Back to Journals » International Journal of Nanomedicine » Volume 17
An Update on Novel Ocular Nanosystems with Possible Benefits in the Treatment of Corneal Neovascularization
Authors Zhang C , Yin Y, Zhao J, Li Y, Wang Y, Zhang Z , Niu L, Zheng Y
Received 27 May 2022
Accepted for publication 2 October 2022
Published 19 October 2022 Volume 2022:17 Pages 4911—4931
DOI https://doi.org/10.2147/IJN.S375570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Chenchen Zhang,1 Yuan Yin,1 Jing Zhao,1 Yanxia Li,1 Yuanping Wang,1 Zhaoying Zhang,1 Lingzhi Niu,2 Yajuan Zheng1
1Department of Ophthalmology, The Second Hospital of Jilin University, Changchun, People’s Republic of China; 2Department of Ophthalmology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, People’s Republic of China
Correspondence: Yajuan Zheng, Email [email protected]
Abstract: Corneal neovascularization (CNV) is an ocular pathological change that results from an imbalance between angiogenic factors and antiangiogenic factors as a result of various ocular insults, including infection, inflammation, hypoxia, trauma, corneal degeneration, and corneal transplantation. Current clinical strategies for the treatment of CNV include pharmacological treatment and surgical intervention. Despite some degree of success, the current treatment strategies are restricted by limited efficacy, adverse effects, and a short duration of action. Recently, gene-based antiangiogenic therapy has become an emerging strategy that has attracted considerable interest. However, potential complications with the use of viral vectors, such as potential genotoxicity resulting from long-term expression and nonspecific targeting, cannot be ignored. The use of ocular nanosystems (ONS) based on nanotechnology has emerged as a great advantage in ocular disease treatment during the last two decades. The potential functions of ONS range from nanocarriers, which deliver drugs and genes to target sites in the eye, to therapeutic agents themselves. Various preclinical studies conducted to date have demonstrated promising results of the use of ONS in the treatment of CNV. In this review, we provide an overview of CNV and its current therapeutic strategies and summarize the properties and applications of various ONS related to the treatment of CNV reported to date. Our goal is to provide a comprehensive review of these considerable advances in ONS in the field of CNV therapy over the past two decades to fill the gaps in previous related reports. Finally, we discuss existing challenges and future perspectives of the use of ONS in CNV therapy, with the goal of providing a theoretical contribution to facilitate future practical growth in the area.
Keywords: ocular nanosystems, nanocarriers, corneal neovascularization, drug delivery, gene therapy
Graphical Abstract:
Introduction
Corneal neovascularization (CNV), also called corneal angiogenesis, involves the formation of new vascular structures in transparent cornea areas that were previously avascular. Such pathological changes can lead to tissue scarring, edema, lipid deposition, and persistent inflammation that may significantly affect visual prognosis and quality of life if not addressed promptly.1 In addition, since it is a crucial risk factor for corneal graft rejection, its treatment before any keratoplasty procedure is essential.2 Corneal angiogenesis results from an imbalance between angiogenic and antiangiogenic factors that preserve corneal transparency (Created with BioRender.com) as a result of various ocular insults, including infection, inflammation, hypoxia, trauma, corneal degeneration, and corneal transplantation.1,3 Current clinical treatment strategies for CNV include pharmacological treatment, such as the use of anti-inflammatory and immunosuppressive agents and vascular endothelial growth factor (VEGF) inhibitors, or surgical intervention, including laser ablation, photodynamic therapy (PDT), and fine-needle diathermy (FND).4 Despite some degree of success, the current treatment strategies are restricted by limited efficacy, adverse effects, and a short duration of action.5 Recently, gene-based antiangiogenic therapy has become an emerging strategy that has attracted considerable interest.6 However, potential complications with the use of viral vectors, such as potential genotoxicity resulting from long-term expression and nonspecific targeting, cannot be ignored.7
Nanotechnology is a scientific nanoscale engineering technology that involves elements at the nanometer scale. By applying nanostructures in various fields of science, nanotechnology has been shown to bridge the barriers of biological and chemical-physical sciences.8 To date, nanotechnology has been used in almost all areas of medical science, including imaging, diagnostics, biosensors and drug delivery.9 The use of nanotechnology-based novel ocular nanosystems (ONS) in various ocular disease treatments has been investigated for the last two decades.10,11 The potential functions of ONS range from nanocarriers—which deliver drugs and genes to the targeted site of the eye—to therapeutic agents themselves. The novel ONS may offer new perspectives in the treatment of ocular diseases by realizing targeted delivery, allowing controlled release, ensuring low eye irritation, improving drug bioavailability or enhancing ocular tissue compatibility.12
Recent advancements in the field of ONS could circumvent the above limitations existing in the current therapeutic strategies for CNV, and various preclinical studies conducted to date have demonstrated promising results of ONS in the treatment of CNV. In this review, we provide an overview of CNV and its current therapeutic strategies and summarize the properties and applications of various ONS related to the treatment of CNV reported to date. Our goal is to provide a comprehensive review of these considerable advances in ONS in the field of CNV therapy over the past two decades to fill the gaps in previous related reports. Finally, we provide considerations around current challenges and future perspectives of the use of ONS in CNV therapy, with the goal of providing a reference for the clinical translation of ONS.
Formation of CNV
A healthy cornea is an avascular transparent tissue nourished by diffusion from the aqueous humor and tear film. The term “angiogenic privilege” is used to describe the maintenance of corneal avascularity,13,14 which means that, in the resting state, healthy corneas can exist in an active process involving the homeostatic balance between low levels of angiogenic and high levels of antiangiogenic factors.15 Once such delicate equilibrium is disturbed by various ocular insults, including infection, inflammation, hypoxia, trauma, corneal degeneration, and corneal transplantation, abnormal new vessels can invade the corneal stroma from preexisting pericorneal structures and subsequently lead to CNV.16 Furthermore, this CNV can disrupt the relative immune privilege of the cornea, causing a positive feedback cycle of inflammation and more CNV.3 Corneal pathologies that can lead to neovascularization include lipid keratopathy, corneal ulcers and scars, herpes eye disease, infectious keratitis, chemical burns, graft rejections and hypoxic insults from contact lens wear.4
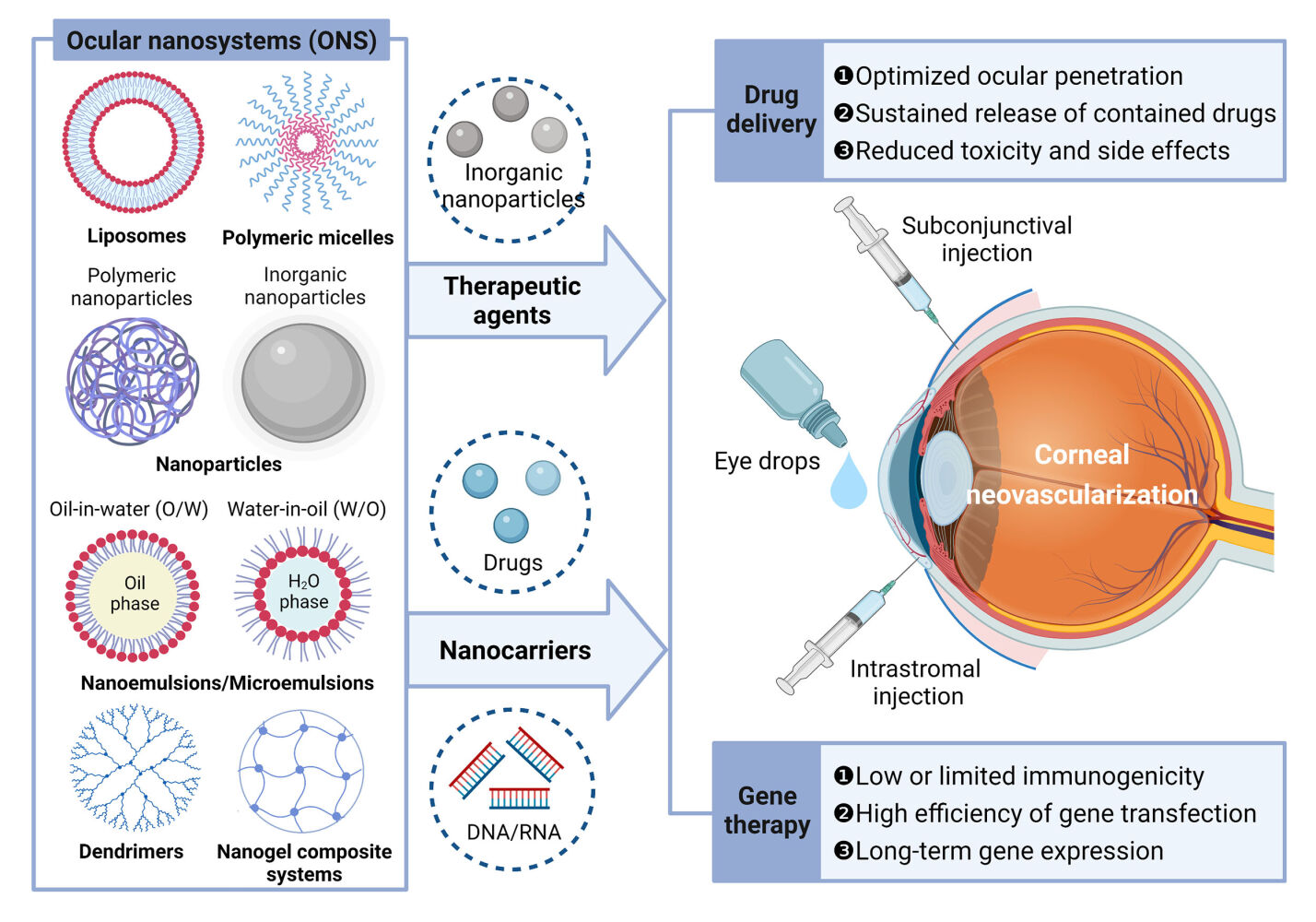
As mentioned above, multiple pathologic insults may result in CNV. These pathologies can be broadly thought of as initiating at least one of two common pathways: (1) loss of the limbal stem cell barrier and (2) inflammation.3 Inflammation is a central pathological process in the formation of CNV. Many articles have discussed inflammatory mechanisms contributing to CNV.3,17 In the following, we provide a brief introduction of the inflammatory mechanisms contributing to CNV (Figure 1).
