Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 12
An Up-to-Date Overview of Therapeutic Agents for the Treatment of COVID-19 Disease
Authors Mulaw Belete T
Received 8 October 2020
Accepted for publication 4 December 2020
Published 14 December 2020 Volume 2020:12 Pages 203—212
DOI https://doi.org/10.2147/CPAA.S284809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Tafere Mulaw Belete
Department of Pharmacology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tafere Mulaw Belete Tel +251 918045943
Email [email protected]
Abstract: Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has a great potential to overwhelm the world healthcare systems that may lead to high morbidity and mortality. It also affects world economic development in the future. Currently, no proven effective drugs or vaccines are available for the management of COVID-19 disease. The pace of normal drug development progression is unacceptable in the context of the current pandemic. Therefore, repurposing the existing drugs that were used for the treatment of malaria, Ebola, and influenza helps rapid drug development for COVID-19. Currently, several repurposing candidate drugs are in a clinical trial including, chloroquine monoclonal antibodies, convalescent plasma, interferon, and antiviral therapies. Antiviral drugs like arbidol, remdesiv and favirnavir are the most promising due to the similarities of the viruses regarding viral entry, fusion, uncoating, and replication. This review article provides an overview of the potential therapeutic agent, which displayed better clinical treatment outcomes. Moreover, with further understanding of the SARS-CoV-2 virus, new drugs targeting specific SARS-CoV-2 viral components arise, and investigations on these novels anti-SARSCoV- 2 agents are also reviewed.
Keywords: corona virus, SARS-CoV-2, SARS-CoV, COVID-19, chloroquine
Background
Coronaviruses (CoVs), belonging to the family Coronaviridae, are positive-sense enveloped RNA viruses and cause infections in birds, mammals, and human beings. The family has four genera: alpha, beta, delta, and gamma. From these genera, beta and alpha CoVs infect human beings. Even though most CoVs infection causes a mild symptom, the infection of the two beta CoVs, namely Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome coronavirus (MERS-CoV), caused greater than 10,000 cases with mortality rates of 37% for MERS-CoV and 10% for SARS-CoV.1–3
Unexpectedly, the world has faced a severe public health problem due to the current pandemic of atypical pneumonia caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Wuhan, China. SARS-CoV-2 is the etiological agent of COVID-19 disease, an acute pulmonary syndrome characterized by dry cough, fever, fatigue, muscle aches, headache, dyspnea, and atypical pneumonia. In the severe state, SARS-CoV-2 causes cute Respiratory Distress Syndrome, ie, a lung inflammation that causes fluid accumulation that leads to septic shock due to decreased blood pressure and oxygen starvation.4,5 The World health organization declared COVID-19 as a pandemic on March 11th, 2020. The COVID-19 outbreak spread all over the world with more than 62,730,726 confirmed cases and 1,459,317 confirmed deaths worldwide as of November 30th, 2020.6
Structure of SARS-CoV-2 Virion
CoVs are enveloped plus single-stranded (positive-sense) RNA viruses. CoVs have the largest genomes (29.9-kilobase) among the known RNA viruses with several open reading frames (ORFs).7 The two ORF (ORF1a/ORF1b) in combination contain two-thirds of the viral genome which directly encodes two polyproteins; namely, pp1a and pp1ab using host cell protein translation machinery. These polyproteins further processed into 16 non-structural proteins (nsps) by viral proteases enzymes like papain-like protease (PLpro) and 3C-like protease (3CLpro). Most nsps involved in the synthesis viral RNA. These nsps are Mpro, cleaves viral polyprotein (nsp5), RNA-dependent RNA polymerase (nsp12), RNA binding protein (nsp12), primase (nsp8), exoribonuclease (nsp14), endoribonuclease (nsp15), RNA helicase (nsp13) and 2_-O-methyltransferase (nsp16) which are important for viral replication. The remaining ORFs posses one-third of the viral genome like SARS and MERS translates the four structural proteins and the accessory proteins.8,9
Spike protein (S) is a transmembrane protein, lies as a trimer on the virion surface, giving the virion a “corona” or crown-like appearance. S protein facilitates the virus's entrance into Type II pneumocyte through interaction with the ACE2 receptor. S protein is the most immunodominant proteins of the viruses that can induce the host immune response. It has two domains, S1 and S2 domain. S1 domain helps in host receptor binding while; S2 domain is responsible for the fusion of the viral membrane with the infected cell. The former (S1) contains two subdomains, namely the N- terminal domain (NTD) and C- terminal domain (CTD) which act as the receptor-binding domains interacting with the host receptors. The S1 CTD contains the receptor-binding domain (RBD).10–12
