Back to Journals » Biologics: Targets and Therapy » Volume 14
An Update for the Clinician on Biologics for the Treatment of Psoriatic Arthritis
Authors Chimenti MS , D'Antonio A, Conigliaro P, Ferrigno S, Vendola A, Ferraioli M, Triggianese P, Costa L , Caso F , Perricone R
Received 30 April 2020
Accepted for publication 5 August 2020
Published 20 August 2020 Volume 2020:14 Pages 53—75
DOI https://doi.org/10.2147/BTT.S260754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shein-Chung Chow
Maria Sole Chimenti,1,* Arianna D’Antonio,1,* Paola Conigliaro,1 Sara Ferrigno,1 Andrea Vendola,1 Mario Ferraioli,1 Paola Triggianese,1 Luisa Costa,2 Francesco Caso,2 Roberto Perricone1
1Rheumatology, Allergology and Clinical Immunology, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy; 2Rheumatology Unit, Department of Clinical Medicine and Surgery, School of Medicine and Surgery, University Federico II, Naples, Italy
*These authors contributed equally to this work
Correspondence: Maria Sole Chimenti Tel +39 06 20900358
Email [email protected]
Abstract: Psoriatic arthritis (PsA) is a chronic inflammatory arthropathy typically associated with psoriasis (PsO). The pathogenesis is strictly related to the association among the presence of genetic risk alleles and innate and acquired immune response with dramatic consequences on bone remodeling. Clinically, PsA patients may present heterogenicity of articular and periarticular manifestations that may be associated with the presence of comorbidities making treatment decision challenging in patients management. The identification of patient-targeted therapies is still a critical issue. Actually, several biological and synthetic drugs are promising in terms of efficacy and safety profile. National and international treatment recommendations support clinicians in the decision of the best treatment, although they may have limits basically related to updates and different outcomes included in the clinical studies evaluated. The aim of this narrative review is therefore to give guidance for clinicians for PsA patients treatment. For this purpose, we evaluated evidence on biological therapies efficacy used for PsA treatment. Specifically, we reviewed data on biological therapies, Janus kinases (JAK) inhibitors, and drugs with a new mechanism of action that are part of the treatment pipeline. The concept of “switching” and “swapping” is also described, as well as data concerning special populations such as pregnant women and elderly patients.
Keywords: psoriatic arthritis, biological therapies, TNF-inhibitors, JAK-inhibitors, phosphodiesterase-4, tofacitinib, tsDMARDs
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis typically associated with psoriasis (PsO) occurring in nearly 30% of patients affected by PsO.1 PsA is characterized by inflammation at joints, tendons, and enthesal levels making the articular involvement extremely diversified.1 The clinical heterogeneity of PsA, as well as the frequent presence and association with several comorbidities, make the treatment choice challenging for rheumatologists.2 Recent evidence suggests a complex interplay between genetic predisposition and innate and acquired immune response.2,3
In the 1990s, findings based on the immunopathogenesis of the disease have led to the development of biological drugs directed against pathogenetic targets, such as Tumor Necrosis Factor (TNF).4 TNF is a pleiotropic cytokine which regulates several inflammatory reactions and immune functions through the control of cellular processes and plays a central role in the pathogenesis of PsA.5 TNF-inhibitors (TNF-i) drugs [Infliximab (IFX), Etanercept (ETA), Adalimumab (ADA), Golimumab (GOL) and Certolizumab Pegol (CZT)], have opened new therapeutic horizons in PsA, proving to be effective in the control of the signs/symptoms of inflammation, in improving the quality-of-life and the functional outcome, in inhibiting the progression of the structural damage in the peripheral joints, and in presenting a good safety profile.5,8 Recently, advances in the role of Interleukin (IL)-23 and IL-17 in PsA pathogenesis and in particular in the pathogenesis of enthesitis and dactylitis, support the use of drugs that have these two cytokines as targets.9 In addition, research has also focused on bone remodeling in PsA, demonstrating the interplay between IL-23 and IL-17 and osteoblasts and osteoclasts in both erosions and osteoproductive lesions.10 Currently, histologic features of PsA synovitis also support the relevance of an autoimmune pathway of the disease.2 However, drugs such as rituximab (RTX) typically used for autoimmune diseases such as rheumatoid arthritis (RA) were only partially effective in PsA treatment. On the contrary, targeted-synthetic DMARDs (tsDMARDs) drugs, approved for RA as Janus kinases inhibitors (JAKi), were demonstrated to be effective for PsA treatment, making the treatment armamentarium richer and the treatment decision intriguing.11 In order to clarify the different therapeutic options for PsA, guidelines help in identification of the best treatment based on the clinical predominant manifestation. International and National Guidelines suggest to start with the use of conventional DMARDs (csDMARDs) and in cases of inadequate response, contraindication, or intolerance to at least one DMARD, treatment with biological DMARDs (bDMARDs) such as TNFi or anti-IL17 and anti-IL23 therapies [ustekinumab (UST), secukinumab (SEC) or ixekizumab (IXE)] should be considered.12,13 However, management of PsA patients with special conditions, such as the elderly, pregnancy, or those with several comorbidities, is still a challenge. Relevant suggestions emerged also from registries and real-life data, which may improve our knowledge in bDMARDs use.14 To date, the position of JAKi and the place of future drugs that will come on the market is still unknown.
The overarching aim of this narrative review was to give guidance for clinicians for PsA patients treatment and to focus on significant insights on potential new therapeutic targets. First of all, we performed a description of the main disease characteristics, both articular and peri-articular, as well as the systemic inflammatory involvement as extra-articular manifestations and comorbidities. Then, we described the main studies demonstrating TNFi efficacy and the efficacy of different mechanisms of action. We also dedicated a section to tsDMARDs, even if they are not considered biologics, but they may have the same place in the treatment armamentarium as bDMARDs. We conclude with a discussion based on our opinion on PsA management as guidance for clinicians.
Clinical Manifestations and Comorbidities
Clinical features of PsA are included in a systemic disease defined as Systemic Psoriatic Disease (SysPsD), highlighting its systemic nature characterized by joints involvement, enthesitis, dactylitis, psoriasis (PsO), and a wide spectrum of extra-cutaneous and -articular manifestations.2 PsA has an extensive variety of clinical presentations, ranging from single “sausage” digits to arthritis mutilans. The classic description of articular involvement, by Moll and Wright in 1973, was based on the main articular site involved, and portrayed five clinical subtypes, described as: axial PsA, symmetrical polyarthritis, asymmetrical oligoarthritis, distal interphalangeal (DIP) arthritis, and arthritis mutilans.15 Patterns may change during time or occur in a combined manner; in particular, DIP arthritis and polyarthritis may overlap with axial disease, leading to the forewritten extension of variety of PsA.16,17 PsA is characterized by a general inflammatory state responsible for associated comorbidities and systemic manifestations, including cardiovascular (CV) disease, diabetes mellitus II (DM), obesity, metabolic syndrome (MetS), uveitis, inflammatory bowel disease (IBD), liver inflammation, osteoporosis, emotional, and psychological symptoms.18, More than half of patients with PsA have at least one comorbidity.21 The prevalence of clinical features and comorbidities are summarized in Table 1. Cardiovascular diseases (CVD) are the leading cause of death in PsA patients, who present a 43% increased risk of CVD over the general population.22 CV risk appears as an independent risk factor for major adverse cardiovascular events (MACE), although an increased rate of CV events in PsA patients correlate also with the presence of traditional CV risk factors (ie, diabetes, hypertension, obesity, dyslipidemia, and metabolic syndrome).22,26 Ocular pathologies related to SysPsD include: conjunctivitis, episcleritis, scleritis, keratitis, macular edema, glaucoma, and cataract.27 However, acute anterior uveitis is the most frequent manifestation.27,29 SysPsD is also associated with IBD, assuming a gut–joint–skin axis.30,31 Recent data have shown patients presenting PsA or PsO have 4-fold increased risk of developing IBD.30 In addition, Scarpa et al32 found microscopic changes in all PsA patients of their study’s population and none of them presented macroscopic mucosal changes or IBD symptoms. Anxiety and depression are common pathologies among SysPsD patients.20 These disorders have a great impact on QoL patients, lowering their pain threshold and reducing adherence to therapies.33,34 Little evidence has been reported on any pulmonary diseases, but evidence supports that PsA patients present a tendency to develop drug-related lung fibrosis, especially in those treated with methotrexate (MTX) or TNFi.35,36 As deducible, PsA represents a significant health issue having a profound impact on QoL: chronic pain together with the effects on bones and cartilages led to a limitation in physical functioning and work abilities, extreme fatigue, and emotional and social impairment.37 The systemic nature of this disease and the high presence of comorbidities make the treatment choice a task for rheumatologists.
 |
Table 1 Prevalence of Comorbidities in PsA Patients |
Biological DMARDs
In recent years, extensive research has showed the pathophysiologic basis of rheumatic diseases, combined with the biopharmaceutical developments, leading to the introduction of biotechnological drugs.38 These agents target specific components of the immune system that are essential for the generation and maintenance of the pathogenetic process.3,9 Their appearance on the therapeutic scene has considerably changed the approach to PsA treatment.
TNF-Inhibitors
TNFi were the first bDMARDs approved, through the progressive improvement in knowledge on TNFα actions in the pathogenesis of PsA. Indeed, TNFα is a key mediator of acute inflammation in PsA, activating pro-inflammatory genes transcription, cytokines secretion, and overexpression of macrophages and other immune cells, thus promoting and perpetuating unbalanced inflammation and articular damage.39 One of the first studies demonstrating TNFi efficacy in modifying synovial cell populations and infiltrates in PsA dates back to 2001, when Baeten et al40,41 showed a reduction of vascularity and inflammatory cell populations following IFX treatment. TNFi have been approved for PsA patients since the 2000s.3 Evidence of their efficacy in treating both PsO and PsA is available from numerous randomized controlled trials (RCTs), being significantly more effective than placebo in improving American College of Rheumatology 20% (ACR20) response rates, PsA Response Criteria (PsARC), and Psoriasis Area Severity Index (PASI).42,46 An improvement in nail disease, dactylitis, and enthesitis, as well as a significant inhibition of radiographic progression were also detected.3 TNFi are also considered a first-line option for the treatment of axial disease in PsA, despite most of data being based on literature from Ankylosing Spondylitis (AS) and axial spondyloarthritis.47 Data concerning head-to-head efficacy are still lacking, but all the drugs in the TNFi group have been indirectly compared to each other, demonstrating similar outcomes and safety profiles.3 With regard to the latter, data on oncologic and infection risk in PsA patients treated with TNFi was derived from RCTs and RCTs-metanalyses, demonstrating a safety profile comparable to the control/placebo.48 A large metanalysis on ADA has recently shown that the overall rate of malignancy for PsA patients treated was similar to those as expected from the general population.49 Real-life studies and Registries have confirmed these data.50,55
With regard to infections, RCTs and observational studies reported a good safety profile of TNFi. However, even if well tolerated, TNFi are associated with an increased infective risk, including opportunistic infections. Monoclonal antibodies, in particular IFX, seem to be responsible for the increased risk of these infections.56 Several recommendations for screening infections before initiating TNFi have been proposed. Latent, acute, and chronic infections represent a contraindication to use a biological therapy. In cases of Latent tuberculosis infection, anti-tubercular prophylaxis is recommended. Furthermore, HBV and HCV virological follow-up should be considered during TNFi treatment. Finally, patients who are at high risk of varicella zoster (HZ) reactivation would benefit from a second vaccination in adulthood when receiving TNFi.57 In recent years, biosimilars for IFX, ETA, and ADA have become available and their licensing studies showed similar pharmacodynamics, pharmacokinetics, and efficacy to the reference product.43,45 Their advantages are mainly related to economic saving.