 |
Figure 1 Simplified schematic pathway of inflammatory CNV. Abbreviations: CNV, Corneal neovascularization; BM, basement membrane; ECM, extracellular matrix. MMP, matrix metalloproteinase; PDGF, platelet derived growth factor; PDGFR, PDGF receptor; VEC, vascular endothelial cell; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor. Notes: (A) A limbal vessel is shown in cross section. Corneal injury leads to production of proangiogenic substances including VEGF, bFGF, and cytokines by a variety of cell types (only VEGF is shown here for simplicity). (B) Activation of VEGFR causes pericyte loss and release of MMPs from VECs, leading to destruction of the VEC BM and surrounding ECM of the cornea, and allowing VECs to migrate into the cornea via chemotaxis. (C) Migration and proliferation of VECs leads to sprouting of a neovascular stalk that invades the corneal stroma. The cells at the tip of this stalk form dendritic projections as they move chemotactically toward the site of inflammation, and continue releasing MMPs. VECs begin secreting BM. The new vessel is tenuous due to lack of pericyte support. (D) As neovascularization progresses, VECs release PDGF, which binds to receptors on pericytes, leading to their proliferation and migration. Pericytes then associate with and stabilize the new vessel. Reproduced from Nicholas MP, Mysore N. Corneal neovascularization. Exp Eye Res. 2021;202:108,363. © 2021 Published by Elsevier Ltd. With permission from Elsevier.3 |
When the cornea is damaged, multiple cell types, such as corneal epithelial and endothelial cells, stromal keratocytes, immune cells, vascular endothelial cells (VECs), and pericytes, release several proangiogenic cytokines, including VEGF, basic fibroblast growth factor (bFGF), chemokines, and adhesion molecules. Moreover, this upregulation of proangiogenic factors is accompanied by the downregulation of antiangiogenic factors such as pigment epithelium-derived factors. The activation of vascular endothelial growth factor receptor (VEGFR) leads to the release of matrix metalloproteinases (MMPs) from VECs, which degrade the vascular basement membrane and surrounding corneal extracellular matrix. In this context, VECs are able to migrate and proliferate into the cornea, causing neovascular stalk sprouting and resulting in invasion of the corneal stroma. The cells at the tip of this stalk form dendritic projections as they move chemotactically toward the site of inflammation and continue releasing MMPs. As neovascularization progresses, VECs release platelet-derived growth factor, causing the proliferation and migration of pericytes. Finally, the new vascular lumen matures and is stabilized as supporting pericytes are recruited, indicating that abnormal new vessels do not require proangiogenic factors for survival.
Strategies for the Treatment of CNV and Corresponding Limitations
Pharmacological Treatment
Pharmacological treatments need to deliver drugs to the eyes. Topical eye drops or subconjunctival injections have been considered conventional delivery routes for anterior segment diseases. However, human eyes have various defensive barriers (such as tear film, cornea, and blood barriers) and clearance mechanisms (such as blinking reflex, tear renewal, and tear drainage).18 Hindered by these ocular barriers and factors, the efficacy of the total amount of administered drugs is less than 5%, thus rendering the poor bioavailability of ocular drugs.19 Low drug penetration, transient residence time at the targeted site, and potential side effects resulting from frequent administration are also common drawbacks with respect to conventional drug delivery.20
To date, there are a wide range of drugs for CNV treatment. According to the properties and targets of drugs, they can be divided into different types, which have been reviewed elsewhere.3,6,21,22 We list several important and emerging drugs below.
Anti-Inflammatory Drugs
Steroids, including dexamethasone (DEX), are potent inhibitors of inflammation and have been used in the treatment of CNV for their anti-inflammatory23 and antiangiogenic24 properties. Steroids are most effective in inhibiting CNV when started before or immediately after tissue injury. However, the complete suppression of CNV with topical steroids is not possible, as steroids do not cause established CNV to regress.25 Moreover, steroids have various potential side effects, such as steroid-induced glaucoma, acceleration of cataract formation, superinfection, and herpes simplex recurrence.26 Therefore, it is crucial to closely monitor patients on long-term steroids for early diagnosis and management of potential side effects.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also anti-inflammatory agents with known antiangiogenic properties, but they are not considered sufficiently effective.27,28 Notably, similar to steroids, long-term use of topical NSAIDs may cause potential corneal side effects that necessitate close monitoring.29
Immunosuppressants
Cyclosporine A (CsA) is an immunosuppressive agent used to control the rejection of organ transplants and to treat multiple autoimmune and inflammatory conditions.30 A study indicated that topical 0.05% CsA shows an inhibitory effect on immune-mediated CNV in rabbits, with the inhibitory effect of topical 0.05% CsA treatment being significantly higher than that of topical isotonic saline or 0.5% bevacizumab (BEV) (the percent area of CNV was 24.4 ± 14.4, 37.1 ± 20.4 and 44.1 ± 25, respectively).31 Notably, systemic administration of CsA in high-risk corneal transplantation led to side effects, including eczema, back pain, chest pain, and nausea.32 The side effects of CsA for CNV treatment need further clinical research.
Tacrolimus is another potent immunosuppressant that effectively blocks the production of cytokines by T cells and immunoglobulins by B cells.22 Systemic, topical and subconjunctival tacrolimus application has been shown to be useful in reducing experimental CNV.33,34
Anti-VEGF Drugs
Suppressing VEGF activity with anti-VEGF antibodies such as BEV is a possible strategy for CNV treatment.35 BEV is a full-length humanized murine monoclonal IgG1 antibody that can recognize all isoforms of VEGF-A.3 Topical BEV has been successfully utilized for the reduction in CNV in cases unresponsive to conventional anti-inflammatory medications in animal studies and clinical trials.22,36 Apart from topical administration, subconjunctival BEV application also achieves a significant reduction in the area of CNV. A significant reduction in CNV area was seen in clinical human studies, with a pooled reduction of 36% [95% confidence interval (CI), 18–54%] overall, of 48% (95% CI, 32–65%) for topical treatment, and 32% (95% CI, 10–54%) for subconjunctival BEV injections.37 Notably, topical administration of BEV may lead to an increased risk of corneal epithelial defects in a time- and dose-dependent manner, while subconjunctival administration has been widely used in clinical studies with no obvious side effects.21 However, CNV may recur following successful management with subconjunctival BEV necessitating repeated injections, especially in cases with lipid deposition.38 In addition, similar to steroids, anti-VEGF antibodies are less effective in treating mature CNV.3
VEGFR tyrosine kinase inhibitors, such as apatinib, cabozantinib and axitinib, can inhibit the combination of receptors and ligands by interacting with the starting point of VEGF signal transduction pathways. It can suppress the formation of CNV that is mediated by VEGF.39–41
Plant Extracts
Several plant extracts, including epigallocatechin gallate (EGCG),42 celastrol,43 curcumin,44 kaempferol45 and naringenin,46 have been found to inhibit experimental CNV. The plant extracts mentioned above (except EGCG, which is a water-soluble active ingredient) are all hydrophobic molecules. Therefore, despite their powerful beneficial bioactivity, their clinical use has been limited mainly due to their poor water solubility. In this context, seeking a next-generation delivery carrier/strategy becomes an urgent issue to improve their water solubility while still retaining their activities in the treatment of CNV.
Surgical Treatment
Physical ablation with lasers, PDT and FND may be considered when the pharmacological treatments described above show no effect, especially in the setting of relatively mature vasculature. The applications of these techniques in the treatment of CNV have been reviewed by other authors.4,22,27 Although the above surgical treatments have shown potential in a clinical setting, they all may evoke an inflammatory response, which can further exacerbate CNV.47 Moreover, in the laser ablation procedure, high laser energy can predispose patients to various complications, including iris atrophy, corneal thinning, pupillary ectasia, peripheral corneal hemorrhage48,49 and necrotizing scleritis.50 Considering the fact that there may be collagen shrinkage and damage to the adjacent stroma of the diathermy site as well as the fact that the long-term effects of FND on the cornea are not yet clear,6 the use of FND has also not gained widespread acceptance.
Gene Therapy
The unique anatomical position and immune privilege of the cornea along with the relative ease of access enable it to be a promising candidate for gene-based therapy—either through transgenic expression of an antiangiogenic factor or inactivation of a proangiogenic factor via gene silencing. Unlike pharmacological or surgical treatment for CNV that only provides limited efficacy, a gene-based strategy offers targeted treatments providing long-term therapeutic correction.51 Studies have suggested that antiangiogenic genes such as endostatin and angiostatin can be transfected into corneal epithelial cells, successfully reducing CNV in animal models.52–54 In addition, small interfering RNA (siRNA) targeting VEGFs has been demonstrated to inhibit CNV in animal models.55 However, gene-based therapies for corneal neovascularization are still largely at the preclinical stage. The success of gene therapy seen in some animal studies is accomplished by early and frequent administration, which is far from ideal for treating ongoing CNV.6
Liu et al6 provided a comprehensive review of therapeutic target genes and potential gene carriers available (eg, adenoviral vectors, lentiviral vectors, adeno-associated viral vectors and nanocarriers) to treat CNV. Although viral-based vectors are well established and effective, there are safety concerns, including the potential genotoxicity of long-term expression and nonspecific targeting.7 Furthermore, lentiviral vectors randomly integrate their genome into host cells, which can lead to insertional mutagenesis.56 Therefore, novel nanocarriers and nonviral delivery methods that are less likely to induce an immune response and can realize targeted gene delivery might offer considerable benefits in ocular gene delivery in the future.
