Back to Journals » Drug Design, Development and Therapy » Volume 14
Analgesic and Antiallodynic Effects of 4-Fluoro-N-(4-Sulfamoylbenzyl) Benzene Sulfonamide in a Murine Model of Pain
Authors Ur Rehman N , al-Rashida M, Tokhi A , Ahmed Z, Subhan F , Abbas M , Arshid MA, Rauf K
Received 28 June 2020
Accepted for publication 10 September 2020
Published 27 October 2020 Volume 2020:14 Pages 4511—4518
DOI https://doi.org/10.2147/DDDT.S269777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Naeem Ur Rehman,1 Mariya al-Rashida,2 Ahmed Tokhi,1 Zainab Ahmed,1 Fazal Subhan,1 Muzaffar Abbas,3 Muhammad Awais Arshid,4 Khalid Rauf1
1Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Pakistan; 2Department of Chemistry, Forman Christian College (A Chartered University), Lahore 54600, Pakistan; 3Department of Pharmacy, Capital University of Science and Technology (CUST), Islamabad, Pakistan; 4Aga Khan University Karachi, Karachi, Pakistan
Correspondence: Khalid Rauf Tel + (92) 345 9824468
Email [email protected]
Introduction: Physical, chemical, thermal injuries along with infectious diseases lead to acute pain with associated inflammation, being the primary cause of hospital visits. Moreover, neuropathic pain associated with diabetes is a serious chronic disease leading to high morbidity and poor quality of life.
Objective: Earlier multiple sulphonamides have been reported to have an antinociceptive and antiallodynic profile. 4-Fluoro-N-(4-sulfamoylbenzyl) Benzene Sulfonamide (4-FBS), a synthetic sulfonamide with reported carbonic anhydrase inhibitory activity, was investigated for its potential effects in mice model of acute and diabetic neuropathic pain.
Methods and Results: 4-FBS was given orally (p.o.) one hour before the test and then mice were screened for antinociceptive activity by using the tail immersion test, which showed significant antinociceptive effect at both 20 and 40 mg/kg doses. To explore the possible mechanisms, thermal analgesia of 4-FBS was reversed by the 5HT3 antagonist ondansetron 1mg/kg intraperitoneally (i.p.) and by the μ receptor antagonist naloxone (1 mg/kg i.p.), implying possible involvement of serotonergic and opioidergic pathways in the analgesic effect of 4-FBS. Diabetes was induced in mice by a single dose of streptozotocin (STZ) 200 mg/kg i.p. After two weeks, animals first became hyperalgesic and progressively allodynic in the fourth week, which was evaluated through behavioral parameters like thermal and mechanical tests. 4-FBS at 20 and 40 mg/kg p.o. significantly reversed diabetes-induced hyperalgesia and allodynia at 30, 60, 90, and 120 minutes.
Conclusion: These findings are significant and promising while further studies are warranted to explore the exact molecular mechanism and the potential of 4-FBS in diabetic neuropathic pain.
Keywords: sulfonamides, streptozotocin; STZ, antinociception, diabetes mellitus; DM, neuropathic pain, von Frey filaments
Introduction
Physical, thermal, and chemical noxious stimuli, and infectious diseases, are the leading cause of pain and associated inflammation.1,2 Acute pain is a protective mechanism of homeostasis but has considerable distress that leads to poor quality of life. Tissue and nerve damage translates into intensified pain states, which are driven by afferent traffic caused by otherwise harmless or slightly aversive mechanical and thermal stimuli evoking a behavioral response consistent with a more pronounced stimulus.3,4 Non-steroidal anti-inflammatory drugs (NSAIDs) are usually used for the treatment of mild to moderate painful states, but these drugs are associated with lasting side effects including gastric ulceration and subsequent bleeding.5 Opiates have also been used as narcotic analgesics but are coupled with serious adverse effects, primarily tolerance, and continued use is translated in the form of addiction and dependence.6