Biological DMARDs Other Than TNFi
Although TNFi therapy remains central in the management of PsA, new insights into its pathogenesis led to identification of new therapeutic targets, including IL-12, IL-23, and IL-17. The IL-17 signaling pathway plays a relevant role in the pathogenesis of PsA. This proinflammatory cytokine is richly expressed in psoriatic skin lesions and in the synovial fluid of patients9 and can induce activation and proliferation of keratinocytes and endothelial cells.58 On the other side, IL-23 has been shown to play an important role in the polarization of CD4+ T-cells to become IL-17 producers.59 In the last few years, therapeutic agents targeting the IL-23/IL17 axis have been studied for the treatment of PsA.
Ustekinumab (UST), a fully human monoclonal antibody directed against IL-12/23, was the first of these novel targeted therapies to be approved for the treatment of PsA in 2013.60 UST showed consistent and sustained clinical efficacy in active PsA.61 Efficacy and safety of UST has been evaluated in two Phase III trials, which enrolled patients TNFi naïve with moderate-to-severe disease that failed NSAIDs or synthetic DMARDs (PSUMMIT-1), or had failed to ≥1 TNFi (PSUMMIT 2).62,63 Results clearly demonstrated the effectiveness of UST, right from the first month, in treating most domains of disease, including dactylitis and enthesitis. However, clinical outcomes were better for the TNFi naïve group compared with the TNFi exposed group.64 Integrated data analysis results indicated that there was a significant and persistent inhibition of radiographic progression in UST treated patients, supporting the role of IL-23 in the radiographic damage of PsA.64 A prospective randomized-controlled open-label study, ECLIPSA, showed that UST achieved superior responses as compared to TNFi regarding enthesitis and psoriatic skin disease, but not for peripheral arthritis.65 These results are confirmed by our direct experience: patients who have previously experienced TNFi without a significant improvement in skin disease and enthesis involvement showed a significant response to UST.14 The best data demonstrating ongoing safety of UST is reported in the 5-year PHOENIX long-term extensions. The most commonly reported adverse events (AEs) were nasopharyngitis, upper respiratory tract infection (URTI), headache, and arthralgia, with similar rates between doses (45 mg and 90 mg).66
Novel antibodies directed against the p19 subunit of IL-23 have been developed. Risankizumab (RSK), tildrakizumab (TLK), and Guselkumab (GSK) have been approved for the treatment of moderate-to severe plaque PsO, but they appear to be effective also in PsA.67,69 Results from two phase III clinical trials, DISCOVER-1 and DISCOVER-2, confirmed its possible role as a therapeutic option for PsA. In DISCOVER-1, which involved patients who were either biologic-naïve or had previously been treated with up to two TNFi, the improvement in peripheral arthritis at week 24 was significantly higher among patients treated with GSK than among those given placebo. DISCOVER-2 was larger than DISCOVER-1 and involved only patients naïve to biologic therapies, giving similarly promising results. The effectiveness of GSK was also demonstrated on dactylitis and enthesitis in the two studies.69 Overall, GSK seems to have a favorable safety profile: phase III trials VOYAGE 1 and VOYAGE 2 have found the most common AEs to include nasopharyngitis, headache, and URTI. Serious infection, malignancy, and MACE do not appear to be increased in patients treated with GSK compared to placebo and ADA.70 Recent clinical trials evaluating the efficacy of UST and other anti-IL23 agents, such as RSK, in AS were performed, but they had surprisingly negative outcomes. These results make anti-IL23 appear to be ineffective on long-standing axial disease, such as AS, even if there is a possibility that UST and RSK might have been underdosed in these trials or that they should be used in early phases of spondyloarthritis (SpA).71 A prospective, single-arm, open-label, proof-of-concept trial, the TOPAS, was conducted to evaluate UST efficacy and safety in AS patients who were naïve to biologic therapies. The results showed that UST improved all continuous parameters related to disease activity at week 24, with 65% of patients reaching the primary outcome of a 40% improvement in disease activity according to the Assessment of Spondyloarthritis International Society (ASAS) and 55% reaching a 50% improvement of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).72 Moreover, there was a substantial (41% and 31% for the sacroiliac joints and for the spine, respectively) reduction of active inflammation as detected by MRI in the entire group. Despite the clear limitations of the study, as its open-label design and small sample size, an important indication of the possible therapeutic efficacy of UST in active AS was received.72 In order to explain the conflicting results from therapeutic trials, it has been speculated that IL-23 might have a pathogenic role in the initiation of AS (or axial SpA) but not in maintaining established disease.71
Secukinumab (SEC) is a fully human monoclonal antibody that selectively binds to IL-17A, approved for the treatment of active PsA since 2015. The efficacy and safety of SEC in PsA were assessed for the first time in a randomized, double-blind, placebo-controlled Phase II clinical trial. The results achieved in terms of reduction of disease activity and lowering of acute phase reactant led to the design of the FUTURE trials.73 According to their results, SEC greatly improved clinical responses regarding arthritis, enthesitis, dactylitis, skin, and nail disease compared to the placebo group, with a comparable safety profile associated with a significant reduction in radiographic progression of structural joint damage relative to placebo.73,75 Another recent phase III RCT, the FUTURE 5, showed an improvement of clinical and radiographic response in PsA patients related to the placebo group.75 In the absence of RCTs data, matching-adjusted indirect comparison can estimate the comparative effectiveness of TNFi and SEC in TNFi naïve PsA patients. One of them showed that TNFi naïve patients have a similar probability of achieving clinical responses with subcutaneous SEC or intravenous IFX in the short-term, while in the mid- to long-term patients receiving SEC were more likely to achieve clinical responses than those receiving IFX.76 Furthermore, data from systematic review and meta-analysis shown as SEC appears to be superior to UST in TNFi naïve but not in TNFi experienced PsA patients.77,78 Recently, we demonstrated that SEC was efficacious in daily clinical practice in patients affected by PsA and AS characterized by several comorbidities and/or previous treatment failures. Moreover, the SEC retention rate was not influenced by Body Mass Index (BMI) or gender, supporting that another mechanism of action other than TNFi may be effective in overweight patients and in women.79 In a recent long-term safety analysis, SEC was associated with a generally low frequency of AEs, with higher incidence of URTI. As expected with IL-17 inhibition, cases of candidiasis were observed given that Th-17 cells play an important role in skin and mucous host defense, particularly against fungi and extracellular bacteria.80 Lower incidence of serious AE and opportunistic infections, comparable between doses of 150 mg or 300 mg, was reported. Neutropenia is an important adverse effect that must be considered when administering SEC to patients, possibly attributed to the effect of IL-17 on granulopoiesis. Anyway, no cases of Grade 3 or 4 neutropenia were reported, and no clinically significant AEs were associated with the development of neutropenia. Grade 1 or 3 neutropenia were registered but it resolved during the time in all cases and no patients discontinued treatment due to neutropenia. Discontinuations due to IBD onset or exacerbation were low but it is important to emphasize that the risk of IBD in this analysis could be different from that observed in the real-world, as patients with active IBD were excluded from all clinical trials.81 Additionally, a recent analysis did not assess any evidence for increased rates of adverse pregnancy outcomes with SEC. However, the analysis was limited by a sizable amount of missing outcome data and relatively short exposure to SEC.82
Ixekizumab (IXE) is a recombinant monoclonal antibody that binds IL-17A with high affinity. Its efficacy in PsA was assessed in two phase III trials (SPIRIT-P1, SPIRIT-P2). In SPIRIT-P1 PsA patients with inadequate response to csDMARDs and naïve to biologic therapies were randomized to receive IXE and ADA. At week 12, IXE achieved complete remission of PsO in more patients than ADA, while the effect on joints and nail psoriasis was comparable between the two biologics.83 A long-term extension of the SPIRIT-P1 study showed a sustained improvement in joints, skin, physical function, and quality-of-life over 52 weeks of IXE treatment. Moreover, the radiographic progression was minimal, particularly in patients who maintained IXE from week 0 to 52.84 In SPIRIT-P2, patients who had an inadequate response to TNFi were randomized to receive IXE versus placebo, with a significant improvement of signs and symptoms of active PsO at week 24 in the first group.85 Both SPIRIT-P1 and SPIRIT-P2 showed that IXE significantly improved dactylitis and enthesitis, despite statistical significance not being reached when compared with placebo at week 24 for all endpoints, likely due to the small number of patients who exhibited these peri-articular features. Of note, the efficacy of the IXE groups was similar regardless of use of concomitant csDMARDs, particularly MTX. SPIRIT-P3 evaluated the efficacy and safety of continuing versus withdrawing IXE in PsA patients naïve to other biologics and who achieved sustained minimal disease activity (MDA) on IXE. The results demonstrated that patients lost MDA after IXE withdrawal, but regained it with IXE re-treatment, while MDA was preserved in patients who continued IXE therapy.86 In our experience, a high proportion of PsA patients at 6 months achieved a skin clearance that was maintained over time and a low disease activity of arthritis was reached rapidly within the first 6 months of treatment, with a sustained efficacy during the 12 months follow-up period.87,88 Recently, SPIRIT-H2H was the first completed head-to-head trial comparing IXE and ADA in patients with active PsA and inadequate response to csDMARDs. The 24-week efficacy data from this study demonstrated that IXE was superior to ADA in simultaneously leading to an ACR50 and PASI100 response, was non-inferior to ADA for achieving ACR50, and was superior to ADA for achieving PASI100. Furthermore, significantly more patients achieved Disease Activity in PSoriatic Arthritis (DAPSA) remission with IXE than ADA, suggesting that skin changes were not the only domain contributing to differences between biologics.89
The side-effect profile for IXE was quite favorable and consistent with the studies on psoriasis.90 The majority of AE were mild and URTI were the most prevalent. Injection site reactions were predominantly mild but more frequent with IXE than SEC, probably for the lower immunogenicity of SEC. Recurrent chronic candidiasis has been reported in individuals with rare genetic defects in the IL-17 pathway and no cases of invasive or opportunistic fungal infections were reported. Grade 1 and Grade 2 neutropenia were noted with higher frequency with IXE, but no case of Grade 3 or Grade 4. No cases of IBD have been reported in the SPIRIT-P1 (including an extension period to week 52) or SPIRIT-P2 studies. Because of the potential protective role of IL-17A in the gut epithelium, vigilance is required when abdominal symptoms develop after the initiation of IXE.91
Pooled meta-analysis was conducted by Mourad et al78 identifying RCTs evaluating the efficacy of TNF-i, anti-IL12/23 (UST), and anti-IL17 (SEC, IXE) in treating PsA and conducting a meta-analysis of these agents for treatment of dactylitis and enthesitis. Their results showed that TNF-i and IL inhibitors brought dactylitis to a significant resolution at week 24, with pooled risk ratios (RR) versus placebo of 2.57 (95% CI=1.36–4.84) and 1.88 (95% CI=1.33–2.65) respectively. For resolution of enthesitis at week 24, RR for TNF-i was 1.93 (95% CI=1.33–2.79) versus 1.95 (95% CI=1.60–2.38) for IL inhibitors. According to these results, TNF-i demonstrated the same efficacy of IL inhibitors in treating the two PsA manifestations.92
The meta-analysis of Wu et al92 instead included RCTs evaluating the efficacy of SEC, UST, and IXE in achieving ACR20 and ACR50 over placebo. The rank probabilities based on the network meta-analysis were summarized for each treatment in order to obtain a surface under the cumulative ranking curve (SUCRA): the higher the SUCRA was, the more effective was considered each treatment. SEC showed a SUCRA of 96.42% according to ACR20, compared to the 38.61% of UST and the 50.84% of IXE. According to these results, SEC seems to be the most efficacious short-term treatment for peripheral PsA, although the authors pointed out several limitations of the study. SEC and IXE were also tested in patients with AS, and the clinical outcomes have clearly shown their superiority over placebo.71
Brodalumab (BRD) is a human anti-IL17 receptor A monoclonal antibody that inhibits IL-17A, IL-17F, and IL-17E. In two phase III clinical trials, AMVISION-1 and AMVISION-2, patients with active PsA despite prior DMARDs therapy, including biologics, were enrolled and randomized to receive placebo and BRD.71 At week 24, a higher rate of patients treated with BRD achieved a significant articular response compared to placebo. These results confirmed that BRD delivers consistent and clinically meaningful improvements in PsA.93 Current evidence suggests a similar safety profile for BRD compared to other IL-17 antagonists, with the most common adverse events represented by nasopharyngitis, URTI, and candidiasis. The US Food and Drug Administration (FDA) issued a warning after six patients treated with BRD across four clinical trials committed suicide, although no causal relationship was identified.94
Bimekizumab (BMK) is a humanized monoclonal antibody that selectively binds to and neutralizes both IL-17A and IL-17F. The BE ACTIVE randomized, double-blind, placebo-controlled phase IIb study showed that BMK was associated with significant improvement in joint involvement compared with placebo, with an acceptable safety profile.95 Although the results are promising, larger studies are required to better characterize the efficacy and safety profile of both BRD and BMK in the treatment of PsA.95 The safety profile of BMK was consistent with previous reports, with no apparent relationship between dose and treatment-emergent adverse events.95
According to the 2015 updated European League Against Rheumatism (EULAR) recommendations for the management of PsA, anti-IL17 and anti-IL12/IL23 biologic agents are indicated as a second-line biologic therapy for PsA treatment after the failure of one or more TNFi, or as a first-line in case they were contraindicated. This preference given to the TNFi is based on the longer duration of experience with these drugs and the largest amount of long-term efficacy and safety data available. However, in the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) recommendations published in the same year, TNFi, anti-IL12/23, and anti-IL-17 are included in the same therapeutic step and their use as first or second-line biologic therapy depends on their effectiveness on the dominant PsA manifestations.13 For clinician guidance, UST is preferred in patients with predominance of PsO, enthesitis and with a concomitant gastrointestinal involvement, such as Crohn’s disease, while we give a leading role to SEC in patients with PsO and/or axial PsA. Both therapeutic strategies have shown positive results in patients naïve to biologic therapies, and in patients with comorbidities. Characteristics such as female gender and high BMI may influence SEC indication. These elements lead us to consider the IL inhibitors as a first-line therapeutic option in selected cases. In contrast, monoclonal TNFi would be preferred over anti-IL17 therapy for patients with repeated uveitis as there is minimal evidence indicating anti-IL-17 therapies in effectively treating uveitis. The main randomized clinical trials available for bDMARDs in PsA are summarized in Table 2.