Different Forms of ONS for CNV Therapy
To address these limitations of current treatment strategies in CNV, novel ONS based on nanotechnology have been investigated for the last two decades. The purpose of these ONS is not only to optimize ocular penetration and bioavailability, but also to prolong drug retention time, thereby achieving sustained delivery and controlled therapeutic release with minimal toxicity and side effects.19 For gene therapy, ONS also provide more biocompatible nanovectors with high efficiency of gene transfection and long-term gene expression, which exert considerable benefits in the treatment of CNV. To date, the use of various ONS, such as liposomes, nanoparticles (NPs), polymeric micelles, nanoemulsions/microemulsions, dendrimers, nanogel composite systems (Figure 2) and nanowafers, has become a popular research topic in the field of CNV therapy.
 |
Figure 2 Different forms of ONS for CNV therapy. Created with BioRender.com. |
Other ONS, such as nanosuspensions,57 cubosomes,58 and spanlastics59 are also promising ocular delivery platforms. However, to date, there have been no documented studies reporting their applications in the treatment of CNV. Therefore, these ONS are not discussed in this review, as the main focus of this review is CNV rather than a wider range of eye diseases. The properties of various ONS closely related to CNV are summarized below.
Liposomes
Liposomes are spherical vesicles formed by one or more phospholipid bilayers.60 They are formulated primarily from phospholipids and cholesterol, which enable liposomes with not only high ocular penetration but also satisfactory biocompatibility, biodegradability, almost no toxicity and low antigenicity.61 Liposomes can be used to simultaneously load both hydrophilic and hydrophobic molecules. Hydrophilic molecules can be encapsulated into the core region, while hydrophobic molecules can be encapsulated in lipid bilayers.62 Currently, liposomes are widely used for the delivery of different drugs,60 therapeutic agents63,64 and genes65 to the eye.
The applicability of liposomes as ONS in specific ocular diseases is determined by their lipid composition, preparation approaches and surface charge.66 For instance, the absorption of encapsulated drugs across corneal membranes can be enhanced by positively charged liposomes.67 In addition, via electrostatic interactions, chitosan-coated deformable liposomes with positive charges have the capacity to improve loading efficacy and to enhance binding affinity with negatively charged corneal surfaces.68
However, despite liposomes having the considerable advantages mentioned above, the low stability, relatively low entrapment, and rapid release of hydrophilic drugs substantially limit the advancement of liposomes in clinical applications. In response, many studies have been conducted in efforts to enhance liposome bioavailability, corneal penetration, stability, and targeted action and have achieved positive results.69,70
NPs
NPs are nanoscale colloidal carriers that are mainly made of natural or synthetic polymer composition, inorganic materials, lipids and proteins and peptides.71,72 NPs can be coated with a hydrophilic polymer or further functionalized with antibodies modified onto the coating.71 In addition, they can be formulated with different sizes, charges, solubilities and other physicochemical characteristics, conferring great versatility in terms of the kind of therapeutic molecules to be loaded. To date, NPs have been investigated in the field of ocular delivery and show great promise as novel ONS in the treatment of ocular diseases.73 NPs can be divided into polymeric NPs and inorganic NPs on the basis of their composition.
Polymeric NPs
For polymeric NPs, commonly used materials include poly(lactide-co-glycoside) (PLGA), poly(lactide) (PLA), poly(ε-caprolactone) (PCL), dextran, albumin, gelatin, alginate, collagen, hyaluronic acid (HA), and chitosan.71 The possibilities for their design are nearly limitless.
Among the various polymers developed to formulate polymeric NPs, PLGA has been extensively investigated as an ONS in the field of ocular delivery due to its biodegradability, biocompatibility and nonantigenic nature. PLGA also provides protection of the drug from degradation and the possibility of sustained release. Sánchez-López et al74 successfully developed the PLGA NPs formulation with a high drug entrapment efficiency of greater than 85% and presented a sustained drug release profile compared to that of conventional eye drops. The positively charged surface of PLGA NPs could be used to uplift the penetration of various drugs, such as DEX75 and pranoprofen,76 into the cornea. Therefore, PLGA NPs have been widely applied in drug delivery to reduce the frequency of drug administration for ocular disease treatment.77
Gelatin is a natural biopolymer prepared and purified from collagen. Gelatin nanoparticles (GNPs) have excellent biocompatibility and biodegradability; hence, they have been previously used as carriers with reported successful drug/gene delivery in ophthalmic applications.77 Since collagen is the major component of corneal stroma, the use of GNPs as the drug carrier in eye drop formulations can improve the bioavailability of drugs or genes by interacting with corneal and conjunctival glycoproteins.78
The increasing use of polymeric NP formulations to increase the cargo molecule residence time inside the cornea with a high permeation rate provides a strong rationale for their translation and clinical application in ocular delivery. However, the limitations posed by their stability, particle size uniformity, release kinetics and bulk production issues should also be considered.62
Inorganic NPs
Common materials used for the production of inorganic NPs include gold,79 silver,80 silica81 and cerium oxide.82
Gold nanoparticles (AuNPs) are promising platforms for biomedical applications, especially for drug delivery83 and gene delivery,84 because of their amenability to synthesis, stabilization, and functionalization; low toxicity; and ease of detection.85 Apart from being employed as effective drug or gene carriers, AuNPs have also been shown to act as anti-angiogenesis agents to inhibit angiogenesis and vascular permeability through the downregulation of VEGFR-2 expression.86 Similar to AuNPs, silver nanoparticles (AgNPs) conjugated with a heparin derivative have demonstrated efficacy as antiangiogenic agents.87
The porous property of mesoporous silica allows a high surface area and high pore volume to absorb and encapsulate molecules.88 Moreover, due to their small size (5–50 nm), silica nanoparticles (SiNPs) can effectively increase penetration into the cornea and provide further access to the vitreous area.
In recent years, cerium oxide nanoparticles (CeNPs) have gained increasing attention in the context of biomedical applications. CeNPs can clear superoxide radical anions, conferring good reduction capability.89 Hence, CeNPs can act as excellent antioxidants to reduce oxidative stress for the treatment of ocular diseases. To date, CeNPs have been used in biomedical materials for vascular inhibition and treatment of photoreceptor degeneration.90–92
However, these inorganic NPs are not biodegradable, leading to a potential issue in biological toxicity. It is critical to develop and/or identify appropriate in vitro and in vivo models to assess the toxicity of these NPs.
Polymeric Micelles
Polymeric micelles are nanostructures formed by block copolymers with amphiphilic properties. The lipophilic portion comprises a micelle core that encapsulates the hydrophobic molecules, while the hydrophilic part comprises the outer surface of the micelle to increase cargo molecule solubility and stabilize and prolong the half-life of the therapeutic agents.93 In addition, polymeric micelles are biodegradable and biocompatible, thus preventing adverse effects.47 Currently, polymeric micelles have attracted considerable interest as ONS and offer significant benefits in ocular delivery.
Various studies have demonstrated that the amphiphilic nature of polymeric micelles enables them to penetrate lipophilic corneal epithelial and endothelial cells, and they can also penetrate a hydrophilic matrix, thus promoting drug penetration and improving bioavailability.94–96 In addition, the small size of polymeric micelles might enhance paracellular transport through conjunctiva and sclera, resulting in higher drug levels in intraocular tissues and a sustained drug delivery effect.97 For gene therapy, an in vitro study showed prolonged gene expression after subconjunctival injection of polymeric micelles containing a reporter gene. In addition, gene transfer into the subconjunctival space by polymeric micelles showed significant inhibition of CNV in mice.98 Importantly, polymeric micelles have been shown to have no cytotoxicity in human corneal epithelial cells.99 Based on these abovementioned advantages, polymeric micelles are believed to be safe ONS for treating corneal diseases.
Nanoemulsions/Microemulsions
Nanoemulsions contain an oil phase, water phase, emulsifier, and coemulsion. The presence of surface active ingredients in nanoemulsions enables enhanced mixing of nanosized droplets with the precorneal constituents and, as a consequence, a greater dispersion of the drug over the cornea.100 Microemulsions are isotropic, transparent, and thermodynamically stable nanosized mixtures of oil, water, surfactant and cosurfactant. The small size of the microemulsions and the presence of surfactant among the components confer properties such as good tissue permeability101 and improvement in solubility and stability of the applied drug.102 Thus, reliable patient compliance is attained, and the number of administrations needed decreases daily.103 Nanoemulsions and microemulsions are currently widely being investigated as ONS for ocular delivery, primarily to the front of the eye.