Diabetic peripheral neuropathy (DPN) is one of the leading causes of morbidity, affecting 30–50% individuals presented with diabetes mellitus (DM) and also contributes a greater cost to the health care systems of both developed and developing countries.7,8 The structural deformity of the peripheral nervous system and abnormal functioning of nerves that supply to organs develops a complex and unique pattern of symptoms and signs that are characteristic of diabetic neuropathy. These involve stocking-glove pattern of pain, burning pain especially at nights, lancinating or sharp pain, heightened pain response to touch, and pain from non-noxious stimuli or allodynia. All of these symptoms are called positive symptoms of DPN, while negative symptoms include numbness, cold, and loss of sensory sensation.9,10 Despite the prevalence of DPN and its contribution to disease burden on societies, several treatment options only relate to glucose control and weight loss. The currently available centrally acting drugs for DPN, like antidepressants, anticonvulsants, and opioids, produce the serious risk of use dependence and addiction.11
Research over recent decades has been conducted to look for non-opioid analgesics that can act via a central mechanism.12 Many studies indicate that the serotonergic pathway, primarily the 5-HT1A receptor activation, leads to a novel mechanism mimicking central neuroadaptive analgesic action upon receiving nociceptive stimulation.13
Sulfonamide derivatives have been documented to have diuretic,14 antiepileptic,15 analgesic and antiallodynic,16 anti-cancer,17 hepatoprotective, and GABAA modulatory activities.18 Additionally, sulfonamides have been documented to inhibit neuropathic pain, reverse mechanical hyperalgesia, and allodynia in a dose-dependent manner.19 Additionally, the carbonic anhydrase inhibitory effects of sulphonamides have been reported to reverse oxaliplatin-induced allodynia through reversing oxaliplatin-induced decrement in intracellular pH in mouse dorsal root ganglion (DRG) neurons.20 4-FBS has been reported to have a dual inhibitory effect on alkaline phosphatase and carbonic anhydrase,21 and recent data suggest carbonic anhydrase inhibitors as a potential newer drug target for the management of neuropathic pain.19 Keeping in view this diverse pharmacological profile of 4-FBS (alkaline phosphatase and carbonic anhydrase inhibitory effect), this study was designed to explore the potential of 4-FBS in a murine model of acute pain and diabetes-induced neuropathic pain.
Materials and Methods
Animals
Male BALB/c (n=6/group) mice 8- to 12-weeks-old (24–30 g), obtained from the National Institute of Health, Pakistan were randomly housed six (6) per cage and maintained at temperature 24 ± 1°C with 12 h dark/light cycle in the animal house facility of COMSATS University Islamabad, Abbottabad Campus. Standard rodent chow diet and water were provided ad libitum. All the behavioral experiments were performed during the light cycle from 8:00 am to 2:00 pm, to avoid disturbance in the diurnal rhythm. The experimentation on animals was performed in compliance with the UK Animals (Scientific Procedures) Act 1986 and accordance with the rules and ethics set by the Ethical Committee of COMSATS University Islamabad, Abbottabad campus under the approval Letter number PHM.Eth/cs-M04/11-34.
Treatment Schedule
Mice (n=6/group) were divided into five groups. All groups received streptozotocin (STZ) as a single high dose of 200 mg/kg i.p. once except the vehicle control group.22–24 Group, I served as a positive control (STZ group). Group II dimethyl sulfoxide (DMSO 5%) p.o. remained as a vehicle control group. Group III received gabapentin 75 mg/kg i.p. as the standard, while group IV and V received 4-FBS suspension 20 and 40 mg/kg p.o. via oral gavage dissolved in DMSO 5% to diabetic neuropathic mice and pain behavioral parameters like allodynia and hyperalgesia were evaluated at different time interval 30, 60, 90 and 120 minutes (min).
Drugs and Chemicals
Sulfonamide compound, i.e., 4-FBS was provided by Mariya Al Rashida,21 STZ was purchased from Sigma (St. Louis, MO, USA), naloxone 0.3 mg/mL (Brand name NALOX by Haji Medicines Co), ondansetron 8 mg/4mL (ONSET by Pharmedic Pvt Ltd) were purchased from the model retail pharmacy established in Ayub Teaching Hospital Abbottabad, Pakistan. One-touch basic blood glucose monitoring system by a Life scan was used for measuring blood glucose levels.