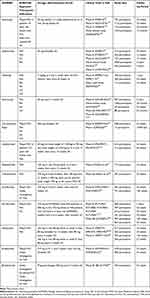 |
Table 2 Therapeutic Indications and Main Clinical Trials on Biologic DMARDs Evaluated and Ongoing for PsA Treatment |
Targeted Synthetic DMARDs
Recent advances provide support that dysfunction of signaling pathways involving the phosphodiesterase 4 (PDE4) enzyme and the kinase (JAK)-signal family pathway play an important role in the complex pathogenesis of PsA.96,97 PDE4, an enzyme belonging to phosphodiesterases, is responsible for the hydrolysis of cyclic adenosine monophosphate (cAMP) into AMP. PDE4 inhibition produces increased cAMP levels in immune and non-immune cells, altering the expression of downstream cascades and modifying inflammatory responses.98,99 Apremilast (APR) is an oral small molecule that inhibits intracellular PDE4 approved in March 2014 for the treatment of adult patients with PsO and active PsA.100 The efficacy and safety of APR were firstly demonstrated in four Phase III, placebo-controlled studies (PALACE 1, 2, 3, and 4). The PALACE 1, 2, and 3 enrolled PsA patients who were previously treated with cs- and/or bDMARDs, PALACE 4 evaluated APR monotherapy in csDMARD-naïve and biological-naïve populations.101,106 In all clinical trials, APR significantly improved PsA signs and symptoms, including enthesitis, dactylitis, PsO, physical function, and Patient-Reported Outcomes (PROs), and response was maintained up to 5 years. Among PsO patients with involvement of ≥3% of the body surface area (BSA) at baseline, cutaneous symptoms improved with apremilast treatment.106 The efficacy of APR was observed regardless of prior biologic experience or concomitant DMARD use, although apremilast was early efficacious in biologic-naïve patients.104,105 However, the time to onset of therapeutic effect has not been reported before week 16.98 A recent phase IIIB study, the Apremilast Monotherapy in a Clinical Trial of BIologic-NaïVE Patients With Psoriatic Arthritis (ACTIVE), showed that in biological-naïve PsA patients, onset of effect with APR was observed at week 2 and continued through week 52.107 Of interest, weight loss was observed at 52 weeks, suggesting that APR could have a positive impact on obesity and metabolic syndrome.108 To date, there is no evidence to demonstrate an impact of APR on structural disease progression: the results from PALACE analysis indicated significant improvements in the numbers of swollen and tender joints over 5 years of treatment, that may have been associated with inhibition of disease progression.104 Nevertheless data from real life studies have recently evidenced that APR is able to induce an early and sustained improvement on ultrasonographic inflammatory status at articular and peri-articular level.109 As other PDE4 inhibitors, APR showed generally an acceptable safety profile.110 The most common reported AEs were diarrhea, nausea, headache, and URTI. The gastrointestinal side-effects generally occurred within the first month of treatment and subsequently subsided.108 While the overall incidence of depression reported was low (≤1.8%), it is nevertheless recommended that the risks and benefits of APR should be carefully weighed prior to initiating therapy in patients with a history of depression and/or suicidal thoughts or behavior.106,108 Marked laboratory abnormalities were infrequent, returning to baseline with continued treatment, and no laboratory monitoring is required in patients receiving APR.106,108 Further, the pharmacokinetics of APR are not influenced by mild or moderate impairment of hepatic and renal function. However, dosage should be reduced in patients with severe renal impairment (creatinine clearance less than 30 mL/mm).106,108 Importantly, there is no interaction with methotrexate, frequently used in patients with PsA and plaque PsO.111
The good safety profile makes APR a convenient option for PsA treatment, especially in patients with high risk of infections, comorbidities, comedication, or with several contraindications including recent history of malignancies.99 Despite this, lack of data from head-to-head trials comparing APR with cs- or bDMARDs agents makes it difficult to place this small molecule in the treatment of PsO and PsA. An indirect comparison with csDMARD has shown more effectiveness of APR, in addition to the benefit of not requiring routine therapeutic drug monitoring. On the contrary, the efficacy of APR is lower than it would be anticipated with biologic therapies as ADA.98 Another indirect comparison indicates that APR, SEC, and UST may have similar efficacy in patients with PsA and an inadequate response to TNFi.112 GRAPPA guidelines strongly recommended bDMARDs and APR in patients with peripheral arthritis and an inadequate response to csDMARDs.113 EULAR guidelines also advise us to consider APR in patients with peripheral arthritis who prefer an orally administered therapy.114
Emerging studies investigated the potential role of JAK/STAT (Signal Transducers and Activators of Transcription) signaling pathway in the pathogenesis of several inflammatory diseases. JAKs activation leads to translocation of STAT proteins into the nucleus, regulating the transcription of pro-inflammatory gene involved in inflammatory and autoimmune disease.115 The tyrosine kinases of the JAK family include four members: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). Following their success in RA, JAKi are emerging as a promising therapeutic option for PsO and PsA. The rationale for using JAKi to treat PsA relies on the central role of cytokines in its pathogenesis. Most of the cytokines involved in PsA pathogenesis, directly or indirectly, are regulated through the JAK-STAT pathway, although JAKs cannot transmit signals provided by IL-1, IL-8 TGFβ, MCSF, and IL-17. Moreover, several studies have shown JAK1 and JAK3 dependent pathway activation in cultured cells from psoriasis skin and synovial/joint tissue, higher than in healthy controls or blood derived samples.116 In this context, targeting all JAKs or different JAK combinations by small-molecule inhibitors is considered a relevant strategy.117 Tofacitinib is an oral inhibitor of JAK1 and JAK-3, but can have some functions on JAK2 as well at higher doses. It was approved for use in combination with MTX in moderate-to-severe active adult PsA who have an inadequate response or intolerance to previous csDMARDs therapy.11 The therapeutic efficacy of tofacitinib has been evaluated in two randomized, multicentric, double-blind, placebo-controlled phase III trials, which enrolled patients with active PsA and either an inadequate response to ≥1 csDMARD and TNFi-naïve (OPAL Broaden), or an inadequate response to ≥1 TNFi (OPAL Beyond).118,119 Primary endpoints (ACR20 response and change from baseline in Health Assessment Questionnaire-Disability Index [HAQ-DI] at month 3) showed significant improvement in patients receiving tofacitinib 5 or 10 mg twice daily vs placebo. Of note, patients on tofacitinib showed an early improvement (2 weeks) from baseline in ACR20 response.119 Significant improvements in HAQ-DI, tender and swollen joints, PsO, enthesitis, and dactylitis vs placebo were observed for both tofacitinib doses at month 3, with the effects being maintained up to 6 months.120 Nevertheless, tofacitinib 10 mg BID was more effective in the treatment of moderate-to-severe plaque type psoriasis compared to the 5 mg BID dosage, as reported also in a dermatology trial.121,122 Post hoc analysis conducted by Strand et al123,124 showed significant improvement, exceeding placebo, across a range of PROs, proving that tofacitinib not only treats signs and symptoms of PsA but can also improve patient function and quality-of-life. In particular, patients treated with tofacitinib had greater improvement in PROs, fatigue, and quality-of-life. Previous studies have shown that tofacitinib inhibits TNF and IL-6-induced osteoclastogenesis and bone destruction, mediated by receptor activator of nuclear factor kappa-B ligand (RANKL), as well as STAT-activated proteins associated with progressive and destructive joint disease.125,127 Moreover, it was speculated that elevated C-reactive Protein (CRP) levels at baseline reflect the systemic inflammation state and could be related with joint destruction in PsA.128 Recently, post hoc analysis of the OPAL Broaden study was conducted for evaluating the effect of baseline risk factors on radiographic progression in enrolled patients. At month 12, >90% of the patients across the tofacitinib groups met the criteria for radiographic non-progression in the joints. However, minimal changes in radiographic outcomes regardless of CRP levels were observed.129 The effect of tofacitinib on RANKL and IL-22 may have an important role in this context, particularly regarding bone loss, but larger and longer studies are required.129 The safety profile and AE related to the use of JAKi have also been evaluated. AE were higher in tofacitinib 10 mg compared to 5 mg and the most common were nasopharyngitis, URTI, headache, and gastrointestinal disorders (diarrhea, nausea, vomiting, constipation). Data taken from RA and ulcerative colitis trials identified increased venous thromboembolic events and pulmonary embolisms in patients treated with tofacitinib, but large observational studies are needed to accurately quantify thromboembolic risks attributable to JAKi.130,131 Of interest for the side-effect profile of tofacitinib, reactivation of endogenous HZ was observed and vaccination against HZ prior to starting the drug may be considered.116 In OPAL trials, an increase from baseline in low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c) was reported, as well as elevations of aspartate and alanine aminotransferase concentrations of three or more times the upper limit of the normal range.99 The association of tofacitinib with lipid lowering agents, such as statins, may increase the safety of JAKs, but additional attention to the hepatic enzymes would be recommended.99 Tofacitinib is an oral drug with fast onset of action and a short half-life. In PsA, the dosage is 5 mg administered BID but a reduction (5 mg once a day) is recommended in patients with moderate/severe renal or hepatic impairment and in patients receiving drugs inhibiting CYP2C19 and/or CYP3A4. Contraindications to the use of tofacitinib are represented by severe hepatic disfunction, serious or opportunistic infections, active tuberculosis (TB), and it should not be initiated in patients with a low level of hemoglobin (<9 g/dL), low absolute leucocyte count (<750 cells/mm3), or low neutrophil count (<1000 cells/mm3). Cautious use of tofacitinib is advised in elderly patients and patients with malignancy history, chronic liver and lung disease, diabetes, and increased risk of GI perforations.99
Other JAK-i, some more specific for JAK1 (eg, upadacitinib, filgotinib, baricitinib), are currently being tested for the treatment of PsA in Phase 2 or Phase 3 clinical trials. EQUATOR is the first clinical trial to investigate a selective JAK1 inhibitor for the treatment of PsA.132 This phase II study explored the effect of filgotinib on patients with active PsA and an insufficient response or intolerance to at least one csDMARD. Data from EQUATOR demonstrated an improvement of disease activity and physical functioning in a PsA population treated with Filgotinib 200 mg once-daily compared to placebo. Particularly, the primary endpoint (ACR20 response at week 16) was achieved in the greater proportion of patients, with measurable improvements in disease activity after 1 week of treatment. ACR50 and ACR70 responses at Week 16 were also significantly higher for filgotinib compared with placebo. The study also found greater improvement of peripheral arthritis, enthesitis, and PsO as measured by MDA and PASI 75, and showed a beneficial effect on physical functioning, fatigue and pain, with significant improvements in psoriatic arthritis related pain intensity at week 1 and in HAQ-DI at week 2.132,133 These results are consistent with the previously observed rapid onset of action reported in the DARWIN1 and DARWIN2 trials of filgotinib in RA and are probably of interest to prospective patients.132,133 Filgotinib was well tolerated and safety-related outcomes were similar to placebo. In the EQUATOR study only one case of fatal pneumonia and of uncomplicated HZ in the filgotinib treatment group were reported, with no case to VTE, PE, malignancies, gastrointestinal perforations, or opportunistic infections/active TB.134 These findings suggest that selective inhibition of JAK1 might theoretically provide an improved safety profile compared with less selective JAKi.132 Upadacitinib, a JAK1 inhibitor approved for treatment of moderate-to-severe RA, is under study in two PsA Phase 3 RCTs. The first trial (SELECT-PsA 1) compared the efficacy and safety of upadacitinib with placebo and ADA in adult patients with active PsA who have had an inadequate response to at least one csDMARD;135 the second one (SELECT-PsA 2) comparing upadacitinib to placebo in PsA patients with inadequate response to at least one bDMARD.136 The results of both trials showed that upadacitinib, at a dosage of 15 mg or 30 mg once daily, achieved noninferiority compared with ADA and statistically significant ACR responses at week 12 vs placebo. Upadacitinib was also associated with improvements of HAQ-DI at week 12, PASI at week 16, and MDA at week 24. Moreover, both doses of upadacitinib showed inhibition of radiographic progression. As regard to safety, more information comes from RA studies.137,140 Preliminary data from SELECT-PsA1 and 2 suggest a low incidence of serious infections and cardiovascular events, but the trial’s long-term extension is still blinded in order to investigate the long-term safety and tolerability.135 Although further research is necessary, the advent of newer mode of action therapies has provided additional choice for clinicians who can choose optimal therapies based on their efficacy for different musculoskeletal and skin manifestations and their side-effect profile. Main randomized clinical trials available for tsDMARDs in PsA are summarized in Table 3.