M protein is the most abundant structural protein that gives a definite shape to the virus. M protein induces the suppression of the IFN-I transcription along with nsp-1 that break down the host’s mRNA. M protein has a vital role in the intracellular formation of virus particles without S protein. In the presence of Tunicamycin, coronavirus produces noninfectious virion that has M protein but without S protein.13 The E protein is the smallest structural protein that has a vital role in the pathogenesis, assembly, and exocytosis of the new virion. Inactivation of E protein affects the virulence of CoVs. The nucleocapsid (N) protein affects complex formation with viral genome, facilitates M protein interaction during assembly, and enhances transcription of the viral genome. Several studies proposed that both the structural and non-structural proteins act as promising novel targets to develop a new drug.14
Current Treatment Strategies for COVID-19
Currently, there is no known antiviral agent approved for the management of COVID-19 disease. However, several studies are undergoing to develop an effective and safe drug to control COVID-19 disease.15,16 Currently, the management of severe COVID-19 cases mainly focused on symptomatic management and supportive care. According to World health organization guidelines, a patient infected with SARS-CoV-2 virus managed with supportive care like adequate nutrition, bed rest, prevention of dehydration, and antibiotics. World health organization endorses extracorporeal membrane oxygenation for patients presenting with refractory hypoxemia.17,18 Preventive measures such as social distancing, maintenance of good immunity, use of masks, awareness, and maintenance of hygiene, isolation, and movement restrictions can help in control of SARS-CoV-2 virus spreading. Besides, several studies are ongoing on vaccine development, repurposing of clinically approved drugs, inhibiting enzymes used during viral replication and transcription, using convalescent plasma, host cell endocytosis inhibitors, interferon-based therapies, mAbs target host cell receptor, neutralizing antibodies, antiviral peptide act at S2 domain and natural product.19
Potential Therapeutic Target for Drug Development for COVID-19
SARS-CoV-2 is characterized by high contagiousness, high morbidity, and mortality. Similar to MERS-CoV and SARS-CoV, no specific antiviral drug developed for COVID-19 disease treatment so far. Many researchers proposed several therapeutic targets of the virus to discover high-efficiency, low-toxicity-targeted drugs.20 Inhibiting the SARS-CoV-2 virus with monoclonal antibodies or convalescent plasma, block the host ACE2 receptor, blocking viral endocytosis, and inhibition of proteolysis of polypeptides that are involved in viral replication and proliferation are some of the proposed target sites as displayed in Figure 1.21,22 But, new drug development from scratch is a long process, thus impossible to face the immediate global challenge. Drug repurposing is an emerging strategy that provides a new treatment option for SARS-CoV-2 at a faster pace than developing a new drug. By relying on the already known pharmacokinetic and pharmacodynamic property of the repurposed drugs, researchers gain a chance to evaluate a large number of drugs for their in vitro and in vivo antiviral activity against novel targets. The repurposing method saves time and money for developing a new drug. In this context, Several Repurposed drugs evaluated and displayed antiviral effects against SARS CoV-2.22,23
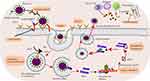 |
Figure 1 SARS-CoV-2 life cycle and potential antiviral drug targets. |
Viral Enter and Fusion Inhibitor
Recombinant Human Angiotensin-Converting Enzyme 2 (APN01)
The S protein is proposed as the main drug target for isolating CoV antiviral agent such as S protein blocker, neutralizing antibodies, S protein cleavage blocker, RBD–ACE2 interaction inhibitor, fusion inhibitor, and protease enzyme inhibitors.11 Angiotensin-converting Enzyme 2 (ACE2) is a membrane protein that is highly expressed in lung alveolar cells. It is the main entry site for the SARS-CoV-2 into cells.24 ACE2 exists in two forms; a transmembrane receptor with an extracellular domain that uses the SARS-CoV-2 spike protein receptor and as a soluble form. So inhibiting the S protein binding to the host cell ACE2 transmembrane receptor is a promising strategy against SARS-CoV-2 Infection. Emodin and promazine interrupted the binding of S protein to the host cell ACE2 receptor. The selective ACE2 inhibitor DX600 may display an effect in SARS-CoV-2 infections; however, its clinical effect in COVID-19 has not been studied. Besides, administering an excessive soluble form of ACE2 recombinant human Angiotensin-converting Enzyme 2 (rhACE2) produced significant blockage SARS-CoV-2 entry by blocking the S protein from interacting with host ACE2 transmembrane receptor and down-regulates transmembrane ACE2 receptor that facilitate virus entry.24,25 APN01 is a rhACE2 agent registered with a clinical trial number (NCT04335136) in Phase II to evaluate the role of rhACE2 in COVID-19 pneumonia. In a similar strategy ACE2 expressing CAR T cells as a decoy with cytotoxic targeting SARS-CoV-2 virus registered on (NCT04324996) as a phase II trial.26,27
Chloroquine and Hydroxychloroquine (CQ/HCQ)
CQ/HCQ is an antimalarial agent used for the management of chronic Q fever and different autoimmune disorders. It has a broad-spectrum antiviral effect including SARS coronavirus, Zika virus, rabies virus, Ebola virus, poliovirus, HIV, influenza A and B, hepatitis A and C virus, Chikungunya virus, and Dengue virus. CQ/HCQ blocks the entrance, fusion, release and replication of different viruses by various modes of action.28,29 CQ/HCQ blocks the viral entrance by inhibiting the synthesis of sialic acids that causes suppressed binding affinity between the host ACE2 receptor and the S protein.