Tail Immersion Test
In the tail immersion test, a noxious pain stimulus was used to quantify thermal pain in naïve male BALB/c mice treated with 4-FBS 20 and 40 mg/kg against the normal saline group. About 1/3rd of the mouse tail was immersed into hot water which was maintained at 54± 0.5ᴼC (noxious pain stimulus for mice) and test recording (tail flick) was noted at 30, 60, 90, and 120 min. The time between the application of thermal stimulus and response, i.e., tail-flick latency was noted via digital stopwatch. A 15 seconds (s) cut-off time was set to avoid tissue injury.25,26
Reversal of Analgesia by Naloxone and Ondansetron
To explore the possible involvement of serotonergic27 and opioidergic pathways,28 selected groups of mice (n=6/group) were given 4-FBS 20 mg/kg p.o. separately. After one hour, the same mice received naloxone 1mg/kg i.p, while other 4-FBS treated group received ondansetron 1mg/kg i.p. and tail-flick latency was quantified against normal saline as described earlier.25,26
Induction and Assessment of Diabetic Neuropathic Pain
Mice were food-deprived overnight for 16 h before administration of a single dose of STZ 200 mg/kg to induce β-cell necrosis.22,24 Mice were immediately provided food and 10% sucrose solution to avoid severe hypoglycemia. After 72 h, mice having random blood glucose levels >250 mg/dl were included in the study.24 Blood glucose and body weight were measured at a different time interval during the experimentation. To avoid infection due to polyuria, mice sawdust bedding was changed on alternate days. After 4 weeks, on the 29th-day post STZ treatment, animals were transferred to a wire mesh cage, given an acclimatization period of 15–45 min, and then evaluated for thermal hyperalgesia and allodynia.29,30
Assessment of Static Allodynia (Paw Withdrawal Threshold (g))
Static allodynia was assessed using von Frey filaments ranging from 0.008 to 4 g. For this purpose, mice right hind paw was exposed through a mesh floor having a pore size of 10×10 cm and an opaque cup was used to avoid visual contact. An acclimatization period of 15–45 min was given for initial exploration and grooming.29,30 Filaments were applied for 6–8 seconds on the plantar surface of the hind paw perpendicularly until it distorted. The careful observation was carried not to touch the less sensitive tori (footpads) of mice with filaments. The von Frey filament was applied 05 times to the hind paw at intervals of several seconds to determine the mean score which also served as the pain related score. After this, the next filament was introduced in a descending pattern using up and down technique.30
Statistics
Data were expressed as means ± S.E.M. Parameters were examined for normality with the Shapiro–Wilk normality test and all data was found normal. One-way ANOVA was followed by Dunnett’s post hoc test. GraphPad Prism v.8.3.1 was used for the analysis of data. The statistical significance level was set as p< 0.05.
Results
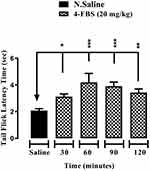
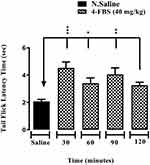
Effects of 4-FBS 20 and 40 mg/kg on Acute Thermal Antinociception Activity
As shown in Figures 1 and 2, 4-FBS at 20 and 40 mg/kg p.o. significantly *p<0.05, **p<0.01, ***p<0.001 elevated tail-flick latency time at 30, 60, 90, and 120 min, when compared with saline group except for 4-FBS 40 mg/kg at 120 min. One way ANOVA followed by Dunnett’s test was used as statistical analysis.
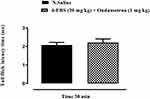
Effects of Ondansetron (1 mg/kg) and Naloxone (1 mg/kg) on 4-FBS 20 mg/kg Acute Thermal Antinociception Activity
As shown in Figures 3 and 4, two mice groups (n=6) received 4-FBS 20 mg/kg p.o. After one hour, they were administered ondansetron and naloxone 1 mg/kg i.p. separately, which abolished the anti-nociceptive effect of 4-FBS 20 mg/kg in the tail-flick latency time although the result was statistically (unpaired t-test) non-significant when compared with normal saline.
Effects of 4-FBS 20 and 40 mg/kg on STZ Induced Diabetic Thermal Hyperalgesia
As shown in Figure 5, 4-FBS at 20 and 40 mg/kg p.o. significantly **p<0.01, ***p<0.001 increased tail-flick latency time in STZ-induced diabetic thermal hyperalgesia at different time points of 30, 60 90, and 120 min except for 4-FBS 20 mg/kg at 120 min. It is pertinent to mention that using one-way ANOVA followed by Dunnett’s test, the 4-FBS 20 mg/kg result was comparable to the standard drug gabapentin 75 mg/kg at 30, 60, and 90 min, while 40mg/kg was comparable at time points of 30, 60, 90, and 120 min.