 |
Table 3 Therapeutical Indications and Main Clinical Trials on Targeted Synthetic DMARDs Treatment for PsA |
Emerging Biological Therapies for PsA
Many treatment options are now available for PsA management considering all its clinical subtypes and comorbidities.1 However some patients have an inadequate response or a relative contraindication to first-line biologic therapy,141 advocating the development of novel medications with different mechanisms of action, such as T-cell modulators and anti-CD20.
Abatacept has been recently introduced as a therapeutic option for treating PsA patients with an inadequate response to DMARDs who do not require additional systemic therapy for PsO lesions.142,143 This drug is a cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4)–Ig human fusion protein that inhibits naïve T-cell activation, and prevents the co-stimulatory binding between CD28 and CD80/CD86. Back in 1999, a role of CTLA4 in the pathogenesis of psoriasis was suggested; a study on patients with stable plaque psoriasis conducted by Abrams et al144 reported a reduction of intralesional T-cells following Abatacept administration, correlated with reductions in epidermal proliferation, epidermal thickness, reversion of keratinocyte maturational abnormalities, and clinical improvement of PsO. Most recently, Mease et al145 evaluated its efficacy and safety in 170 PsA patients, in a 6-month, double-blind, placebo-controlled phase II trial, with less than 50% of participants reaching the ACR20 at 6 months, with a high response in TNFi naïve patients. Abatacept-treated patients also achieved a trend of improvement on additional measures of arthritis severity (ACR50 and ACR70), PsO severity, magnetic resonance imaging (MRI) scores, physical function, and quality-of-life. Following these results, in 2017 Mease et al146 conducted a Phase III RCT (ASTRAEA), in which 424 PsA patients with inadequate response to DMARDs (including TNFi) were randomized to receive a weekly subcutaneous 125 mg dose of abatacept or placebo for 24 weeks. Less than 40% of patients treated with abatacept achieved the ACR20 as a primary endpoint. High ACR20 responses were seen in a TNFi-naïve subpopulation and in those with baseline-elevated CRP. Resolution of enthesitis and dactylitis in the abatacept group compared to the placebo-one was seen.146 The efficacy of abatacept was sustained through the follow-up period. Modest results were observed in skin manifestation. Another study by Szentpetery et al147 randomized 15 TNFi-naïve PsA patients to receive intravenous abatacept or placebo with the aim to assess changes in immunohistochemical expression markers of synovial and skin inflammation, clinical outcomes, and MRI scores. Results reported EULAR responses in 90% of patients at 6 months, PASI50 response in 30% of cases, significant reduction in synovitis on MRI and synovial biopsies, and a decrease of expression of CD4+ Foxp3+ T-cells in synovial lymphoid follicles.147 Nowadays there are no clinical trials evaluated the efficacy of abatacept on axial-PsA, but a case report by Olivieri et al148 and a clinical study on AS patients by Lekpa et al,149 suggest a higher response in HLA-B27 negative women with no-radiological sacroiliitis. It is possible to assume that abatacept is a promising agent for the immunotherapy of PsA. However, stratification of patients and head-to-head comparisons among old and new biologics are still required for fully establishing his exact role in the management of PsA.150 Analysing PsA trials, there is an overall low risk of infection. The risk of opportunistic infections was higher in patients with additional comorbidities, including a history of tobacco use and chronic obstructive pulmonary disease (COPD) as well as recent use of high-dose glucocorticoids. Most of the long-term data of abatacept are derived from RA trials. Pneumonia, bronchitis, and urinary tract infections have been some of the most reported serious infections. Most recommend TB screening prior to treatment initiation, although there is no clear risk of TB infection with abatacept. Moreover, Abatacept should be used with caution in patients with history of recurrent infections, COPD, active pregnancy, or malignancy. Interestingly, data from RA patients estimate the development of treatment-related PSO at an incidence rate of <0.6 in those treated with abatacept.142
Rituximab (RTX) is an immunosuppressive drug used in autoimmune diseases such as RA, granulomatosis with polyangiitis, microscopic polyangiitis, and systemic lupus erythematosus.151 In an open-label study, 30% of PsA patients treated with RTX achieved the ACR20 response with another 30% achieving the PASI50, with a high improvement in TNFi-naïve patients.152 In a prospective study by Jimenez‑Boj et al,153 nine patients with PsA and 14 with RA received rituximab. In PsA patients, the Disease Activity Score (DAS) 28 improved from 6.2 to 4.9 (medians), HAQ was reduced from 1.5 to 1.0, and the Disease Activity Index for psoriatic arthritis was reduced from 52.0 to 32.5. All these improvements were statistically significant. In conclusion, in all these exploratory studies RTX exhibited efficacy in PsA patients with long-standing disease, above all in TNFi-naïve patients.153 Thus, controlled trials will be needed for more definitive understanding of the RTX role in the treatment of PsA, especially in patients who may have a relative contraindication to TNFi. In this small study, no serious adverse event was observed but more data concerning side-effects derive from extensive clinical experience of RTX in the treatment of lymphoma. The majority of patients receiving their first infusion of RTX experience flu-like symptoms; other common symptoms include nausea, headache, fatigue, and rash. About 10% of patients develop more severe symptoms such as bronchospasm, hypoxia, and hypotension. The profile of AEs in patients with RA receiving RTX was similar to that observed in the oncology setting, but the overall incidence was notably lower, and AEs were also less severe. This may be explained by the absence of the cytokine release syndrome associated with tumor cell lysis seen in patients with B cell malignancies, as well as by often associated use of steroids and other immunosuppressive drugs.154 Decreases in Ig levels were observed in some patients following RTX treatment, although the clinical consequences of this fact are unclear. Analysis of registry data has shown that a low IgG level (<6 g/L) before RTX treatment was associated with an increased risk of serious infection events; on the contrary, patients with low IgM had no increased risk of infections. The myocardial infarction rate reported for RTX-treated patients is consistent with epidemiological data from a general RA population receiving TNFi and there was no evidence to suggest an increased risk of MI or other CVD associated with rituximab treatment.155
Tocilizumab (TCZ) showed efficacy in RA, and some studies suggested a potential pathogenic role for IL-6 in PsA, describing increased IL-6 serum levels in PsA patients and a correlation between its levels and disease severity.156 Several case reports have been published showing conflicting results concerning the efficacy in PsA treatment.157 Ogata et al158,159 reported two cases of peripheral PsA refractory to several DMARDs treated with TCZ every 4 weeks (for 7 and 9 month durations, respectively), without achieving disease remission. Madureira et al160 presented three case reports of PsA patients with a personal history of psoriasis and exclusive peripheral joint involvement treated with TCZ: all patients experienced an improvement of the articular disease activity, inflammatory markers, and HAQ, without deterioration of the cutaneous disease (PASI). Based on these conflicting data, TCZ cannot be recommended as an alternative treatment for PsA with predominant peripheral involvement. Only a few cases showed a significant improvement with TCZ therapy and the reduction of DAS28 score may be due to the reduction of inflammation markers, used in DAS28 calculation.161 Nevertheless, further studies are necessary to validate these observations. Studies on the pathogenesis of immune-mediated diseases have been leading to the hypothesis that the concurrent blockade of more inflammatory cytokines may be a winning strategy of treatment, resulting in a more efficient suppression of critical pathogenic pathways and in a reduced risk of developing alternative circuits driving disease inflammation.162 Specifically, TNF and IL-17A may represent two targets to be simultaneously neutralized as they are considered the most relevant mediators in PsA pathogenesis.163,164 In a double-blind study by Mease et al,165 ABT-122 was superior to placebo in all clinical outcomes and superior to adalimumab on ACR50/70 and PASI75 responses. Frequencies of adverse events, were similar across all treatment groups, causing no discontinuations. No serious infections or systemic hypersensitivity reactions were reported with ABT-122. Efficacy assessed by ACR response was maintained over the 24 weeks, with no differences in safety and tolerability assessments.165 Another bispecific agent is COVA322, a TNF-α/IL-17A inhibitor that is currently being tested in psoriasis patients.166 In preclinical models, COVA322 has been shown to improve acute inflammation, with a good safety profile and pharmacokinetics similar to ADA.167 To evaluate the role that bispecific agents could have in the treatment of PsA, large-scale population, and head-to-head studies are necessary. A promising therapeutic target for the treatment of inflammatory diseases is represented by the A3 adenosine receptor (A3 AR). A3AR is a Gi protein-associated receptor, which is over-expressed in inflammatory cells and is involved in the regulation of mitogen-activated protein kinase (MAPK) pathways, managing functions of almost all immune cells.168 CF101 is an A2AR agonist and inhibits the activation of inflammatory pathways, preventing the release of cytokines and metalloproteinases.169 It was found to be safe and well tolerated in all preclinical and human clinical studies and showed promising results, particularly in PsO and RA.170,171 Randomized clinical trials available for emerging therapies in PsA are summarized in Table 4.