CQ/HCQ binds with host cell sialic acids, inhibiting S protein interaction with the host cell membrane. CQ/HCQ also blocks the endocytosis of SARS-CoV-2 by suppressing the phosphatidylinositol binding clathrin assembly protein. CQ/HCQ stored in endosomes, Golgi apparatus, and lysosomes where it increases the pH of these acidic organelles. The increased pH inhibits acid-dependent proteolytic breakdown by protease enzymes like cathepsin or transmembrane serine protease 2 (TMPRSS2) that are crucial for viral fusion and entry by endocytosis into the host cells. Recent studies showed that CQ/HCQ inhibits replication and post-translational modification of SARS-CoV-2 proteins in simian Vero cells by increasing endosomal pH crucial for virus/host cell fusion.30,31 CQ/HCQ inhibits endosome-lysosome membrane fusion that leads to viral membrane uncoating and genome exocytosis into the host cell cytoplasm as shown in SARS-CoV. The immunomodulatory effect of CQ may also important in regulating the cytokine storm that occurs in critically ill COVID-19 patients.31,32 Gao et al showed that CQ has a significant effect in controlling the exacerbation of pneumonia, improves lung imaging, facilitate the virus-negative conversion, and decrease the disease course.33 Thus, several countries initiated clinical trials to determine the efficacy of CQ/HCQ in COVID-19 disease.
Andrea Cortegiani et al reviewed the efficacy and safety CQ/HCQ on 32 studies (6 RCTs, 26 nonrandomized) for a total of 29,192 participants. Among 32 studies only two studies had a low risk of bias. So, the quality of these studies remains poor and data cannot be synthesized to clear conclusions regarding the efficacy and safety of CQ/HCQ. Data from Low and moderate risk of bias studies proposed treatment of hospitalized COVID-19 patients with CQ/HCQ may not decrease the risk of death compared to standard care. Besides, higher dose regimens and combination with macrolide may associate with harm. Post-exposure prophylaxis with CQ/HCQ may not decrease infection rate but the quality of the study is low.34 The Axfors et al meta-analysis, based on 28 published and unpublished RCTs, including 10,319 patients, shows that treatment with HCQ was associated with increased mortality in COVID-19 patients, and there was no benefit from treatment with CQ.35 Generally, the available data do not support the effectiveness of HCQ/CQ for the treatment or prophylaxis of COVID-19. However, still limited number of well-powered and well-conducted observational studies, the heterogeneity of findings from observational studies, and the scant evidence from RCTs – practically based on a single trial – proposed that the current data on the efficacy of HCQ/CQ in reducing mortality among COVID-19 hospitalized patients are not conclusive.36
Arbidol (Umifenovir)
Arbidol is a broad-spectrum antiviral agent marketed in Russia and China for the management and prevention of influenza virus infection. Arbidol displays antiviral effect against several RNA and DNA viruses, including SARS-CoV, Lassa virus, Chikungunya virus, respiratory syncytial virus, adenovirus, parainfluenza type 5, poliovirus 1, hantaan virus, hepatitis B virus, and C virus. Arbidol is a haemagglutinin inhibitor that blocks the fusion of the influenza virus with the host cell membrane after endocytosis. Arbidol also induces interferon production against virus replication; enhance the phagocytic activity of macrophages, and natural killer cells.37,38
A study has shown that arbidol blocks the SARS-CoV-2 virus replication at 10–30 μM concentration.39 The structural similarities between the Arbidol binding receptor sites for SARS-CoV-2 virus S protein and H3N2 haemagglutinin proposed that Arbidol may be effective to manage COVID-19 disease.40 Arbidol is now added to China’s NHC guide for the management of COVID-19. A pilot clinical trial conducted in China showed Arbidol reduced viral load and mortality.41 Another study with Arbidol plus lopinavir/ritonavir for 2-week treatment, 94% of the Arbidol plus lopinavir/ritonavir treated patients was negative vs 53% in the control group, and the chest tomography scans improved for 69% of the arbidol treated groups vs 29% in the control group.42
Huang D et al conducted meta-analysis on 12 studies showed arbidol was safe and associated with a higher negative rate of PCR on day 14 in COVID-19 patients. But, it could not significantly decrease nucleus acid negative conversion time, improve symptoms, or decrease the risk of disease progression. Generally, there is no enough evidence to support the therapeutic use of arbidol for patients with COVID 19 and need to be verified in future studies.43 Currently, several clinical trials are ongoing on the efficacy of arbidol as a single agent (NCT04255017, NCT04260594, and NCT04254874) and in combination with lopinavir/ritonavir, favipiravir, chloroquine or carrimycin.