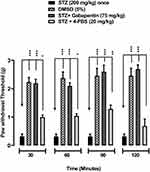
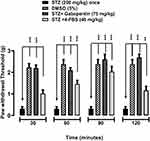
Effects of 4-FBS 20 and 40 mg/kg on STZ Induced Diabetic Static Allodynia
As shown in Figures 6 and 7, oral administration of 4-FBS 20 mg/kg to diabetic mice resulted in a significant **p<0.01, ***p<0.001 reversal of static allodynia by increasing paw withdrawal threshold at 30, 60, 90 min, while 4-FBS at 40 mg/kg increased paw withdrawal at 60, 90, 120 min. Application of one-way ANOVA followed by Dunnett’s test showed the response at 4-FBS 20 mg/kg 120 min and 4-FBS 40 mg/kg 30 min only as non-significant, although considerable paw withdrawal latency can be observed.
Discussion
Our findings showed that 4-FBS has significant antihyperalgesic, and antiallodynic effects in the murine model of diabetic neuropathic pain Figures 5–7. The compound also showed an analgesic effect in the acute thermal pain mice model at 30, 60, 90, and 120 minutes, which is a significant effect in terms of both potency and longevity (Figures 1 and 2). To ascertain the mechanism and involvement of serotonergic pathways, we tried to reverse the effect with ondansetron. Serotonergic receptors agonists specifically have been documented to reverse both hyperalgesia and allodynia in neuropathic pain models.31,32 The thermal pain effect was reversed with ondansetron dose, implying the role of serotonergic pathways primarily 5HT3 receptors in Figure 3. Serotonin plays a diverse role in pain modulation and control; primarily 5HT1B antagonists are used clinically in pain, for instance, tramadol, which partially imparts its analgesia by blocking 5HT1B.33 Tramadol along with other opiates are extensively used for the management of acute, chronic, and neuropathic pain.34–36 Additionally, tramadol has a serotonin reuptake inhibitor (SRI) effect that contributes to its analgesic effect.36 Studies have also documented that, tramadol analgesia quality and duration were reversed by ondansetron.27,37 More detailed receptor-specific studies are warranted using specified receptor antagonists for broader mechanistic studies. The acute thermal effect was also reversed by naloxone, implying some interactions with opioidergic pathways in Figure 4. It is pertinent to mention that reversal was made with 1 mg/kg naloxone and more studies are warranted to ascertain the involvement of specific opioid receptors.
Earlier studies have reported that some sulfonamides have analgesic, antihyperalgesic and antiallodynic effects, as well as a carbonic anhydrase inhibitory effect.17,19 In modulation of pain and hyperalgesia, dopamine plays a major role in different areas of the brain including basal ganglia,38 and it is one of the main sensitive pathways implicated in pain management.39 Dopamine D1 receptor antagonist SCH-23,390 attenuate prostaglandin E2 (PGE2) induced hyperalgesia and neuropathic allodynia.40 Computationally, 4-FBS is a D1 receptor antagonist (support file 1) and it may be suggested that its anti-nociceptive, anti-allodynic activity is due to its antagonistic activity on the D1 receptor. Further studies are warranted to validate both the behavioral and in-silico studies on receptor level using D1 receptor antagonist.
Diabetic neuropathic pain (DPN) is manifested via distal symmetrical polyneuropathy, which is characterized by numbness, stabbing sensations, tingling pain, and weakness of nerves in a stocking-and-glove pattern, beginning in the distal extremities. DPN leads to substantial pain, hyperalgesia, and allodynia which leads to poor quality of life and has been reported to have involvement of the peripheral nervous system and alter central pain processing.41 The polymorphic nature of this disease deleteriously affects different areas in the central nervous system compromising the performance of other body systems. Overexpression of cyclooxygenases seen in diabetes significantly contributes to the severity of pain, hyperalgesia, and discomfort.42,43 Its comorbidities including depression, anxiety, and insomnia make the treatment regimen problematic and challenging.8,44 At present, more than 415 million people suffer from diabetes worldwide and 30–50% have chronic diabetic neuropathy, and approximately 15% with allodynia.44,45 4-FBS significantly ameliorated thermal hyperalgesia at all doses in the animal model of DPN Figure 5 except at 120 min for 4-FBS 20 mg/kg. The compounds also showed a significant antiallodynic effect in diabetic neuropathic pain by reversing mechanical allodynia (Figures 6 and 7) except at 120 min for 4-FBS 20 mg/kg and 30 min for 4-FBS 40 mg/kg. Very few compounds have such a profile of reversing diverse types of pain and the compound effects resembling that of opiates.
