2-AuNRs, respectively. The corona formation completely depended on the material surface properties. The MS data suggested the presence of cell matrix adhesion proteins on the AuNRs, and the absence of those proteins on the SiO2-AuNRs. Cell matrix adhesion proteins such as immunoglobulin are associated with the recognition and phagocytosis of NRs into the cells. The adsorbed proteins regulate the NR–cellular interactions, thus making them toxic or nontoxic to cells. In addition, different reports have shown that the corona could force the toxicity of materials.20 The presence of such proteins could be the reason for excessive free-radical production in cells leading to cell death. The list of proteins involved in corona formation are mentioned in the supplementary information. Hence, we concluded that the biological impact of the AuNRs was not exactly associated with their properties but associated with the attributes of the corona NR complexes. Further studies of the protein corona and its behavior could provide a clearer picture of this relationship.
Acknowledgments
This work was supported by the grants of National Research Foundation of Korea (NRF), funded by Korean government (MEST) (2012R1A2A2A03046819), and (2013R1A1A2005329). The authors would like to thank Kyu Hwan Shim for his help in generating ClueGo figures.
Disclosure
The authors report no conflicts of interest in this work.
References
Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5(6):701–708. | ||
Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4(1):153–159. | ||
Takahashi H, Niidome T, Kawano T, Yamada S, Niidome Y. Surface modification of gold nanorods using layer-by-layer technique for cellular uptake. J Nanopart Res. 2008;10:221–228. | ||
Kleps Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. | ||
Marquis BJ, Love SA, Braun KL, Haynes CL. Analytical methods to assess nanoparticle toxicity. Analyst. 2009;134(3):425–439. | ||
Chang Y, Yang ST, Liu JH, Dong E, Wang Y, Cao A, Liu Y, Wang H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett. 2011;200(3):201–210. | ||
Lee JY, Park W, Yi DK. Immunostimulatory effects of gold nanorod and silica-coated gold nanorod on RAW 264.7 mouse macrophages. Toxicol Lett. 2012;209(1):51–57. | ||
Tedesco S, Doyle H, Blasco J, Redmond G, Sheehan D. Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat Toxicol. 2010;100(2):178–186. | ||
Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol In Vitro. 2009;23(5):808–815. | ||
Sau TK, Murphy CJ. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir. 2004;20(15):6414–6420. | ||
Pérez-Juste J, Correa-Duarte MA, Liz-Marzán LM. Silica gels with tailored, gold nanorod-driven optical functionalities. Applied Surface Science. 2004;226:137–143. | ||
Cong H, Toftegaard R, Arnbjerg J, Ogilby PR. Silica-coated gold nanorods with a gold overcoat: controlling optical properties by controlling the dimensions of a gold-silica-gold layered nanoparticle. Langmuir. 2010;26(6):4188–4195. | ||
Gorelikov I, Matsuura N. Single-step coating of mesoporous silica on cetyltrimethyl ammonium bromide-capped nanoparticles. Nano Lett. 2008;8(1):369–373. | ||
Obare SO, Jana NR, Murphy CJ. Preparation of polystyrene- and silica-coated gold nanorods and their use as templates for the synthesis of hollow nanotubes. Nano Lett. 2001;1:601–603. | ||
Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110(14):7238–7248. | ||
Tansey W, Ke S, Cao XY, Pasuelo MJ, Wallace S, Li C. Synthesis and characterization of branched poly(L-glutamic acid) as a biodegradable drug carrier. J Control Release. 2004;94(1):39–51. | ||
Guo D, Wu C, Li X, Jiang H, Wang X, Chen B. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. J Nanosci Nanotechnol. 2008;8(5):2301–2307. | ||
Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. | ||
Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. | ||
Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano. 2012;6(7):5845–5857. |
Supplementary materials
As shown in this section, a list of proteins involved in the protein corona layer due to the incubation of gold nanorods (AuNRs) (bare) and gold nanorods functionalized with silica (SiO2-AuNRs) (Si) in Dulbecco’s Modified Eagle’s Medium (DMEM) or Roswell Park Memorial Institute (RPMI) medium is provided. As shown in the tables, “common proteins” indicates proteins involved in both DMEM-bare and DMEM-Si (Tables S3, S4, S7 and S8). On the other hand, “uncommon proteins” indicates unique proteins involved in either DMEM-bare or DMEM-Si. Similar designations are specified for the RPMI samples (Tables S5, S6, S9 and S10).
Here, we compared DMEM-bare with DMEM-Si (Table S1) (to show the difference between “bare” and “Si” in DMEM), RPMI-bare with RPMI-Si (Table S2) (to show the difference between “bare” and “Si” in RPMI), DMEM-bare with RPMI-bare (Table S9) (to show the difference between “DMEM” and “RPMI” in bare), and DMEM-Si with RPMI-Si (to show the difference between “DMEM” and “RPMI” in Si) (Table S10).
  | Table S1 Protein corona list of DMEM-bare and DMEM-Si |
  | Table S2 Protein corona list of RPMI-bare and RPMI-Si |
  | Table S9 List of AuNRs attached unique proteins involved in either DMEM or RPMI |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.