 |
Table 4 Therapeutical Indications and Main Clinical Trials for Emerging Therapies for PsA Treatment |
Discussion
The thorough knowledge of the pathogenic mechanisms together with the systemic features of the disease suggest tailored treatments for a better management for PsA patients.1,2,172 The therapeutic paradigm is further expanded by new emerging targets: new drugs capable of inhibiting several inflammatory pathways are appearing in the pharmacological scenario.9 Challenges in PsA treatment arose from different clinical manifestations, the presence of comorbidities and patient characteristics such as the elderly, BMI, and gender.3 PsA patients may present several comorbidities due to the systemic nature of the disease and consequently their presence influences treatment choice. The presence of CVD considerably reduced in both PsA and PsO patients, treated with TNFi.39,173 Pharmacological characteristics among TNFi may influence their use in different clinical pictures.39 TNFi use, in particular of monoclonal antibodies, is also suggested when patients present uveitis or gastrointestinal involvement due to their efficacy and mechanism of action in these pathologies.174 Concerning uveitis, a 2-year follow-up analysis reported a better efficacy and safety profile of ADA than IFX for the treatment of refractory juvenile idiopathic arthritis-associated uveitis.175 As well as, in fertile and pregnant women or in child-bearing women, TNFi, in particular ETN and CZP, may be preferred due to their physical characteristics and their safety profile. ETN and CZP may be considered for use throughout pregnancy due to the low rate of transplacental passage. In particular, among bDMARDs, continuation of TNFi during the first part of pregnancy should be considered. SpA tend to be stable or to get worse during pregnancy, even though the available literature is scarce.176 According to the EULAR overarching principles, family planning must be part of our routine practice in each patient of reproductive age and adjustment of therapy must be considered before a planned pregnancy.177,178 On the contrary, female gender influence bDMARDs efficacy: female sex is a negative predictive factor for TNF-i response in PsA.179 This was not demonstrated in real life data on PsA patients concerning SEC and UST treatment. We recently evaluated gender influence in PsA patients treated with UST and in PsA patients treated with SEC: gender did not influenced the efficacy of SEC nor the efficacy of UST.14,79 The presence of moderate-to-severe skin or nails PsO in “difficult areas” benefits treatment with inhibitors of Il-17 and IL-23 drug axes.3 However, the same superiority in treating articular and axial manifestations in PsA or in radiographic progression is still missing. In patients with comorbidities such as IBD treatment with UST suggests the patient is affected by PsA or Crohn's Disease.180 In addition, tofacitinib may be indicated in PsA patients presenting ulcerative colitis.181 Up to 50% of SpA patients present documented microscopic subclinical gut inflammation, although it is still unclear if the proportion of PsA patients presenting this bowel mucosa changes; even though Scarpa et al 32 found microscopic changes in all PsA patients among a small population and none of them presented macroscopic mucosal changes or IBD symptoms. The screening of gastrointestinal involvement has been suggested in PsA patients, in particular in patients undergoing IL-17 inhibitors or ETN, in order to avoid onset or recrudescence of IBD. The safety profile and infections clearly have an impact on the treatment choice. In this context, latent TB is not a contraindication to the use of other bDMARDs over TNFi, as well as patients with malignancies who underwent APR treatment. According to all this, the well-known screening procedures, for TB and B and C hepatitis, should be applied for all of the bDMARDs but not for APR.12 As suggested by treatment recommendations, Heart failure class III or IV and demyelinating disorders, drugs such as UST, SEC, and APR did not reported contraindications their use. In particular, SEC may have a good effect in neurological disease, such as multiple sclerosis.182 TNFi efficacy is influenced by BMI.12 Gremese et al183 demonstrated that patients affected by SpA presenting overweight or obesity showed a reduced response to TNF-i. Clinical phenotype, such as BMI, should address the treatment choice. We support the hypothesis, from real life data on SEC and UST treatment, that for both treatments, their efficacy is not influenced by high BMI nor by the presence of comorbidities.184 Not uncommonly, the disease can also start in individuals aged >60 years, defined as late-onset PsA. The increase in life expectancy and the improvement of diagnostic imaging tools will likely lead to an increase in the number of elderly subjects with PsA.185
However, treatment opportunities in these patients differ from the general population. Studies have shown that NSAIDs are associated with mild or poor responses in elderly SpAs patients and corticosteroids are not strongly recommended because of the risk of serious long-term adverse effects, such as hypertension, diabetes, osteoporosis, skin atrophy, and cataracts.186 Moreover, csDMARDs effectiveness and safety derived from randomized placebo-controlled trials (RCTs) and longitudinal observational cohorts are scarce and mainly focused on methotrexate and sulfasalazine.187 Esposito et al188 retrospectively evaluated the effectiveness and safety of subcutaneous etanercept and adalimumab in 89 elderly patients with PsO and PsA, aged between 65 and 82 years (mean age 69.7 years). The results showed that TNF-i are appropriate in the long-term management of elderly patients. Additionally, in this study, a good safety profile was reported.187 When appropriate, APR can be useful and relatively safe. On the contrary, data on tofacitinib in elderly patients are very few, and, among those, age older than 65 years has been reported as an independent predictor of increased risk of serious infections.189
Here, we summarized different studies supporting the efficacy and safety of TNFi in PsA, but discontinuation or switching is quite common.190 PsA patients can experience TNFi, lack of efficacy, or adverse events: a mean of 40% of PsA reported switching to a second TNFi.190 However, several studies exist on the effectiveness of switching among different TNFi. Recently, the concept of “swapping” emerged in PsA management: switching to another mode of action, such as to APR, SEC, UST, and IXE. In clinical trials considering these drugs, response to the drug was considered, both in patients naïve to TNFi and in patients failure to TNFi. All trials confirm their efficacy, both in TNF-naïve patients and those previously treated with TNFi. Data from real-life settings also support these results: UST, SEC, and IXE treatment were effective in a population of PsA patients who previously experienced failure to TNF-i.14,88 The use of biosimilars was introduced in our daily clinical practice, with the advantage of economic benefit and savings for the national health system. TNFi biosimilar drugs have been investigated in PsA switching from the originator drug to a biosimilar molecule, demonstrating that switching from the originator molecule to a biosimilar is not inferior to continued treatment with the originator drug.190,193
PsA is a multifaceted disease with a complex pathogenesis involving both innate and acquired immune response. In the near future, the advent of newest therapeutic antibodies neutralizing more than one cytokine (TNF and IL-17) may have the advantage of contemporarily blocking multiple key steps of the pathogenic cascade conversely to one-cytokine-blocking antibody.1,9 Of note, oral small molecules may already block different pathways, however their efficacy should be validated by relevant clinical data in the real-life practice. Treatment options, such as rituximab and abatacept, effective in treating RA, demonstrated their efficacy in only a few cases of PsA patients, bounding their use only in limited cases.153 Guidance for clinicians is directed by patients clinical characteristics, such as age, gender, BMI, and the presence of comorbidities. bDMARDs and tsDMARDs variety, at present and in the near future, give us the possibility for the best choice in the best moment in PsA patients management. The drugs used for PsA patients management should be tailored based on the characteristics of any single patient and to the presence of complex age- and disease-related aspects.
Abbreviations
ACR, American College of Rheumatology; ADA, Adalimumab; AEs, adverse events; APR, Apremilast; AS, Ankylosing Spondylitis; ASAS, Assessment of Spondyloarthritis International Society; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARDs, biological DMARDs; BMI, Body Mass Index; BMK, Bimekizumab; BRD, Brodalumab; BSA, body surface area; cAMP, cyclic adenosine monophosphate; COPD, chronic obstructive pulmonary disease; CRP, C-reactive Protein; csDMARDs, conventional DMARDs; CTLA-4, cytotoxic-T-lymphocyte-associated antigen 4; CV, cardiovascular; CVD, cardiovascular disease; CZT, Certolizumab pegol; DAPSA, Disease Activity in PSoriatic Arthritis; DAS, Disease Activity Score; DIP, distal interphalangeal; DM, diabetes mellitus; ETA, Etanercept; EULAR, European League Against Rheumatism; FDA, US Food and Drug Administration; GOL, Golimumab; GRAPPA, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis; GSK, Guselkumab; HAQ-DI, Health Assessment Questionnaire-Disability Index; HDL-c, high-density lipoprotein cholesterol; HZ, varicella zoster; IBD, inflammatory bowel disease; IFX, Infliximab; IL, interleukin; IXE, Ixekizumab; JAK, Janus kinases; JAKi, Janus kinases inhibitors; LDL-c, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; MDA, minimal disease activity; MAPK, mitogen-activated protein kinase; MetS, metabolic syndrome; MTX, methotrexate; PASI, Psoriasis Area Severity Index; PDE4, phosphodiesterase 4; PROs, Patient-Reported Outcomes; PsA, Psoriatic Arthritis; PSARC, PsA Response Criteria; PsO, psoriasis; RA, Rheumatoid Arthritis; RCTs, randomized controlled trials; RR, risk ratios; RSK, Risankizumab; RTX, Rituximab; SEC, Secukinumab; SpA, spondyloarthritis; SUCRA, surface under the cumulative ranking curve; SysPsD, Systemic Psoriatic Disease; TB, tuberculosis; TCZ, Tocilizumab; TLK, Tildrakizumab; TNF, Tumor Necrosis Factor; TNFi, Tumor Necrosis Factor inhibitors; tsDMARDs, targeted-synthetic DMARDs; TYK, tyrosine kinase; URTI, upper respiratory tract infection; UST, Ustekinumab.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64:14–17.
2. Chimenti MS, Caso F, Alivernini S, et al. Amplifying the concept of psoriatic arthritis: the role of autoimmunity in systemic psoriatic disease. Autoimmun Rev. 2019;18(6):565–575. doi:10.1016/j.autrev.2018.11.007
3. Chimenti MS, Triggianese P, De Martino E, et al. An update on pathogenesis of psoriatic arthritis and potential therapeutic targets. Expert Rev Clin Immunol. 2019;15(8):823–836. doi:10.1080/1744666X.2019.1627876
4. Chimenti MS, Ballanti E, Perricone C, Cipriani P, Giacomelli R, Perricone R. Immunomodulation in Psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev. 2013;12(5):599–606. doi:10.1016/j.autrev.2012.10.002
5. D’Angelo S, Cantini F, Ramonda R, et al. Effectiveness of adalimumab for the treatment of psoriatic arthritis: an Italian real-life retrospective study. Front Pharmacol. 2019;10:1497. doi:10.3389/fphar.2019.01497
6. Papoutsaki M, Costanzo A, Chimenti MS, Chimenti S. Adalimumab for the treatment of severe psoriasis and psoriatic arthritis. Expert Opin Biol Ther. 2008;8(3):363–370. doi:10.1517/14712598.8.3.363
7. Papoutsaki M, Chimenti MS, Costanzo A, et al. Adalimumab for severe psoriasis and psoriatic arthritis: an open-label study in 30 patients previously treated with other biologics. J Am Acad Dermatol. 2007;57(2):269–275. doi:10.1016/j.jaad.2006.12.003
8. Chimenti MS, Saraceno R, Chiricozzi A, Giunta A, Chimenti S, Perricone R. Profile of certolizumab and its potential in the treatment of psoriatic arthritis. Drug Des Devel Ther. 2013;15(7):339–348. doi:10.2147/DDDT.S31658
9. Novelli L, Chimenti MS, Chiricozzi A, Perricone R. The new era for the treatment of psoriasis and psoriatic arthritis: perspectives and validated strategies. Autoimmun Rev. 2014;13(1):64–69. doi:10.1016/j.autrev.2013.08.006
10. Araujo EG, Schett G. Enthesitis in psoriatic arthritis (Part 1): pathophysiology. Rheumatology (Oxford). 2020;1:59.
11. Chiricozzi A, Saraceno R, Novelli L, et al. Small molecules and antibodies for the treatment of psoriasis: a patent review (2010–2015). Expert Opin Ther Pat. 2016;26(7):757–766. doi:10.1080/13543776.2016.1192129
12. Marchesoni A, Olivieri I, Salvarani C, et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin Exp Rheumatol. 2017;35(6):991–1010.