4-FBS is a Carbonic anhydrase inhibitor21 and has been documented for a diverse role in neuropathic pain.19 Diabetic neuropathy leads to hyperalgesia and progressively results in allodynia. DPN is characterized by peripheral nerve injury, which negatively modulates spinal γ-aminobutyric (GABA)-ergic neuronal networks and causes a significant decrease in the neuron-specific potassium-chloride (K+-Cl−) co-transporter (KCC2), that translates in hyperalgesia and allodynia.10 Carbonic anhydrase inhibitors (CAIs) have been documented to decrease the bicarbonate-dependent depolarization of γ-aminobutyric (GABA) GABAA receptors, producing antiallodynic effects in neuropathic pain.18,19
Acetazolamide along with many other carbonic anhydrase inhibitors has been reported for a role in the management of neuropathic pain through various pathways.16 4-FBS has been reported to have a carbonic anhydrase inhibitory effect21 and this effect might be contributing partially to the analgesic properties of 4-FBS in the acute pain model as well as in diabetic neuropathic pain including both hyperalgesia and allodynia.
Limitations
These are purely behavioral studies and require more specific studies at the molecular level to explore the involvement of any specific opioid receptor, serotonergic receptor, or carbonic anhydrase inhibitory effects for its analgesic, antihyperalgesic and anti-allodynic, role in neuropathic pain models.
Acknowledgment
We thankfully acknowledge the language editing of the manuscript by Dr. Garcia Rivas University of Yale, USA.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Keating L, Smith S. Acute pain in the emergency department: the challenges. Rev Pain. 2011;5(3):13–17. doi:10.1177/204946371100500304
2. Michaelides A, Zis P. Depression, anxiety, and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–444. doi:10.1080/00325481.2019.1663705
3. Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurons and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi:10.1016/S0301-0082(99)00051-9
4. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3):S2–S15. doi:10.1016/j.pain.2010.09.030
5. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal anti-inflammatory drugs: an update of gastrointestinal, cardiovascular, and renal complications. J Pharm Pharm Sci. 2013;16(5):821–847. doi:10.18433/J3VW2F
6. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405. doi:10.1037/a0013628
7. Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff MR, Matthews DR. The prevalence, patterns, and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr. 2012;4(1):21. doi:10.1186/1758-5996-4-21
8. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
9. Vincent AM, Calabek B, Roberts L, Feldman EL. Biology of diabetic neuropathy. In: Handbook of Clinical Neurology. Vol. 115. Elsevier; 2013:591–606.
10. Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007;3(6):331–340. doi:10.1038/ncpneuro0504
11. Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol. 2020;72(7):863–872. doi:10.1111/jphp.13241
12. Colpaert F, Tarayre J, Koek W, et al. Large-amplitude 5-HT1A receptor activation: a new mechanism of profound, central analgesia. Neuropharmacol. 2002;43(6):945–958. doi:10.1016/S0028-3908(02)00119-3
13. Nadeson R, Goodchild C. Antinociceptive role of 5‐HT1A receptors in rat spinal cord. Br J Anaesth. 2002;88(5):679–684. doi:10.1093/bja/88.5.679
14. Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat. 2013;23(6):681–691. doi:10.1517/13543776.2013.780598
15. Aggarwal M, Kondeti B, McKenna R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat. 2013;23(6):717–724. doi:10.1517/13543776.2013.782394
16. Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148(2):302–308. doi:10.1016/j.pain.2009.11.015
17. Krall N, Pretto F, Decurtins W, Bernardes GJ, Supuran CT, Neri D. A small‐molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed. 2014;53(16):4231–4235. doi:10.1002/anie.201310709
18. Asiedu MN, Mejia GL, Hübner CA, Kaila K, Price TJ. Inhibition of carbonic anhydrase augments GABAA receptor-mediated analgesia via a spinal mechanism of action. J Pain. 2014;15(4):395–406. doi:10.1016/j.jpain.2014.01.001
19. Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother. 2016;16(8):961–968.
20. Potenzieri A, Riva B, Rigolio R, et al. Oxaliplatin-induced neuropathy occurs through impairment of haemoglobin proton buffering and is reversed by carbonic anhydrase inhibitors. Pain. 2020;161(2):405–415.