The properties of nanoemulsions and microemulsions depend on the nature and composition of their components. Kalam’s work revealed that the optimized microemulsion possessed good stability, showed greater adherence to the corneal surface and good permeation of gatifloxacin in the anterior chamber of the eye, resulting in a twofold higher gatifloxacin concentration than that of the conventional dose.101
However, microemulsions are unsuitable when the therapeutic molecules are water-soluble or insoluble (does not dissolve in water or oil) or thermolabile or if the therapeutic molecules appear transparent externally. In addition, unlike liposomes, microemulsions are unsuitable for long-term sustained drug release.61
Dendrimers
Dendrimers are repeated/individual molecules with a regularly branched structure that contain many sidechain moieties arranged in a highly regular branching pattern, typically symmetrically around a central core. Owing to their regularly arranged framework, the unique branched topologies of dendrimers afford properties such as high solubility, availability of tremendous internal cavities loaded with various therapeutic molecules, and controllable molecular weight.70 To date, dendrimers have been widely used as ONS for the delivery of different drugs and therapeutic agents to the eye.104,105
Poly(amidoamine) (PAMAM) dendrimers are most commonly used among dendrimers for ocular delivery due to improved biological response, tolerability and relatively low clearance from the ocular surface.106 In addition, PAMAM dendrimers can not only solubilize drugs that have poor water solubility but also increase the ocular residence time upon topical administration.107 Moreover, in vitro studies have revealed that the interactions between negatively charged ocular mucins and PAMAM dendrimers can lead to subsequent intensified corneal penetration.108
Nonetheless, although dendrimers have several advantages, as mentioned above, they may cause chemical modifications to drug molecules, leading to cytotoxicity issues. In addition, multistep syntheses and high preparation costs have prohibited further advancement of dendrimers from the laboratory to the clinic.104
Nanowafers
Nanowafers are very small, transparent circular discs fabricated by different polymers (Figure 3). The composition of nanowafers enables them to be readily applied to the ocular surface with a fingertip and to withstand constant blinking without being displaced. In addition, nanowafers contain arrays of drug-loaded nanoreservoirs from which the drug is released in a tightly controlled manner for a few hours to days to enhance drug residence time and subsequent absorption into ocular tissues. Moreover, at the end of the stipulated period of drug release, nanowafers dissolve and are carried away.109 Good tolerability and stability, together with the enhanced therapeutic effect of the drug with minimal side effects, make nanowafers ideal ONS for ophthalmic drug delivery. To date, nanowafers have offered considerable benefits in the stable delivery of axitinib for the effective treatment of CNV.109
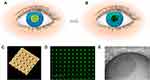 |
Figure 3 Ocular drug delivery nanowafer. Notes: (A) Schematic of the nanowafer instilled on the cornea. (B) Diffusion of drug molecules into the corneal tissue. (C) AFM image of a nanowafer demonstrating an array of 500 nm diameter nanoreservoirs. (D) Fluorescence micrograph of a nanowafer filled with doxycycline (scale bar 5 μm). (E) Nanowafer on a fingertip. Reproduced with permission from Yuan X, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC, Acharya G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;24;9(2):1749–1758. © 2015 American Chemical Society.109 |
Nanogel Composite Systems
To further improve the ocular therapeutic duration and bioavailability of therapeutic agents released from the abovementioned ONS, different nanocarriers such as NPs110,111 and dendrimers112 have been embedded in hydrogels to form nanogel composite systems in recent years. The drug release duration of the composition system is longer than that of the nanocarriers alone and the hydrogel matrix alone. In addition, the composite system can also improve the biocompatibility of the nanocarriers by hiding them within the hydrogel and minimizing drug metabolism from the enzymes present in tears or on the corneal surface.113
Since the formulations of nanogel composite systems are versatile and complex, the quality control of such systems is comprehensive. It is therefore necessary to consider the quality of the entire system, including the stability of the gel and the gelling performance of the in situ gel at the macro level. Importantly, due to the composition of many auxiliary materials in nanogel composite systems, safety issues should not be ignored.93
Applications of ONS in CNV Therapy
ONS as Nanocarriers
Drug Delivery Based on ONS
Multiple ONS have been utilized as nanocarriers for the delivery of different drugs, including DEX, CsA, tacrolimus, BEV, apatinib, cabozantinib, axitinib, plant extracts and some signaling pathway inhibitors, to the eye in the field of CNV therapy (Table 1).
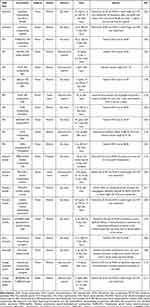 |
Table 1 Drug Delivery Based on ONS for CNV |
The antiangiogenic effect of various steroids, including DEX, has been shown in multiple animal models and in clinical practice.28 However, inhibiting and/or treating CNV requires frequent administration of steroids, which results in patient incompliance, particularly in elderly individuals. Therefore, the development of a sustained steroid delivery system to the anterior segment with high bioavailability to reduce dosing frequency is needed. PLGA NPs,114 polymeric micelles115 and the PAMAM dendrimer-gel composite system112 were able to deliver DEX or dexamethasone sodium phosphate (DSP) to the mouse cornea via subconjunctival injection or topical administration. These nanoformulations achieved sustained drug release and showed more robust efficacy on CNV suppression and inflammation elimination than free DEX or DSP, with a negligible effect on normal tissues. Thus, the number of administrations needed decreases daily.112,114
CsA and tacrolimus are emerging immunosuppressive agents used to inhibit CNV.31,33,34 Di et al116 developed novel micelle formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactides) (MPEG-hexPLA) copolymers and used the nanosized polymeric micelles as drug carriers of CsA in a rat model of cornea transplantation. The results showed that when applied topically, MPEG-hexPLA micelles with 0.5% CsA can significantly decrease graft rejection and reduce CNV. Lin et al64 prepared cationic liposomal tacrolimus with a surface potential of approximately +30 mV; the authors claimed that tacrolimus liposomes (0.2 mg/mL) inhibited CNV and reduced corneal inflammation. Moreover, its therapeutic effect was better than those of the commercial tacrolimus eye drops (1 mg/mL) and the free drug (0.2 mg/mL).
The use of anti-VEGF antibodies such as BEV is a possible strategy for CNV treatment.21,27 PLGA NPs,117 albumin NPs118 and mesoporous silica nanoparticles (MSNs)119 were utilized as nanocarriers to deliver BEV to enhance antiangiogenic effects in CNV therapy. These novel strategies of encapsulating BEV could increase the bioavailability and decrease the toxicity of BEV in animal models of CNV. Recently, Lyu et al111 successfully embedded MSNs loaded with BEV in a CsA-containing thermosensitive hydrogel matrix. This nanogel composite system can regulate the in vitro release of both BEV and CsA in a sustainable way for up to four weeks, thus enhancing CNV inhibition through synergistic anti-VEGF and anti-inflammation effects.
Apatinib, cabozantinib and axitinib are VEGFR tyrosine kinase inhibitors that have the ability to suppress CNV formation mediated by VEGF.39–41 However, as hydrophobic drugs, their application to the ocular surface needs to be improved. To surmount the ocular surface barriers and release the drug for extended periods of time, Lee et al39 encapsulated water-insoluble apatinib in NPs composed of human serum albumin (HSA)-conjugated poly(ethylene glycol) (PEG). The authors demonstrated that a subconjunctival injection of apatinib-loaded HSA-PEG NPs efficiently led to lower levels of alkali burn injury-induced CNV in rats than that observed with an injection of free apatinib solution. Han et al40 developed cationic polypeptide micelles with mucoadhesive ability that carry lipophilic cabozantinib to enhance the bioavailability of lipophilic cabozantinib and remarkably inhibit CNV. Shi et al41 used methoxy poly(ethylene glycol)-poly(ε-caprolactone) (MPEG-PCL) to encapsulate axitinib to prepare axitinib-loaded micelles. The results showed that axitinib-loaded micelles exerted superior antiangiogenic effects with remarkable reductions in the CNV area, which were as effective as clinical DEX but without apparent side effects, offering a new prospect for safe and effective CNV treatment. To enhance therapeutic efficacy and improve patient compliance, Yuan et al109 developed nanowafer-loaded axitinib. As expected, axitinib nanowafers administered once a day were therapeutically twice as effective as axitinib delivered twice a day by topical eye drop therapy in treating CNV in a murine ocular burn model.
EGCG, the major active component of green tea, has been shown to inhibit angiogenesis via inhibition of vascular endothelial cell growth, thus exhibiting considerable potential in the field of inhibiting CNV.120 Miyagawa et al42 designed an eye drop formulation containing EGCG NPs for targeted therapy in CNV. The authors claimed that this eye drop formulation can effectively target corneal vessels and thereby inhibit chemical cauterized-induced CNV by a once-daily treatment. Other plant extracts, including celastrol, curcumin, kaempferol and naringenin, are hydrophobic molecules that limit their clinical use. In response, they were encapsulated in micelles,43 NPs,44 GNPs45 and microemulsions46 respectively to greatly improve their water solubility while still retaining their activities in CNV therapy.
The Wnt signaling pathway, which is an upstream signaling pathway regulating VEGF, plays an indispensable role in developmental and pathological ocular angiogenesis.121,122 Inhibitors of Wnt signaling have displayed therapeutic potential in treating Wnt pathway–dependent diseases. Zhong et al63 constructed liposomes loaded with the Wnt/β-catenin pathway inhibitor XAV939 (XAV939 NPs). After being topically applied to alkali-burned corneas, XAV939 NPs enhanced corneal wound healing and suppressed CNV. Moreover, the expression of angiogenic and inflammatory-related genes was inhibited by XAV939 NPs.
TAK1 is an emerging therapeutic target for pathologic angiogenesis since it closely engages in several important angiogenic activities.123 Wang et al124 developed a novel eye drop formulation based on GNPs to encapsulate the selective TAK1 inhibitor 5Z-7-oxozeaenol to treat CNV in a rodent model. Topical administration of GNP-encapsulated 5Z-7-oxozeaenol led to significantly greater suppression of CNV in a mouse model than the free form of 5Z-7-oxozeaenol.