13. Gossec L, Coates L, de Wit M, et al. Management of psoriatic arthritis in 2016: a comparison of EULAR and GRAPPA recommendations. Nat Rev Rheumatol. 2016;12:743–750. doi:10.1038/nrrheum.2016.183
14. Chimenti MS, Ortolan A, Lorenzin M, et al. Effectiveness and safety of ustekinumab in naïve or TNF-inhibitors failure psoriatic arthritis patients: a 24-month prospective multicentric study. Clin Rheumatol. 2018;37:397–405. doi:10.1007/s10067-017-3953-6
15. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi:10.1016/0049-0172(73)90035-8
16. Napolitano M, Caso F, Scarpa R, et al. Psoriatic arthritis and psoriasis: differential diagnosis. Clin Rheumatol. 2016;35(8):1893–1901. doi:10.1007/s10067-016-3295-9
17. Khan M, Schentag C, Gladman DD. Clinical and radiological changes during psoriatic arthritis disease progression. J Rheumatol. 2003;30:1022–1026.
18. Caso F, Chimenti MS, Navarini L, et al. Metabolic Syndrome and psoriatic arthritis: considerations for the clinician. Expert Rev Clin Immunol. 2020;16:409–420. doi:10.1080/1744666X.2020.1740593
19. Costa L, Caso F, D’Elia L, et al. Psoriatic arthritis is associated with increased arterial stiffness in the absence of known cardiovascular risk factors: a case control study. Clin Rheumatol. 2012;31:711–715. doi:10.1007/s10067-011-1892-1
20. Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol. 2015;27(2):118–126. doi:10.1097/BOR.0000000000000152
21. Scarpa R, Caso F, Costa L, et al. Psoriatic disease: clinical staging. J Rheumatol Suppl. 2015;93:24–26. doi:10.3899/jrheum.150629
22. Husted JA, Thavaneswaran A, Chandran V, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken). 2011;63(12):1729–1735. doi:10.1002/acr.20627
23. Queiro R, Lorenzo A, Pardo E, Brandy A, Coto P, Ballina J. Prevalence and type II diabetes-associated factors in psoriatic arthritis. Clin Rheumatol. 2018;37:1059–1064. doi:10.1007/s10067-018-4042-1
24. Sobchak C, Eder L. Cardiometabolic disorders in psoriatic disease. Curr Rheumatol Rep. 2017;19(10):63. doi:10.1007/s11926-017-0692-2
25. Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi:10.1001/jamadermatol.2013.5015
26. Mok CC, Ko GT, Ho LY, Yu KL, Chan PT, To CH. Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthritis Care Res (Hoboken). 2011;63(2):195–202. doi:10.1002/acr.20363
27. Murray PI, Rauz S. The eye and inflammatory rheumatic diseases: the eye and rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. Best Pract Res Clin Rheumatol. 2016;30:802–825. doi:10.1016/j.berh.2016.10.007
28. Chimenti MS, Triggianese P, Salandri G, et al. A multimodal eye assessment in psoriatic arthritis patients sine-psoriasis: evidence for a potential association with systemic inflammation. J Clin Med. 2020;9(3):719. doi:10.3390/jcm9030719
29. Wakefield D, Chang JH, Amjadi S, et al. What is new HLA-B27 acute anterior uveitis? Ocul Immunol Inflamm. 2011;19:139–144. doi:10.3109/09273948.2010.542269
30. Schreiber S, Colombel JF, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78(4):473–479. doi:10.1136/annrheumdis-2018-214273
31. Chimenti MS, Perricone C, Novelli L, et al. Interaction between microbiome and host genetics in psoriatic arthritis. Autoimmun Rev. 2018;17(3):276–283. doi:10.1016/j.autrev.2018.01.002
32. Scarpa R, Manguso F, D’Arienzo A, et al. Microscopic inflammatory changes in colon of patients with both active psoriasis and psoriatic arthritis without bowel symptoms. J Rheumatol. 2000;27(5):1241–1246.
33. Freire M, Rodríguez J, Möller I, et al. Prevalence of symptoms of anxiety and depression in patients with psoriatic arthritis attending rheumatology clinics. Reumatol Clin. 2011;7:20–26. doi:10.1016/j.reuma.2010.03.003
34. Chimenti MS, Fonti GL, Conigliaro P, et al. Evaluation of Alexithymia in patients affected by Rheumatoid arthritis and Psoriatic arthritis: a cross-sectional study. Medicine (Baltimore). 2019;98(4):e13955. doi:10.1097/MD.0000000000013955
35. Caso F, Costa L, Peluso R, Del Puente A, Scarpa R. Psoriatic Arthritis. Mosaic of Autoimmunity. The Novel Factors of Autoimmune Diseases. Elsevier; 2019:527–540.
36. Conway R, Low C, Coughlan R, et al. Methotrexate use and risk of lung disease in psoriasis, psoriatic arthritis, and inflammatory bowel disease: systematic literature review and meta-analysis of randomised controlled trials. BMJ Br Med J. 2015;350:h1269. doi:10.1136/bmj.h1269
37. Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(5):405–441. doi:10.1080/1744666X.2018.1468252
38. Addimanda O, Possemato N, Caruso A, Pipitone N, Salvarani C. The role of tumor necrosis factor-α blockers in psoriatic disease. Therapeutic options in psoriatic arthritis. J Rheumatol. 2015;93:73–78.
39. Caso F, Lubrano E, Del Puente A, et al. Progress in understanding and utilizing TNF-α inhibition for the treatment of psoriatic arthritis. Expert Rev Clin Immunol. 2016;12(3):315–331. doi:10.1586/1744666X.2016.1117941
40. Baeten D, Kruithof E, Van den Bosch F, et al. Immunomodulatory effects of anti-tumor necrosis factor alpha therapy on synovium in spondyloarthropathy: histologic findings in eight patients from an open-label pilot study. Arthritis Rheum. 2001;44:186–195. doi:10.1002/1529-0131(200101)44:1<186::AID-ANR25>3.0.CO;2-B
41. Giacomelli R, Passacantando A, Perricone R, et al. T lymphocytes in the synovial fluid of patients with active rheumatoid arthritis display CD134-OX40 surface antigen. Clin Exp Rheumatol. 2001;19(3):317–320.
42. Damjanov N, Karpati S, Kemeny L, et al. Efficacy and safety of etanercept in psoriasis and psoriatic arthritis in the PRESTA study: analysis in patients from Central and Eastern Europe. J Dermatol Treat. 2018;29(1):8–12. doi:10.1080/09546634.2017.1329509
43. Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis. 2009;68(5):702–709. doi:10.1136/ard.2008.092767
44. Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum. 2005;52(4):1227–1236. doi:10.1002/art.20967
45. Kavanaugh A, Mease P. Treatment of psoriatic arthritis with tumor necrosis factor inhibitors: longer-term outcomes including enthesitis and dactylitis with golimumab treatment in the longterm extension of a randomized, placebo-controlled study (GO-REVEAL). J Rheumatol. 2012;89:90–93.
46. Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73:48–55. doi:10.1136/annrheumdis-2013-203696
47. Elyoussfi S, Thomas BJ, Ciurtin C. Tailored treatment options for patients with psoriatic arthritis and psoriasis: review of established and new biologic and small molecule therapies. Rheumatol Int. 2016;36:603–612. doi:10.1007/s00296-016-3436-0
48. Lemos LL, de Oliveira Costa J, Almeida AM, et al. Treatment of psoriatic arthritis with anti- TNF agents: a systematic review and meta-analysis of efficacy, effectiveness and safety. Rheumatol Int. 2014;34:1345–1360. doi:10.1007/s00296-014-3006-2
49. Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517–524. doi:10.1136/annrheumdis-2011-201244
50. Gross RL, Schwartzman-Morris JS, Krathen M, et al. A comparison of the malignancy incidence among patients with psoriatic arthritis and patients with rheumatoid arthritis in a large US cohort. Arthritis Rheum. 2014;66:1472–1481. doi:10.1002/art.38385
51. Saad AA, Ashcroft DM, Watson KD, et al. BSRBR: efficacy and safety of anti-TNF therapies in psoriatic arthritis: an observational study from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 2010;49:697–705. doi:10.1093/rheumatology/kep423
52. Mariette X, Tubach F, Bagheri H, et al. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann Rheum Dis. 2010;69:400–408. doi:10.1136/ard.2009.117762
53. Hellgren K, Smedby KE, Backlin C, et al. Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: a cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheum. 2014;66:1282–1290. doi:10.1002/art.38339
54. Carmona L, Abasolo L, Descalzo MA, et al. Cancer in patients with rheumatic diseases exposed to TNF antagonists. Semin Arthritis Rheum. 2011;41:71–80. doi:10.1016/j.semarthrit.2010.08.005
55. Ventura-Ríos L, Bañuelos-Ramírez D, Hernández-Quiroz Mdel C, et al. Patient survival and safety with biologic therapy. Results of the Mexican National Registry Biobadamex 1.0. Reumatol Clin. 2012;8:189–194. doi:10.1016/j.reuma.2012.02.010
56. Murdaca G, Spanò F, Contatore M, et al. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf. 2015;14(4):571–582. doi:10.1517/14740338.2015.1009036
57. Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79–99. doi:10.2147/DHPS.S28801
58. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med. 2017;17(1):65–70. doi:10.7861/clinmedicine.17-1-65
59. Caso F, Costa L, Chimenti MS, Navarini L, Punzi L. Pathogenesis of psoriatic arthritis. Crit Rev Immunol. 2019;39:361–377. doi:10.1615/CritRevImmunol.2020033243
60. Scarpa R, Costa L, Atteno M, Del Puente A, Caso F, Moll JM. Psoriatic arthritis: advances in pharmacotherapy based on molecular target. Expert Opin Pharmacother. 2013;14:2311–2313. doi:10.1517/14656566.2013.840292
61. Dobbin-Sears I, Roberts J, O’Rielly DD, Rahman P. Ustekinumab in psoriatic arthritis and related phenotypes. Ther Adv Chronic Dis. 2018;9(10):191–198. doi:10.1177/2040622318781760
62. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi:10.1016/S0140-6736(13)60594-2
63. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999.
64. Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000–1006.
65. Araujo EG, Englbrecht M, Hoepken S, et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum. 2019;48(4):632–637. doi:10.1016/j.semarthrit.2018.05.011
66. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854. doi:10.1111/bjd.12214
67. Mease PJ, Kellner H, Morita A, et al. OP0307 Efficacy and safety of risankizumab, a selective il-23p19 inhibitor, in patients with active psoriatic arthritis over 24 weeks: results from a phase 2 trial. BMJ. 2018;77(2):200–201.
68. Mease PJ, Chohan S, Fructuoso FJG, et al. LB0002 randomised, double-blind, placebo-controlled, multiple-dose, phase 2b study to demonstrate the safety and efficacy of tildrakizumab, a high-affinity anti-interleukin-23p19 monoclonal antibody, in patients with active psoriatic arthritis. Ann Rheum Dis. 2019;78(2):78–79.
69. Deodhar A, Helliwell P, Boencke WH, et al. Guselkumab, an anti-interleukin-23p19 monoclonal antibody, in biologic-naïve patients with active psoriatic arthritis: week 24 results of the phase 3, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2019;71(10).