21. al-Rashida M, Ejaz SA, Ali S, et al. Diarylsulfonamides and their bioisosteres as dual inhibitors of alkaline phosphatase and carbonic anhydrase: structure activity relationship and molecular modelling studies. Bioorg Med Chem. 2015;23(10):2435–2444. doi:10.1016/j.bmc.2015.03.054
22. Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):
23. Biessels G, Bril V, Calcutt N, et al. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab). J Peripher Nerv Syst. 2014;19(2):77–87. doi:10.1111/jns5.12072
24. Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193(4251):415–417. doi:10.1126/science.180605
25. Ramabadran K, Bansinath M, Turndorf H, Puig MM. Tail immersion test for the evaluation of a nociceptive reaction in mice: methodological considerations. J Pharmacol Methods. 1989;21(1):21–31. doi:10.1016/0160-5402(89)90019-3
26. Awe E, Adeloye A, Idowu T, Olajide OA, Makinde J. Antinociceptive effect of Russelia equisetiformis leave extracts: identification of its active constituents. Phytomedicine. 2008;15(4):301–305. doi:10.1016/j.phymed.2007.03.012
27. Arcioni R, Della Rocca M, Romanò S, Romano R, Pietropaoli P, Gasparetto A. Ondansetron inhibits the analgesic effects of tramadol: a possible 5-HT3 spinal receptor involvement in acute pain in humans. Anesth Analg. 2002;94(6):1553–1557. doi:10.1213/00000539-200206000-00033
28. Santos AR, Gadotti VM, Oliveira GL, et al. Mechanisms involved in the antinociception caused by agmatine in mice. Neuropharmacol. 2005;48(7):1021–1034. doi:10.1016/j.neuropharm.2005.01.012
29. Aman U, Subhan F, Shahid M, et al. Passiflora incarnata attenuation of neuropathic allodynia and vulvodynia apropos GABA-ergic and opioidergic antinociceptive and behavioral mechanisms. BMC Complement Altern Med. 2016;16(1):77. doi:10.1186/s12906-016-1048-6
30. Chaplan SR, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
31. Pero JE, Rossi MA, Lehman HD, et al. Benzoxazolinone aryl sulfonamides as potent, selective Nav1. 7 inhibitors with in vivo efficacy in a preclinical pain model. Bioorg Med Chem Lett. 2017;27(12):2683–2688. doi:10.1016/j.bmcl.2017.04.040
32. Panczyk K, Golda S, Waszkielewicz A, Zelaszczyk D, Gunia-Krzyzak A, Marona H. Serotonergic system and its role in epilepsy and neuropathic pain treatment: a review based on receptor ligands. Curr Pharm Des. 2015;21(13):1723–1740. doi:10.2174/1381612821666141121114917
33. Fowler M, Clifford JL, Garza TH, et al. A rat model of full-thickness thermal injury characterized by thermal hyperalgesia, mechanical allodynia, pronociceptive peptide release, and tramadol analgesia. Burns. 2014;40(4):759–771. doi:10.1016/j.burns.2013.10.011
34. Tiwari V, Anderson M, Yang F, et al. Peripherally acting μ-opioid receptor agonists attenuate ongoing pain-associated behavior and spontaneous neuronal activity after nerve injury in rats. Anesthesiology. 2018;128(6):1220–1236. doi:10.1097/ALN.0000000000002191
35. Cooper TE, Chen J, Wiffen PJ, et al. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;(5). doi:10.1002/14651858.CD011669.pub2
36. Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg. 2017;124(1):44–51. doi:10.1213/ANE.0000000000001683
37. Murmua A, Kundua SB, Pahari A, et al. Effect of ondansetron on the analgesic efficacy of tramadol used for postoperative analgesia: a randomised controlled study. South Afr J Anaesth Analg. 2015;21(5):16–20.
38. Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8(5):781–797. doi:10.1586/14737175.8.5.781
39. Faramarzi G, Zendehdel M, Haghparast A. D1‐and D2‐like dopamine receptors within the nucleus accumbens contribute to stress‐induced analgesia in formalin‐related pain behaviours in rats. Eur J Pain. 2016;20(9):1423–1432. doi:10.1002/ejp.865
40. Megat S, Shiers S, Moy JK, et al. A critical role for dopamine D5 receptors in pain chronicity in male mice. J Neurosci. 2018;38(2):379–397. doi:10.1523/JNEUROSCI.2110-17.2017
41. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi:10.1016/S1474-4422(12)70065-0
42. Ramos KM, Jiang Y, Svensson CI, Calcutt NA. Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes. 2007;56(6):1569–1576. doi:10.2337/db06-1269
43. Daulhac L, Mallet C, Courteix C, et al. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70(4):1246–1254. doi:10.1124/mol.106.025478
44. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
45. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. 2011;34(10):2220–2224. doi:10.2337/dc11-1108
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.