Gene Therapy Based on ONS
Various ONS have been investigated, and they offer considerable benefits in gene therapy for CNV (Table 2).
 |
Table 2 Gene Therapy Based on ONS for CNV |
Among multiple targets of gene therapy in CNV, VEGF is the most common therapeutic target.125 Antiangiogenic soluble VEGFR-1, also called sFlt-1, is essential for preserving the avascular ambit of the cornea. Normal human corneas strongly express sFlt-1 in the corneal epithelium and weakly in the corneal stroma close to the limbus.7 Albumin NPs,126 PLGA127 and polymeric micelles98 were able to deliver plasmid DNA encoding sFlt-1 or Flt23k (the VEGF-binding domains 2–3 of Flt-1) to the mouse cornea via intrastromal injection or subconjunctival injection. These strategies all showed prolonged gene expression with low cytotoxicity and significant inhibition of CNV in mice. VEGF-A is a key proangiogenic signal that can bind and activate VEGFR-2 to promote angiogenesis.128 Qazi et al129 loaded PLGA NPs with a plasmid containing a short hairpin RNA (shRNA) expression cassette against VEGF-A and applied it to the mouse cornea via intrastromal injection. They claimed that this nanoscale therapeutic strategy can provide highly efficacious, sustainable, nontoxic regression of CNV. Similarly, ocular delivery of antisense oligonucleotide by cationic nanoemulsion directed at VEGFR-2 elicited a significant inhibition of CNV in mice.130 Vascular endothelial cell growth inhibitor (VEGI) was reported to be a novel cytokine that can inhibit the proliferation of endothelial cells and angiogenesis.131 Mediated by liposomes, VEGI cDNA was successfully delivered into all layers of the cornea and achieved 13.8 mm2 less rabbit CNV after a silk suture was placed, which was unlike the results of the controls.132
GA-binding protein (GABP) is a transcription factor that regulates the expression of VEGF.6 Yoon et al133 used a lipid-based vector to deliver a plasmid DNA-encoding GABP to the mouse cornea via subconjunctival injection. This approach was shown to decrease VEGF gene expression and delay CNV for up to 2 weeks in a mouse model of deliberate corneal injury.
Another transcription factor, nuclear factor kappa B (NFκB), was reported to regulate the expression of various genes implicated in inflammation and angiogenesis.134 Han et al135 selected siRNA targeting NFκB p50 and loaded it within reducible branched polyethylenimine nanoparticles (rBPEI-NPs) to develop a new siRNA carrier as a hope for CNV therapy. This novel siRNA carrier achieved a desirable distribution of therapeutic siRNA inside the diseased CNV region and showed successful gene knockdown of angiogenesis without significant cytotoxicity in rat models of corneal alkaline injury.
Previous studies have reported that both very-low-density lipoprotein receptor (VLDLR) and VLDLR N-terminal ectodomain (VLN) have an inhibitory effect on the Wnt signaling pathway.136,137 Wang et al138 encapsulated an expression plasmid of VLN with PLGA NPs and applied it to treat mouse CNV induced by alkali burns. They demonstrated that this nanoscale therapeutic strategy can suppress Wnt signaling and ameliorate CNV. The VLN-NP-treated group developed fewer vessels in the cornea with alkali burns than the controls.
ONS as Therapeutic Agents
Some ONS, such as inorganic NPs, are rarely used to deliver any therapeutic agents but are themselves the therapeutic agents.139–141 Cho et al142 applied AuNPs in a mouse model and found that topical administration of AuNPs significantly reduced the development of CNV by inhibiting the ERK pathway. Since inflammation is a central pathological process in the formation of CNV, Luo et al80 developed dual-functional (antibacterial and antiangiogenic) gelatin-capped AgNPs and demonstrated that intrastromal administration of highly biocompatible gelatin-capped AgNPs alleviates S. aureus-induced bacterial keratitis in rabbit eyes and bacterial infection-induced CNV. Zheng et al143 synthesized CeNPs with antioxidant efficacy and attempted to use their antioxidant capacity for the treatment of inflammation-associated CNV. The synthesized CeNPs showed good biocompatibility and were capable of controlling inflammation and neovascularization by blocking oxidative stress. Topical SiNPs can also effectively decrease the extent of CNV.144 Tang et al145 prepared c(RGDyC)-tagged silicon nanoparticles (SiNPs-RGD), a theranostic agent made of peptide-functionalized SiNPs that is suitable for simultaneous ocular neovascularization imaging and therapy. Their results indicated that intravenous injection of SiNPs-RGD can selectively detect angiogenic blood vessels in vivo as well as inhibit alkali burn-induced CNV.
Conclusion and Future Perspectives
The novel ONS based on nanotechnology represent a new avenue for the treatment of CNV. Compared to traditional drug delivery, ONS can not only optimize ocular penetration and bioavailability, but also prolong drug retention time, thereby achieving sustained delivery and controlled therapeutic release with minimal toxicity and side effects (Table 3). Some inorganic nanomaterials, such as AuNPs,142 AgNPs,80 CeNPs143 and carbon-based nanomaterials,146 are themselves the antiangiogenic nanoagents for antiangiogenic applications. In the field of gene therapy for CNV, various ONS have been utilized as biocompatible nanocarriers to achieve high efficiency of gene transfection and long-term gene expression. Notably, ONS for CNV therapy are still in the preliminary research stage; thus, appropriately designed clinical trials for various ONS in the treatment of CNV are needed in the future. Several challenges remain to be addressed for translation from the bench into clinical products, including selection of various nanocarrier forms, safety control of potential toxicity associated with ONS and large-scale production of ONS.
 |
Table 3 Features of ONS for Drug Delivery in CNV Therapy |
Nanocarriers are designed to overcome the limitations associated with current CNV therapy and ensure targeted and controlled drug delivery.147,148 Therefore, therapeutic drugs are of top priority in nanoformulations and full consideration about their properties should be given when selecting nanocarrier forms. For instance, some antiangiogenic drugs are hydrophilic, while others are hydrophobic; some nanocarrier forms can load both hydrophilic and hydrophobic molecules, while others can deliver only either hydrophilic or hydrophobic molecules. Hence, it is indispensable to carefully consider the characteristics of each antiangiogenic drug to select the most suitable nanocarrier forms to optimize the antiangiogenic efficacy of the tailored nanoformulation to meet specific clinical needs.20
Although various efforts have been made to understand the toxicity of ONS to eyeball tissues, preclinical toxicology data of animal models are usually used, and human clinical trials are lacking.11,12 With the further development of ONS for drug delivery and gene therapy for CNV, appropriately designed clinical trials for various ONS should be conducted in the future. Nanotoxicity depends heavily on a variety of properties, such as material composition, concentration, biodistribution, size, shape, surface charge, attached chemical groups and zeta potential.149,150 For example, positively charged biopolymers containing nanoformulations prolong the drug retention time at the ocular surface.20 However, prolonged residence time might also potentially provoke corneal toxicity that necessitates further investigation. In addition, nanoformulations that have a surfactant concentration that is too high could cause corneal damage.151 In some cases, the presence of surfactants may cause a sticky sensation and blurred vision upon instillation, hence impeding patient compliance.152 Most inorganic nanoparticles are relatively hard to degrade and eliminate from the human body, which may cause excess retention in living organisms and harmful effects such as inflammation and tissue damage.70 Therefore, it is essential to carefully evaluate the strengths and limitations of each formulation to maximize the therapeutic effects of active drugs while minimizing the potential systemic or local toxic side effects. Continuous optimization with appropriate modification and functionalization of the formulation would be beneficial for clinical translation.20 Special considerations should also be given to the techniques for sterilizing the ONS for CNV therapy, such as aseptic processing/sterile filtration or terminal sterilization by autoclaving.113 Furthermore, regulatory bodies need to establish guidelines to ensure the safety and quality of various ONS formulations for CNV therapy.153
The formulations of ONS for CNV therapy are versatile. The unique customizable feature of each nanoformulation may result in a lack of standardized protocols for large-scale production. In addition, the production of ONS usually involves a multistep and complex process that includes multiple components. It is difficult to achieve a robust process to maintain repeatability. Moreover, the complex production process also greatly increases production costs.11 In response, recent advances in technology, such as microfluidic, high-pressure homogenization, particle replication in nonwetting template and hydrogel template methods, have been investigated and have provided hope for large-scale production.154 In the future, cost-effective technology also needs to be developed to scale up the production of ONS designed for CNV therapy.
Taking all aspects into account, more meticulous work is needed before ONS-based products for CNV therapy are successfully on the market. Nevertheless, various preclinical studies conducted to date have demonstrated promising results of the use of ONS in the treatment of CNV. We believe that, in the future, ONS will play a more significant role in the treatment of CNV in different stages, with the hope of circumventing the common limitations of currently available treatment strategies in the clinic.
Abbreviations
ONS, Ocular nanosystems; CNV, Corneal neovascularization; VEGF, Vascular endothelial growth factor; PDT, Photodynamic therapy; FND, Fine-needle diathermy; VECs, Vascular endothelial cells; VEGFR, Vascular endothelial growth factor receptor; MMPs, matrix metalloproteinases; bFGF, basic fibroblast growth factor; DEX, Dexamethasone; NSAIDs, Nonsteroidal anti-inflammatory drugs; CsA, Cyclosporine A; BEV, Bevacizumab; EGCG, Epigallocatechin gallate; siRNA, Small interfering RNA; NPs, Nanoparticles; PLGA, Poly(lactide-co-glycoside); PLA, Poly(lactide); PCL, Poly(ε-caprolactone); HA, Hyaluronic acid; PEG, Poly(ethylene glycol); GNPs, Gelatin nanoparticles; AuNPs, Gold nanoparticles; AgNPs, Silver nanoparticles; SiNPs, Silica nanoparticles; CeNPs, Cerium oxide nanoparticles; PAMAM, Poly(amidoamine); DSP, Dexamethasone sodium phosphate; MPEG-hexPLA, Methoxy poly(ethylene glycol)-hexylsubstituted poly(lactides); MSNs, Mesoporous silica nanoparticles; HSA, Human serum albumin; MPEG-PCL, Methoxy poly(ethylene glycol)-poly(ε-caprolactone); shRNA, Short hairpin RNA; VEGI, Vascular endothelial cell growth inhibitor; GABP, GA-binding protein; NFκB, Nuclear factor kappa B; rBPEI-NPs, Reducible branched polyethylenimine nanoparticles; VLDLR, Very-low-density lipoprotein receptor; VLN, VLDLR N-terminal ectodomain; SiNPs-RGD, c(RGDyC)-tagged silicon nanoparticles.