70. Nakamura M, Lee K, Jeon C, et al. Guselkumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther (Heidelb). 2017;7(3):281–292. doi:10.1007/s13555-017-0187-0
71. Sieper J, Poddubnyy D, Miossec P. The IL-23/IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol. 2019;15/12:747–757. doi:10.1038/s41584-019-0294-7
72. Poddubnyy D, Hermann KGA, Callhoff J, et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis. 2014;73:817–823. doi:10.1136/annrheumdis-2013-204248
73. Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year follow up from a phase III, randomized, double-blind placebo-controlled study: PsA and long-term treatment with secukinumab. Arthritis Care Res. 2017;69:347–355. doi:10.1002/acr.23111
74. McInnes IB, Mease PJ, Ritchlin CT, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology. 2017;56:1993–2003. doi:10.1093/rheumatology/kex301
75. Mease P, Van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77:890–897.
76. Strand V, McInnes I, Mease P, et al. Matching-adjusted indirect comparison: secukinumab versus infliximab in biologic-naïve patients with psoriatic arthritis. J Comp Eff Res. 2019;8(7):497–510. doi:10.2217/cer-2018-0141
77. Kawalec P, Holko P, Mocko P, Pilc A. Comparative effectiveness of abatacept, apremilast, secukinumab and ustekinumab treatment of psoriatic arthritis: a systematic review and network meta-analysis. Rheumatol Int. 2018;38(2):189–201. doi:10.1007/s00296-017-3919-7
78. Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and meta-analysis. J Rheumatol. 2019;47(1):59–65. doi:10.3899/jrheum.180797
79. Chimenti MS, Fonti GL, Conigliaro P, et al. One-year effectiveness, retention rate and safety of secukinumab in ankylosing spondylitis and psoriatic arthritis: a real-life multicenter study. Expert Opin Biol Ther. 2020;20(7):813–821. doi:10.1080/14712598.2020.1761957
80. Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47–62. doi:10.1111/bjd.15015
81. Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111. doi:10.1186/s13075-019-1882-2
82. Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179(5):1205–1207. doi:10.1111/bjd.16901
83. Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an anti-IL17A specific monoclonal antibody, for the treatment of biologic-naïve patients with active psoriatic arthritis: results from 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87.
84. van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367–377. doi:10.3899/jrheum.170429
85. Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-weeks, randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;386(10086):2317–2327. doi:10.1016/S0140-6736(17)31429-0
86. Coates L, Pillai S, Zhang L, et al. Continuing versus withdrawing Ixekizumab in patients with psoriatic arthritis who achieved sustained minimal disease activity: results from the SPIRIT-P3 study. Arthritis Rheum. 2019;71(10).
87. Giunta A, Ventura A, Chimenti MS, Bianchi L, Esposito M. Spotlight on ixekizumab for the treatment of moderate-to-severe plaque psoriasis: design, development, and use in therapy. Drug Des Devel Ther. 2017;11:1643–1651. doi:10.2147/DDDT.S92128
88. Manfreda V, Chimenti MS, Canofari C, et al. Efficacy and safety of ixekizumab in psoriatic arthritis: a retrospective, single-centre, observational study in a real-life clinical setting. Clin Exp Rheumatol. 2020.
89. Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131. doi:10.1136/annrheumdis-2019-215386
90. Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679–685. doi:10.1111/jdv.13990
91. O’Rielly DD, Rahman P. A review of ixekizumab in the treatment of psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(12):993–1002. doi:10.1080/1744666X.2018.1540931
92. Wu D, Yue J, Tam LS. Efficacy and safety of biologics targeting interleukin-6, −12/23 and −17 pathways for peripheral psoriatic arthritis: a network meta-analysis. Rheumatology (Oxford). 2019;57(3):563–571. doi:10.1093/rheumatology/kex452
93. McInnes I, Mease P, Hjuler KF, et al. Brodalumab in psoriatic arthritis (PsA): 24-week results from the phase III AMVISON-1 and −2 trials. JAAD. 2019;81(4):AB28.
94. Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23(2):1–3.
95. Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395:427–440. doi:10.1016/S0140-6736(19)33161-7
96. Chimenti MS, Gramiccia T, Saraceno R, et al. Apremilast for the treatment of psoriasis. Expert Opin Pharmacother. 2015;16:2083–2094. doi:10.1517/14656566.2015.1076794
97. Fiocco U, Martini V, Accordi B, et al. Ex vivo signaling protein mapping in T lymphocytes in the psoriatic arthritis joints. J Rheumatol Suppl. 2015;93:48–52. doi:10.3899/jrheum.150636
98. Reed M, Crosbie D. Apremilast in the treatment of psoriatic arthritis: a perspective review. Ther Adv Musculoskelet Dis. 2017;9(2):45–53. doi:10.1177/1759720X16673786
99. Caso F, Navarini L, Ruscitti P, et al. Targeted synthetic pharmacotherapy for psoriatic arthritis: state of the art and evidence from a systematic review. Expert Opin Pharmacother. 2020;14:1–12.
100. Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40(8):495–500.
101. Schett G, Wollenhaupt J, Papp K, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64(10):3156–3167. doi:10.1002/art.34627
102. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–1026. doi:10.1136/annrheumdis-2013-205056
103. Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724–1734. doi:10.3899/jrheum.151376
104. Kavanaugh A, Gladman DD, Edwards CJ, et al. Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1–3 pooled analysis. Arthritis Res Ther. 2019;21(1):118. doi:10.1186/s13075-019-1901-3
105. Gladman DD, Kavanaugh A, Gomez Reino JJ, et al. The 5-year results from a PALACE 1-3 pooled analysis showing apremilast efficacious in maintaining clinical benefit on enthesitis and dactylitis. RMD Open. 2018;4:e000669. doi:10.1136/rmdopen-2018-000669
106. Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naïve psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57(7):1253–1263. doi:10.1093/rheumatology/key032
107. Nash P, Ohson K, Walsh J, et al. Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE). Ann Rheum Dis. 2018;77(5):690–698. doi:10.1136/annrheumdis-2017-211568
108. Adebajo A, Gladmann D, Kavanaugh A, et al. Long-term (104-week) efficacy and safety profile of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results from a phase III, randomized, controlled trial and open-label extension (PALACE 1). Rheumatol. 2015;74(2):350–352.
109. Ceccarelli F, Lucchetti R, Perricone C, et al. Musculoskeletal ultrasound in monitoring response to apremilast in psoriatic arthritis patients: results from a longitudinal study. Clin Rheumatol. 2019;38(11):3145–3151. doi:10.1007/s10067-019-04674-3
110. Mazzilli S, Lanna C, Chiaramonte C, et al. A real life experience of apremilast in psoriasis and arthritis psoriatic patients: preliminary results on metabolic biomarkers. J Dermatol. 2020;47(6):578–582. doi:10.1111/1346-8138.15293
111. Liu Y, Zhou S, Nissel J, et al. The pharmacokinetic effect of coadministration of apremilast and methotrexate in individuals with rheumatoid arthritis and psoriatic arthritis. Clin Pharmacol Drug Dev. 2014;3(6):456–465. doi:10.1002/cpdd.109
112. Ungprasert P, Thongprayoon C, Davis JM. Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol. 2016;35(7):1795–1803. doi:10.1007/s10067-016-3204-2
113. Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheum. 2016;68(5):1060–1071.
114. Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi:10.1136/annrheumdis-2015-208337
115. Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318–328.
116. Berekmeri A, Mahmood F, Wittmann M, Helliwell P. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expert Rev Clin Immunol. 2018;14:719–730. doi:10.1080/1744666X.2018.1512404
117. Jamilloux Y, El Jammal T, Vuitton L, et al. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019;18(11):102390. doi:10.1016/j.autrev.2019.102390
118. Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. doi:10.1056/NEJMoa1615975
119. Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–1536. doi:10.1056/NEJMoa1615977
120. Nash P, Coates LC, Fleischmann R, et al. Efficacy of tofacitinib for the treatment of psoriatic arthritis: pooled analysis of two phase 3 studies. Rheumatol Ther. 2018;5(2):567–582. doi:10.1007/s40744-018-0131-5
121. Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo‐controlled, phase III trials. Br J Dermatol. 2015;173(4):949–961. doi:10.1111/bjd.14018
122. Feldman SR, Thaçi D, Gooderham M, et al. Tofacitinib improves pruritus and health-related quality of life up to 5weeks: results from 2 randomized phase III trials in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(6):1162–70.e3. doi:10.1016/j.jaad.2016.07.040
123. Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open. 2019;5:e000806. doi:10.1136/rmdopen-2018-000806
124. Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL beyond. RMD Open. 2019;5:e000808. doi:10.1136/rmdopen-2018-000808
125. Yokota K, Sato K, Miyazaki T, et al. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheum. 2014;66(1):121–129. doi:10.1002/art.38218
126. La Branche TP, Jesson MI, Radi ZA, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 2012;64(11):353.
127. Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. 2016;75(1):311–315. doi:10.1136/annrheumdis-2014-207201
128. McArdle A, Flatley B, Pennington SR, FitzGerald O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther. 2015;17:141. doi:10.1186/s13075-015-0652-z
129. van der Heijde D, Gladman DD, FitzGerald O, et al. Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis treated with tofacitinib or adalimumab. J Rheumatol. 2019;46(9):1089–1096. doi:10.3899/jrheum.180971
130. Scott IC, Hider SL, Scott DL. Thromboembolism with Janus Kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf. 2018;41(7):645. doi:10.1007/s40264-018-0651-5
131. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. doi:10.1056/NEJMoa1606910
132. Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2367–2377. doi:10.1016/S0140-6736(18)32483-8
133. Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis. 2017;76(6):998–1008. doi:10.1136/annrheumdis-2016-210104
134. Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis. 2017;76(6):1009–1019. doi:10.1136/annrheumdis-2016-210105
135. ClinicalTrials.gov. Identifier: NCT03104400.
136. ClinicalTrials.gov. Identifier: NCT03104374.
137. Klunder B, Mohamed MF, Othman AA. Population pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I and II clinical trials. Clin Pharmacokinet. 2018;57(8):977–988. doi:10.1007/s40262-017-0605-6
138. Kremer JM, Emery P, Camp HS, et al. Phase IIB study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to antitumor necrosis factor therapy. Arthritis Rheum. 2016;68(12):2867–2877. doi:10.1002/art.39801
139. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study, in patients with rheumatoid arthritis and inadequate response to methotrexate. Arthritis Rheum. 2016;68(12):2857–2866. doi:10.1002/art.39808
140. Burmester GR, Kremer JM, den Bosch V, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi:10.1016/S0140-6736(18)31115-2
141. Navarini L, Costa L, Tasso M, et al. Retention rates and identification of factors associated with anti-TNFα, anti-IL17, and anti-IL12/23R agents discontinuation in psoriatic arthritis patients: results from a real-world clinical setting. Clin Rheumatol. 2020. doi:10.1007/s10067-020-05027-1
142. Noisette A, Hochberg MC. Abatacept for the treatment of adults with psoriatic arthritis: patient selection and perspectives. Psoriasis (Auckl). 2018;8:31–39.
143. Ursini F, Russo E, De Giorgio R, De Sarro G, D’Angelo S. Current treatment options for psoriatic arthritis: spotlight on abatacept. Ther Clin Risk Manag. 2018;14:1053–1059. doi:10.2147/TCRM.S148586
144. Abrams JR, Lebwhol MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103(9):1243–1252. doi:10.1172/JCI5857
145. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63(4):939–948. doi:10.1002/art.30176
146. Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76(9):1550–1558. doi:10.1136/annrheumdis-2016-210724
147. Szentpetery A, Heffernan E, Gogarty M, et al. Abatacept reduces synovial regulatory T-cell expression in patients with psoriatic arthritis. Arthritis Res Ther. 2017;19(1):158. doi:10.1186/s13075-017-1364-3
148. Olivieri I, D’Angelo S, Mennillo GA, et al. Abatacept in spondyloarthritis refractory to tumour necrosis factor α inhibition. Ann Rheum Dis. 2009;68:151–152. doi:10.1136/ard.2008.097030
149. Lekpa FK, Farrenq V, Canou¨ı-Poitrine F, et al. Lack of efficacy of abatacept in axial spondylarthropathies refractory to tumor-necrosis-factor inhibition. Joint Bone Spine. 2012;79(1):47–50. doi:10.1016/j.jbspin.2011.02.018
150. Zizzo G, Gremese E, Ferraccioli G. Abatacept in the treatment of psoriatic arthritis: biological and clinical profiles of the responders. Immunotherapy. 2018;10(9):807–821. doi:10.2217/imt-2018-0014
151. Wcisło-Dziadecka D, Zbiciak M, Brzezińska-Wcisło L, Mazurek U. Anti‑cytokine therapy for psoriasis - not only TNF‑α blockers. Overview of reports on the effectiveness of therapy with IL‑12/IL‑23 and T and B lymphocyte inhibitors. Postepy Hig Med Dosw (Online). 2016;70:1198–1205.