Acknowledgments
We thank American Journal Experts (https://www.aje.cn/) for its linguistic assistance during the preparation of this manuscript. We also thank Biorender (https://biorender.com/) and Yifan Bao from University of Vienna for helping provide the figures in this manuscript.
Funding
This work was supported by the Science and Technology Development Plan Project of Jilin Province (Grant No. 20190303186SF) and the Science and Technology Project of Education Department of Jilin Province (Grant No. JJKH20201089KJ).
Disclosure
The authors declare that they have no competing interests.
References
1. Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(4):242–249. doi:10.1097/00055735-200108000-00002
2. Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117(7):1300–1305.e1307.
3. Nicholas MP, Mysore N. Corneal neovascularization. Exp Eye Res. 2021;202:108363.
4. Sharif Z, Sharif W. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom J Ophthalmol. 2019;63(1):15–22.
5. Gote V, Sikder S, Sicotte J, Pal D. Ocular drug delivery: present innovations and future challenges. J Pharmacol ExpTher. 2019;370(3):602–624.
6. Liu S, Romano V, Steger B, Kaye SB, Hamill KJ, Willoughby CE. Gene-based antiangiogenic applications for corneal neovascularization. Surv Ophthalmol. 2018;63(2):193–213.
7. Torrecilla J, Del Pozo-Rodríguez A, Vicente-Pascual M, Solinís M, Rodríguez-Gascón A. Targeting corneal inflammation by gene therapy: emerging strategies for keratitis. Exp Eye Res. 2018;176:130–140.
8. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71.
9. Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443.
10. Tang Z, Fan X, Chen Y, Gu P. Ocular nanomedicine. Adv Sci. 2022;9(15):e2003699. doi:10.1002/advs.202003699
11. Lyu Q, Peng L, Hong X, et al. Smart nano-micro platforms for ophthalmological applications: the state-of-The-art and future perspectives. Biomaterials. 2021;270:120682. doi:10.1016/j.biomaterials.2021.120682
12. Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm Sin B. 2017;7(3):281–291. doi:10.1016/j.apsb.2016.09.001
13. Di Zazzo A, Gaudenzi D, Yin J, et al. Corneal angiogenic privilege and its failure. Exp Eye Res. 2021;204:108457. doi:10.1016/j.exer.2021.108457
14. Zhong W, Montana M, Santosa SM, et al. Angiogenesis and lymphangiogenesis in corneal transplantation-A review. Surv Ophthalmol. 2018;63(4):453–479. doi:10.1016/j.survophthal.2017.12.008
15. Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29(3):208–248. doi:10.1016/j.preteyeres.2010.01.002
16. Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19(2):125–133. doi:10.1016/j.semcdb.2007.08.014
17. Ma DH, Chen HC, Lai JY, et al. Matrix revolution: molecular mechanism for inflammatory corneal neovascularization and restoration of corneal avascularity by epithelial stem cell transplantation. Ocul Surf. 2009;7(3):128–144.
18. Yadav KS, Rajpurohit R, Sharma S. Glaucoma: current treatment and impact of advanced drug delivery systems. Life Sci. 2019;221:362–376.
19. Srinivasarao DA, Lohiya G, Katti DSJ. Fundamentals, challenges, and nanomedicine‐based solutions for ocular diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(4):e1548.
20. Liu YC, Lin MT, Ng AHC, Wong TT, Mehta JS. Nanotechnology for the treatment of allergic conjunctival diseases. Pharmaceuticals. 2020;13(11):351.
21. Liu X, Wang S, Wang X, Liang J, Zhang Y. Recent drug therapies for corneal neovascularization. Chem Biol Drug Des. 2017;90(5):653–664.
22. Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and emerging therapies for corneal neovascularization. Ocul Surf. 2018;16(4):398–414.
23. Mukwaya A, Mirabelli P, Lennikov A, et al. Repeat corneal neovascularization is characterized by more aggressive inflammation and vessel invasion than in the initial phase. Invest Ophthalmol Vis Sci. 2019;60(8):2990–3001.
24. Langendorf EK, Rommens PM, Drees P, Ritz U. Dexamethasone inhibits the pro-angiogenic potential of primary human myoblasts. Int J Mol Sci. 2021;22(15):7986.
25. Leopold IH, Purnell JE, Cannon EJ, Steinmetz CG, Mc DP. Local and systemic cortisone in ocular disease. Am J Ophthalmol. 1951;34(3):361–371.
26. Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–512.
27. Feizi S, Azari AA, Safapour S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017;4:28.
28. Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011;30(8):927–938.
29. Raj N, Panigrahi A, Alam M, Gupta N. Bromfenac-induced neurotrophic keratitis in a corneal graft. BMJ Case Rep. 2022;15(7):e249400.
30. Leonardi A, Doan S, Amrane M, et al. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126(5):671–681.
31. Bucak YY, Erdurmus M, Terzi EH, Kükner A, Çelebi S. Inhibitory effects of topical cyclosporine A 0.05% on immune-mediated corneal neovascularization in rabbits. Graefes Arch Clin Exp Ophthalmol. 2013;251(11):2555–2561.
32. Shimazaki J, Den S, Omoto M, Satake Y, Shimmura S, Tsubota K. Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol. 2011;152(1):33–39.e31.
33. Turgut B, Guler M, Akpolat N, Demır T, Celıker U. The impact of tacrolimus on vascular endothelial growth factor in experimental corneal neovascularization. Curr Eye Res. 2011;36(1):34–40.
34. Park JH, Joo CK, Chung SK. Comparative study of tacrolimus and bevacizumab on corneal neovascularization in rabbits. Cornea. 2015;34(4):449–455.
35. Merz PR, Röckel N, Ballikaya S, Auffarth GU, Schmack I. Effects of ranibizumab (Lucentis®) and bevacizumab (Avastin®) on human corneal endothelial cells. BMC Ophthalmol. 2018;18(1):316.
36. Cheng SF, Dastjerdi MH, Ferrari G, et al. Short-term topical bevacizumab in the treatment of stable corneal neovascularization. Am J Ophthalmol. 2012;154(6):940–948.e941.
37. Papathanassiou M, Theodoropoulou S, Analitis A, Tzonou A, Theodossiadis PG. Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: a meta-analysis. Cornea. 2013;32(4):435–444.
38. Chu HS, Chen TC, Hu FR, Chen WL. Recurrence of corneal neovascularization associated with lipid deposition after subconjunctival injection of bevacizumab. Cornea. 2013;32(11):1446–1453.
39. Lee JE, Kim KL, Kim D, et al. Apatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularization. Int J Nanomedicine. 2017;12:4813–4822.
40. Han H, Yin Q, Tang X, et al. Development of mucoadhesive cationic polypeptide micelles for sustained cabozantinib release and inhibition of corneal neovascularization. J Mater Chem B. 2020;8(23):5143–5154.
41. Shi S, Peng F, Zheng Q, et al. Micelle-solubilized axitinib for ocular administration in anti-neovascularization. Int J Pharm. 2019;560:19–26.
42. Miyagawa T, Chen ZY, Chang CY, et al. Topical application of hyaluronic acid-RGD Peptide-Coated gelatin/epigallocatechin-3 gallate (EGCG) nanoparticles inhibits corneal neovascularization via inhibition of VEGF production. Pharmaceutics. 2020;12(5):404.
43. Li Z, Yao L, Li J, et al. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int J Nanomedicine. 2012;7:1163–1173.
44. Pradhan N, Guha R, Chowdhury S, Nandi S, Konar A, Hazra S. Curcumin nanoparticles inhibit corneal neovascularization. J Mol Med. 2015;93(10):1095–1106.
45. Chuang YL, Fang HW, Ajitsaria A, et al. Development of kaempferol-loaded gelatin nanoparticles for the treatment of corneal neovascularization in mice. Pharmaceutics. 2019;11(12):635.
46. Ma Y, Yang J, Zhang Y, et al. Development of a naringenin microemulsion as a prospective ophthalmic delivery system for the treatment of corneal neovascularization: in vitro and in vivo evaluation. Drug Deliv. 2022;29(1):111–127.
47. Gonzalez L, Loza RJ, Han KY, et al. Nanotechnology in corneal neovascularization therapy–a review. J Ocul Pharmacol Ther. 2013;29(2):124–134.
48. Epstein RJ, Stulting RD, Hendricks RL, Harris DM. Corneal neovascularization. Pathogenesis and inhibition. Cornea. 1987;6(4):250–257.
49. Nirankari VS, Baer JC. Corneal argon laser photocoagulation for neovascularization in penetrating keratoplasty. Ophthalmology. 1986;93(10):1304–1309.
50. Pai VH, Handary SV. Necrotizing scleritis following laser therapy for corneal vascularization. Ann Ophthalmol. 2009;41(1):50–51.
51. Mohan RR, Martin LM, Sinha NR. Novel insights into gene therapy in the cornea. Exp Eye Res. 2021;202:108361.
52. Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14(3):313–322.
53. Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol Vis. 2007;13:2344–2352.
54. Fouladi N, Parker M, Kennedy V, et al. Safety and efficacy of OXB-202, a genetically engineered tissue therapy for the prevention of rejection in high-risk corneal transplant patients. Hum Gene Ther. 2018;29(6):687–698.
55. Zuo L, Fan Y, Wang F, Gu Q, Xu X. A siRNA targeting vascular endothelial growth factor-A inhibiting experimental corneal neovascularization. Curr Eye Res. 2010;35(5):375–384.
56. Amador C, Shah R, Ghiam S, Kramerov AA, Ljubimov AV. Gene therapy in the anterior eye segment. Curr Gene Ther. 2022;22(2):104–131.
57. Jacob S, Nair AB, Shah J. Emerging role of nanosuspensions in drug delivery systems. Biomater Res. 2020;24:3.
58. Teba HE, Khalil IA, El Sorogy HM. Novel cubosome based system for ocular delivery of Acetazolamide. Drug Deliv. 2021;28(1):2177–2186.
59. Abdelbari MA, El-Mancy SS, Elshafeey AH, Abdelbary AA. Implementing spanlastics for improving the ocular delivery of clotrimazole: in vitro characterization, ex vivo permeability, microbiological assessment and in vivo safety study. Int J Nanomedicine. 2021;16:6249–6261.
60. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571.
61. Honda M, Asai T, Oku N, Araki Y, Tanaka M, Ebihara N. Liposomes and nanotechnology in drug development: focus on ocular targets. Int J Nanomedicine. 2013;8:495–503.
62. Lalu L, Tambe V, Pradhan D, et al. Novel nanosystems for the treatment of ocular inflammation: current paradigms and future research directions. J Control Release. 2017;268:19–39.
63. Zhong Y, Wang K, Zhang Y, et al. Ocular Wnt/β-catenin pathway inhibitor XAV939-loaded liposomes for treating alkali-burned corneal wound and neovascularization. Front Bioeng Biotechnol. 2021;9:753879.
64. Lin X, Yu X, Chen X, et al. Inhibition of neovascularization and inflammation in a mouse model of corneal alkali burns using cationic liposomal tacrolimus. Front Bioeng Biotechnol. 2021;9:791954.
65. Kattar A, Concheiro A, Alvarez-Lorenzo C. Diabetic eye: associated diseases, drugs in clinic, and role of self-assembled carriers in topical treatment. Expert Opin Drug Deliv. 2021;18(11):1589–1607.
66. Sheikholeslami B, Lam NW, Dua K, Haghi M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022;300:120574.
67. Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16(5–6):270–277.
68. Chen H, Pan H, Li P, et al. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf B Biointerfaces. 2016;143:455–462.
69. Lôbo G, Paiva KLR, Silva ALG, Simões MM, Radicchi MA, Báo SN. Nanocarriers used in drug delivery to enhance immune system in cancer therapy. Pharmaceutics. 2021;13(8):1167.
70. Zhai Z, Cheng Y, Hong J. Nanomedicines for the treatment of glaucoma: current status and future perspectives. Acta Biomater. 2021;125:41–56.
71. Diebold Y, Calonge M. Applications of nanoparticles in ophthalmology. Prog Retin Eye Res. 2010;29(6):596–609.
72. Willem de Vries J, Schnichels S, Hurst J, et al. DNA nanoparticles for ophthalmic drug delivery. Biomaterials. 2018;157:98–106.
73. Nguyen DD, Lai J-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym Chem. 2020;11(44):6988–7008.
74. Sánchez-López E, Esteruelas G, Ortiz A, et al. Dexibuprofen biodegradable nanoparticles: one step closer towards a better ocular interaction study. Nanomaterials. 2020;10(4):720.
75. Zhang L, Li Y, Zhang C, Wang Y, Song C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomedicine. 2009;4:175–183.
76. Cañadas C, Alvarado H, Calpena AC, et al. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int J Pharm. 2016;511(2):719–727.
77. Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL. Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci. 2018;19(9):2830.
78. Hathout RM, Omran MK. Gelatin-based particulate systems in ocular drug delivery. Pharm Dev Technol. 2016;21(3):379–386.
79. Masse F, Desjardins P, Ouellette M, et al. Synthesis of ultrastable gold nanoparticles as a new drug delivery system. Molecules. 2019;24(16):2929.
80. Luo LJ, Lin TY, Yao CH, et al. Dual-functional gelatin-capped silver nanoparticles for antibacterial and antiangiogenic treatment of bacterial keratitis. J Colloid Interface Sci. 2019;536:112–126.
81. Hu C, Sun J, Zhang Y, et al. Local delivery and sustained-release of nitric oxide donor loaded in mesoporous silica particles for efficient treatment of primary open-angle glaucoma. Adv Healthc Mater. 2018;7(23):e1801047.
82. Choi SW, Cha BG, Kim J. Therapeutic contact lens for scavenging excessive reactive oxygen species on the ocular surface. ACS Nano. 2020;14(2):2483–2496.
83. Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):410–422.
84. Kong L, Qiu J, Sun W, et al. Multifunctional PEI-entrapped gold nanoparticles enable efficient delivery of therapeutic siRNA into glioblastoma cells. Biomater Sci. 2017;5(2):258–266.
85. Amina SJ, Guo B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int J Nanomedicine. 2020;15:9823–9857.
86. Darweesh RS, Ayoub NM, Nazzal S. Gold nanoparticles and angiogenesis: molecular mechanisms and biomedical applications. Int J Nanomedicine. 2019;14:7643–7663.
87. Kemp MM, Kumar A, Mousa S, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20(45):455104.
88. Lee GH, Kim YS, Kwon E, Yun JW, Kang BC. Toxicologic evaluation for amorphous silica nanoparticles: genotoxic and non-genotoxic tumor-promoting potential. Pharmaceutics. 2020;12(9):826.
89. Yadav N. Cerium oxide nanostructures: properties, biomedical applications and surface coatings. 3 Biotech. 2022;12(5):121.
90. Das S, Singh S, Dowding JM, et al. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33(31):7746–7755.
91. Nelson BC, Johnson ME, Walker ML, Riley KR, Sims CM. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants. 2016;5(2):15.
92. Cai X, Seal S, McGinnis JF. Sustained inhibition of neovascularization in vldlr-/- mice following intravitreal injection of cerium oxide nanoparticles and the role of the ASK1-P38/JNK-NF-κB pathway. Biomaterials. 2014;35(1):249–258.
93. Zhang J, Jiao J, Niu M, et al. Ten years of knowledge of nano-carrier based drug delivery systems in ophthalmology: current evidence, challenges, and future prospective. Int J Nanomedicine. 2021;16:6497–6530.
94. Li X, Zhang Z, Li J, Sun S, Weng Y, Chen H. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale. 2012;4(15):4667–4673.
95. Alvarez-Rivera F, Fernández-Villanueva D, Concheiro A, Alvarez-Lorenzo C. α-lipoic acid in soluplus(®) polymeric nanomicelles for ocular treatment of diabetes-associated corneal diseases. J Pharm Sci. 2016;105(9):2855–2863.
96. Li J, Li Z, Zhou T, et al. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. Int J Nanomedicine. 2015;10:6027–6037.
97. Grimaudo MA, Pescina S, Padula C, et al. Poloxamer 407/TPGS mixed micelles as promising carriers for cyclosporine ocular delivery. Mol Pharm. 2018;15(2):571–584.
98. Iriyama A, Usui T, Yanagi Y, et al. Gene transfer using micellar nanovectors inhibits corneal neovascularization in vivo. Cornea. 2011;30(12):1423–1427.