152. Mease P, Kanavaugh A, Genovese M, et al. Rituximab in psoriatic arthritis provides modest clinical improvement and reduces expression of inflammatory biomarkers in skin lesions. Arthritis Rheum. 2020;62(10 suppl):S818.
153. Jimenez-Boj E, Stamm TA, Sadlonova M, et al. Rituximab in psoriatic arthritis: an exploratory evaluation. Ann Rheum Dis. 2012;71(11):1868–1871. doi:10.1136/annrheumdis-2012-201897
154. Kimby E. Tolerability and safety of rituximab (MabThera). Cancer Treat Rev. 2005;31(6):456–473. doi:10.1016/j.ctrv.2005.05.007
155. van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB. Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol. 2015;42(10):1761–1766. doi:10.3899/jrheum.150051
156. Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi:10.1002/art.20303
157. Costa L, Caso F, Cantarini L, Del Puente A, Scarpa R, Atteno M. Efficacy of tocilizumab in a patient with refractory psoriatic arthritis. Clin Rheumatol. 2014;33(9):1355–1357. doi:10.1007/s10067-014-2603-5
158. Ogata A, Kumanogoh A, Tanaka T. Pathological role of interleukin-6 in psoriatic arthritis. Arthritis. 2012;2012:713618. doi:10.1155/2012/713618
159. Ogata A, Umegaki N, Katayama I, Kumanogoh A, Tanaka T. Psoriatic arthritis in two patients with an inadequate response to treatment with tocilizumab. Joint Bone Spine. 2012;79(1):85–87. doi:10.1016/j.jbspin.2011.06.011
160. Madureira P, Pimenta SS, Bernardo A, Brito JS, Bernardes M, Costa L. Off-label use of tocilizumab in psoriatic arthritis: case series and review of the literature. Acta Reumatol Port. 2016;41(3):251–255.
161. Hughes M, Chinoy H. Successful use of tocilizumab in a patient with psoriatic arthritis. Rheumatology (Oxford). 2013;52(9):1728–1729. doi:10.1093/rheumatology/kes432
162. Torres T, Romanelli M, Chiricozzi A. A revolutionary therapeutic approach for psoriasis: bispecific biological agents. Expert Opin Investig Drugs. 2016;25(7):751–754. doi:10.1080/13543784.2016.1187130
163. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheum. 2017;69(12):2283–2291. doi:10.1002/art.40319
164. Genovese MC, Weinblatt ME, Aelion JA, et al. ABT-122, a bispecific dual variable domain immunoglobulin targeting tumor necrosis factor and interleukin-17A, in patients with rheumatoid arthritis with an inadequate response to methotrexate: a randomized, double-blind study. Arthritis Rheum. 2018;70(11):1710–1720. doi:10.1002/art.40580
165. Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheum. 2018;70(11):1778–1789. doi:10.1002/art.40579
166. Genovese MC, Weinblatt ME, Mease PJ, et al. Dual inhibition of tumour necrosis factor and interleukin-17A with ABT-122: open-label long-term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatology (Oxford). 2018;57(11):1972–1981. doi:10.1093/rheumatology/key173
167. Silacci M, Lembke W, Woods R, et al. Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs. 2016;8:141–149. doi:10.1080/19420862.2015.1093266
168. Jacobson KA, Merighi S, Varani K, et al. A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med Res Rev. 2018;38(4):1031–1072.
169. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter,randomized, controlled trial. J Drugs Dermatol. 2016;15:931–938.
170. van Troostenburg AR, Clark EV, Carey WD, et al. Tolerability, pharmacokinetics and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther. 2004;42(10):534–542. doi:10.5414/CPP42534
171. Silverman MH, Strand V, Markovits D, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol. 2008;35(1):41–48.
172. Chimenti MS, Triggianese P, Nuccetelli M, et al. Auto-reactions, autoimmunity and psoriatic arthritis. Autoimmun Rev. 2015;14(12):1142–1146. doi:10.1016/j.autrev.2015.08.003
173. Scarpa R, Caso F, Costa L, Peluso R, Del Puente A, Olivieri I. Psoriatic disease 10 years later. J Rheumatol. 2017;44(9):1298–1301. doi:10.3899/jrheum.161402
174. Caso F, Del Puente A, Peluso R, et al. Emerging drugs for psoriatic arthritis. Expert Opin Emerg Drugs. 2016;21(1):69–79. doi:10.1517/14728214.2016.1146679
175. Cecchin V, Zannin ME, Ferrari D, et al. Longterm safety and efficacy of adalimumab and infliximab for uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2018;45(8):1167–1172. doi:10.3899/jrheum.171006
176. Andreoli L, Gerardi MC, Fernandes MA, et al. Disease activity assessment of rheumatic diseases during pregnancy: a comprehensive review of indices used in clinical studies. Autoimmun Rev. 2019;18(2):164–176. doi:10.1016/j.autrev.2018.08.008
177. Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. doi:10.1136/annrheumdis-2015-208840
178. Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55(9):1693–1697. doi:10.1093/rheumatology/kev404
179. Chimenti MS, Triggianese P, Conigliaro P, et al. A 2-year observational study on treatment targets in psoriatic arthritis patients treated with TNF inhibitors. Clin Rheumatol. 2017;36(10):2253–2260. doi:10.1007/s10067-017-3769-4
180. Casas Deza D, García López S, Lafuente Blasco M, et al. Efficacy and safety of ustekinumab in real clinical practice. Retrospective multicentre study. ARAINF cohort. Gastroenterol Hepatol. 2020;43(3):126–132. doi:10.1016/j.gastrohep.2019.09.011
181. Honap S, Chee D, Chapman TP. et al. LEO (London, Exeter, Oxford) IBD research consortium. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multi-centre UK experience. J Crohns Colitis;2020;
182. Macaluso F, Guggino G, Mauro D, Rizzo C, Bignone R, Ciccia F. Safety and efficacy of secukinumab treatment in a patient with ankylosing spondylitis and concomitant multiple sclerosis. Clin Exp Rheumatol. 2019;37(6):1096.
183. Gremese E, Bernardi S, Bonazza S, et al. Body weight, gender and response to TNF-α blockers in axial spondyloarthritis. Rheumatology (Oxford). 2014;53(5):875–881. doi:10.1093/rheumatology/ket433
184. Pantano I, Iacono D, Favalli EG, et al. Secukinumab efficacy in patients with PsA is not dependent on patients’ body mass index. Ann Rheum Dis. 2020;
185. Caso F, Tasso M, Chimenti MS, et al. Late-onset and elderly psoriatic arthritis: clinical aspects and management. Drugs Aging. 2019;36(10):909–925. doi:10.1007/s40266-019-00688-3
186. Caso F, Costa L, Del Puente A, et al. Pharmacological treatment of spondyloarthritis: exploring the effectiveness of nonsteroidal anti-inflammatory drugs, traditional disease-modifying antirheumatic drugs and biological therapies. Ther Adv Chronic Dis. 2015;6:328–338. doi:10.1177/2040622315608647
187. Costa L, Lubrano E, Ramonda R, et al. Elderly psoriatic arthritis patients on TNF-α blockers: results of an Italian multicenter study on minimal disease activity and drug discontinuation rate. Clin Rheumatol. 2017;36:1797–1802. doi:10.1007/s10067-017-3697-3
188. Esposito M, Giunta A, Mazzotta A, et al. Efficacy and safety of subcutaneous anti-tumor necrosis factor-alpha agents, etanercept and adalimumab, in elderly patients affected by psoriasis and psoriatic arthritis: an observational long-term study. Dermatology. 2012;225:312–319. doi:10.1159/000345623
189. Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, long-term extension studies. J Rheumatol. 2014;41:837–852. doi:10.3899/jrheum.130683
190. Costa L, Perricone C, Chimenti MS, et al. Switching between biological treatments in psoriatic arthritis: a review of the evidence. Drugs R D. 2017;17(4):509–522. doi:10.1007/s40268-017-0215-7
191. Braun J, Kudrin A. Switching to biosimilar infliximab (CT-P13): evidence of clinical safety, effectiveness and impact on public health. Biologicals. 2016;44(4):257–266. doi:10.1016/j.biologicals.2016.03.006
192. Switching from innovator to biosimilar (subsequent entry) infliximab: an updated review of the clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): Canadian agency for drugs and technologies in health; 2017. Available from http://www.ncbi.nlm.nih.gov/books/NBK442045/.
193. Faccin F, Tebbey P, Alexander E, Wang X, Cui L, Albuquerque T. The design of clinical trials to support the switching and alternation of biosimilars. Expert Opin Biol Ther. 2016;16(12):1445–1453. doi:10.1080/14712598.2017.1238454
194. Frankel EH, Strober BE, Crowley JJ, et al. Etanercept improves psoriatic arthritis patient reported outcomes: results from EDUCATE. Cutis. 2007;79:322–326.
195. ClinicalTrials.gov. Identifier: NCT02486302.
196. Gladman DD, Bombardier C, Thorne C, et al. Effectiveness and safety of etanercept in patients with psoriatic arthritis in a Canadian clinical practice setting: the REPArE trial. J Rheumatol. 2011;38:1355–1362. doi:10.3899/jrheum.100698
197. ClinicalTrials.gov. Identifier: NCT00235885.
198. Gladman DD, Sampalis JS, Illouz O, Guérette B; ACCLAIM Study Investigators. Responses to adalimumab in patients with active psoriatic arthritis who have not adequately responded to prior therapy: effectiveness and safety results from an open-label study. J Rheumatol. 2010;37:1898–1906. doi:10.3899/jrheum.100069
199. ClinicalTrials.gov. Identifier: NCT01078558.
200. ClinicalTrials.gov. Identifier: NCT02814175.
201. Baranauskaite A, Raffayová H, Kungurov NV, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis. 2012;71(4):541–548. doi:10.1136/ard.2011.152223
202. Vieira-Sousa E, Alves P, Rodrigues AM, et al. GO-DACT: a phase 3B randomised, double-blind, placebo-controlled trial of golimumab plus methotrexate (MTX) versus placebo plus MTX in improving dactylitis in MTX-naive patients with psoriatic arthritis. Ann Rheum Dis. 2020;79(4):490–498. doi:10.1136/annrheumdis-2019-216500
203. ClinicalTrials.gov. Identifier: NCT01313858.
204. ClinicalTrials.gov. Identifier: NCT04108468.
205. ClinicalTrials.gov. Identifier: NCT02154425.
206. McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet. 2020;395(10235):1496–1505. doi:10.1016/S0140-6736(20)30564-X
207. ClinicalTrials.gov Identifier: NCT03747939.
208. ClinicalTrials.gov Identifier: NCT03828045.
209. ClinicalTrials.gov Identifier: NCT03106051.
210. ClinicalTrials.gov. Identifier: NCT01976364.
211. ClinicalTrials.gov. Identifier: NCT03736161.
212. ClinicalTrials.gov. Identifier: NCT04115748.
213. ClinicalTrials.gov. Identifier: NCT04115839.
214. ClinicalTrials.gov. Identifier: NCT04106804.
215. ClinicalTrials.gov. Identifier: NCT03419143.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