99. Kutlehria S, Vhora I, Bagde A, et al. Tacrolimus loaded PEG-cholecalciferol based micelles for treatment of ocular inflammation. Pharm Res. 2018;35(6):117. doi:10.1007/s11095-018-2376-7
100. Gawin-Mikołajewicz A, Nartowski KP, Dyba AJ, Gołkowska AM, Malec K, Karolewicz B. Ophthalmic nanoemulsions: from composition to technological processes and quality control. Mol Pharm. 2021;18(10):3719–3740. doi:10.1021/acs.molpharmaceut.1c00650
101. Kalam MA, Alshamsan A, Aljuffali IA, Mishra AK, Sultana Y. Delivery of gatifloxacin using microemulsion as vehicle: formulation, evaluation, transcorneal permeation and aqueous humor drug determination. Drug Deliv. 2016;23(3):896–907. doi:10.3109/10717544.2014.920432
102. Lidich N, Garti-Levy S, Aserin A, Garti N. Potentiality of microemulsion systems in treatment of ophthalmic disorders: keratoconus and dry eye syndrome - In vivo study. Colloids Surf B Biointerfaces. 2019;173:226–232. doi:10.1016/j.colsurfb.2018.09.063
103. Momin MM, Afreen SD. Nanoformulations and highlights of clinical studies for ocular drug delivery systems: an overview. Crit Rev Ther Drug Carrier Syst. 2021;38(4):79–107. doi:10.1615/CritRevTherDrugCarrierSyst.2021035767
104. Mandal A, Pal D, Agrahari V, Trinh HM, Joseph M, Mitra AK. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67–95. doi:10.1016/j.addr.2018.01.008
105. Akhter MH, Ahmad I, Alshahrani MY, et al. Drug delivery challenges and current progress in nanocarrier-based ocular therapeutic system. Gels. 2022;8(2):82. doi:10.3390/gels8020082
106. Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):151–165. doi:10.1089/jop.2012.0232
107. Cheng Y, Wu Q, Li Y, Xu T. External electrostatic interaction versus internal encapsulation between cationic dendrimers and negatively charged drugs: which contributes more to solubility enhancement of the drugs? J Phys Chem B. 2008;112(30):8884–8890. doi:10.1021/jp801742t
108. Bravo-Osuna I, Noiray M, Briand E, et al. Interfacial interaction between transmembrane ocular mucins and adhesive polymers and dendrimers analyzed by surface plasmon resonance. Pharm Res. 2012;29(8):2329–2340. doi:10.1007/s11095-012-0761-1
109. Yuan X, Marcano DC, Shin CS, et al. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;9(2):1749–1758. doi:10.1021/nn506599f
110. Liu W, Borrell MA, Venerus DC, Mieler WF, Kang-Mieler JJ. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol. 2019;8(1):12. doi:10.1167/tvst.8.1.12
111. Lyu N, Zhao Y, Xiang J, et al. Inhibiting corneal neovascularization by sustainably releasing anti-VEGF and anti-inflammation drugs from silica-thermogel nanohybrids. Mater Sci Eng C Mater Biol Appl. 2021;128:112274. doi:10.1016/j.msec.2021.112274
112. Soiberman U, Kambhampati SP, Wu T, et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials. 2017;125:38–53. doi:10.1016/j.biomaterials.2017.02.016
113. Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31–64. doi:10.1016/j.addr.2017.04.001
114. Wang B, Tang Y, Oh Y, et al. Controlled release of dexamethasone sodium phosphate with biodegradable nanoparticles for preventing experimental corneal neovascularization. Nanomedicine. 2019;17:119–123. doi:10.1016/j.nano.2019.01.001
115. Zhang Y, Yu Y, Li G, Zhang X, Wu Z, Lin L. Bioadhesive glycosylated nanoformulations for extended trans-corneal drug delivery to suppress corneal neovascularization. J Mater Chem B. 2021;9(20):4190–4200. doi:10.1039/D1TB00229E
116. Di Tommaso C, Bourges JL, Valamanesh F, et al. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur J Pharm Biopharm. 2012;81(2):257–264. doi:10.1016/j.ejpb.2012.02.014
117. Zhang XP, Sun JG, Yao J, et al. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed Pharmacother. 2018;107:1056–1063. doi:10.1016/j.biopha.2018.08.092
118. Luis de Redín I, Boiero C, Recalde S, et al. In vivo effect of bevacizumab-loaded albumin nanoparticles in the treatment of corneal neovascularization. Exp Eye Res. 2019;185(107697):107697. doi:10.1016/j.exer.2019.107697
119. Sun JG, Jiang Q, Zhang XP, et al. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int J Nanomedicine. 2019;14:1489–1501. doi:10.2147/IJN.S195504
120. Sánchez-Huerta V, Gutiérrez-Sánchez L, Flores-Estrada J. (-)-Epigallocatechin 3-gallate (EGCG) at the ocular surface inhibits corneal neovascularization. Med Hypotheses. 2011;76(3):311–313. doi:10.1016/j.mehy.2010.09.020
121. Ouyang H, Xue Y, Lin Y, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511(7509):358–361. doi:10.1038/nature13465
122. Wang Z, Liu CH, Huang S, Chen J. Wnt Signaling in vascular eye diseases. Prog Retin Eye Res. 2019;70:110–133. doi:10.1016/j.preteyeres.2018.11.008
123. Zhu L, Lama S, Tu L, GJ Dusting, JH Wang, GS Liu. TAK1 signaling is a potential therapeutic target for pathological angiogenesis. Angiogenesis. 2021;24(3):453–470. doi:10.1007/s10456-021-09787-5
124. Wang JH, Tseng CL, Lin FL, et al. Topical application of TAK1 inhibitor encapsulated by gelatin particle alleviates corneal neovascularization. Theranostics. 2022;12(2):657–674. doi:10.7150/thno.65098
125. Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57(5):415–429. doi:10.1016/j.survophthal.2012.01.007
126. Jani PD, Singh N, Jenkins C, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48(5):2030–2036. doi:10.1167/iovs.06-0853
127. Cho YK, Uehara H, Young JR, et al. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest Ophthalmol Vis Sci. 2012;53(4):2328–2336. doi:10.1167/iovs.11-8393
128. Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–467.
129. Qazi Y, Stagg B, Singh N, et al. Nanoparticle-mediated delivery of shRNA.VEGF-a plasmids regresses corneal neovascularization. Invest Ophthalmol Vis Sci. 2012;53(6):2837–2844. doi:10.1167/iovs.11-9139
130. Hagigit T, Abdulrazik M, Valamanesh F, Behar-Cohen F, Benita S. Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatment of ocular neovascularization: an in-vivo study in rats and mice. J Control Release. 2012;160(2):225–231. doi:10.1016/j.jconrel.2011.11.022
131. Duan L, Yang G, Zhang R, Feng L, Xu C. Advancement in the research on vascular endothelial growth inhibitor (VEGI). Target Oncol. 2012;7(1):87–90. doi:10.1007/s11523-012-0206-0
132. Wang H, Wang B. Inhibition of corneal neovascularization by vascular endothelia growth inhibitor gene. Int J Ophthalmol. 2010;3(4):295–298. doi:10.3980/j.issn.2222-3959.2010.04.04
133. Yoon KC, Bae JA, Park HJ, et al. Subconjunctival gene delivery of the transcription factor GA-binding protein delays corneal neovascularization in a mouse model. Gene Ther. 2009;16(8):973–981. doi:10.1038/gt.2009.50
134. Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi:10.1196/annals.1352.026
135. Han H, Son S, Son S, et al. Reducible polyethylenimine nanoparticles for efficient siRNA delivery in corneal neovascularization therapy. Macromol Biosci. 2016;16(11):1583–1597. doi:10.1002/mabi.201600051
136. Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007;282(47):34420–34428. doi:10.1074/jbc.M611289200
137. Lee K, Shin Y, Cheng R, et al. Receptor heterodimerization as a novel mechanism for the regulation of Wnt/β-catenin signaling. J Cell Sci. 2014;127(Pt 22):4857–4869. doi:10.1242/jcs.149302
138. Wang Z, Cheng R, Lee K, et al. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler Thromb Vasc Biol. 2015;35(4):855–864. doi:10.1161/ATVBAHA.114.304627
139. Nguyen DD, Lai J-YJCEJ. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem Eng J. 2022;435(2):134970. doi:10.1016/j.cej.2022.134970
140. Luo LJ, Nguyen DD, Lai JY. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics. 2021;11(11):5447–5463. doi:10.7150/thno.54525
141. Luo L-J, Jian H-J, Harroun SG, Lai J-Y, Unnikrishnan B, Huang C-C. Targeting nanocomposites with anti-oxidative/inflammatory/angiogenic activities for synergistically alleviating macular degeneration. Appl Mater Today. 2021;24:101156. doi:10.1016/j.apmt.2021.101156
142. Cho WK, Kang S, Choi H, Rho CR. Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea. 2015;34(4):456–459. doi:10.1097/ICO.0000000000000343
143. Zheng Q, Fang Y, Zeng L, et al. Cytocompatible cerium oxide-mediated antioxidative stress in inhibiting ocular inflammation-associated corneal neovascularization. J Mater Chem B. 2019;7(43):6759–6769. doi:10.1039/C9TB01066A
144. Mohammadpour M, Jabbarvand M, Hashemi H, Delrish E. Prophylactic effect of topical silica nanoparticles as a novel antineovascularization agent for inhibiting corneal neovascularization following chemical burn. Adv Biomed Res. 2015;4:124. doi:10.4103/2277-9175.158039
145. Tang M, Ji X, Xu H, et al. Photostable and biocompatible fluorescent silicon nanoparticles-based theranostic probes for simultaneous imaging and treatment of ocular neovascularization. Anal Chem. 2018;90(13):8188–8195. doi:10.1021/acs.analchem.8b01580
146. Lai P-X, Chen C-W, Wei S-C, et al. Ultrastrong trapping of VEGF by graphene oxide: anti-angiogenesis application. Biomaterials. 2016;109:12–22. doi:10.1016/j.biomaterials.2016.09.005
147. Khiev D, Mohamed ZA, Vichare R, et al. Emerging nano-formulations and nanomedicines applications for ocular drug delivery. Nanomaterials. 2021;11(1):173. doi:10.3390/nano11010173
148. Sousa F, Castro P, Fonte P, Kennedy PJ, Neves-Petersen MT, Sarmento B. Nanoparticles for the delivery of therapeutic antibodies: dogma or promising strategy? Expert Opin Drug Deliv. 2017;14(10):1163–1176. doi:10.1080/17425247.2017.1273345
149. Domingues C, Santos A, Alvarez-Lorenzo C, et al. Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS Nano. 2022;16(7):9994–10041. doi:10.1021/acsnano.2c00128
150. Sarma A, Bania R, Devi JR, Deka S. Therapeutic nanostructures and nanotoxicity. J Appl Toxicol. 2021;41(10):1494–1517. doi:10.1002/jat.4157
151. Suresh PK, Sah AK. Nanocarriers for ocular delivery for possible benefits in the treatment of anterior uveitis: focus on current paradigms and future directions. Expert Opin Drug Deliv. 2014;11(11):1747–1768. doi:10.1517/17425247.2014.938045
152. Ako-Adounvo AM, Nagarwal RC, Oliveira L, et al. Recent patents on ophthalmic nanoformulations and therapeutic implications. Recent Pat Drug Deliv Formul. 2014;8(3):193–201. doi:10.2174/1872211308666140926112000
153. Gorantla S, Rapalli VK, Waghule T, et al. Nanocarriers for ocular drug delivery: current status and translational opportunity. RSC Adv. 2020;10(46):27835–27855. doi:10.1039/D0RA04971A
154. Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360. doi:10.3389/fchem.2018.00360
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