Viral Protease Inhibitor
Protease enzymes like 3CLpro and PLpro have a key role in the maturation, and replication of different viruses in the host cell. Protease also inhibits the host innate immune system. Protease inhibitors, such as diarylheptanoids, cinanserin, and flavonoids are promising agent for the treatment SARS-CoV- 2.28 Based on modes of infection and genomic structure of SARS-CoV-2, there are several promising repurposed agents. Most agents are in the preclinical stage to be used as anti-COVID-19 agents.
Lopinavir–Ritonavir
LPV/r is a protease inhibitor used for management of HIV infection. Based on evidences in managing SARS-CoV during the 2003 pandemic and MERS, LPVr is supposed to manage COVID-19 patients.44 Besides, Chu et al showed the activity of LPV/r on SARS-CoV by in vitro antiviral susceptibility testing. Hence, LPV/r was explored for the management of COVID-19 disease. A retrospective cohort study, done by Deng et al showed LPV/r with arbidol was significantly better than LPV/r alone for nasopharyngeal swab PCR conversion to negative and improvement on CT chest.45 In another study, LPV/r was not significantly than the standard of care for the management of COVID-19.46 Generally, the data on the therapeutic use of LPV/r for the management of COVID-19 is limited. Larger controlled clinical studies are required to determine the role of LPV/r in COVID-19 disease. Nelfinavir is another protease inhibitor that blocks SARS-CoV replication may also inhibit SAR-CoV-2 virus.47 Darunavir is a protease inhibitor usually used in combination with ritonavir or cobicistat, for the management of HIV. The in-vitro studies proposed the inhibitory effect of SARS-CoV-2 replication. It is still under clinical trials for the effectiveness of the drug against SARS-CoV-2 virus.48
Camostat Mesylate and Nafamostat Mesilate
The SARS-CoV-2 virus binds to ACE2 by its S proteins to enter the host cells.24 After binding into the ACE2 receptor, the viral S proteins require to be primed by proteases enzyme like TMPRSS2 for the viral s protein and host membranes fusion, which is crucial for the virus to infect the different cells. Hence, inhibiting the entrance of virus by inhibiting viral protease has a promising potential to identify a novel therapeutic agent.49 The serine protease inhibitor agents such as Bromhexine, Nafamostat, Camostat, and Aprotinin inhibit TMPRSS2. Camostat mesylate first developed for the management of postoperative reflux esophagitis and chronic pancreatitis significantly reduced infection with SARS-CoV-2.25 Nafamostat mesylate is another TMPRSS2 inhibitor developed for the management of acute pancreatitis, disseminated intravascular coagulation and anticoagulation in extracorporeal circulation. A study done on simian Vero E6 cells infected with SARS-CoV-2, nafamostat mesylate inhibited SARS-CoV-2 infection.50
Viral Nucleic Acid Synthesis Inhibitors
Antivirals are the major therapeutic agent understudy for the management of COVID-19. Even if SARS-CoV and SARS-CoV-2 share 82% RNA sequence, their RNA-dependent RNA polymerase (RdRp) shares a 96% sequence identity.51 Therefore, drugs inhibiting viral RdRp proteins of SARS-CoV may have promising effect on SARS-CoV- 2 virus. Several nucleoside analogs agents available that inhibit RdRp including, favipiravir, remdesivir, ribavirin, Sofosbuvir, and galidesivir showed promising in vitro effects against SARS-CoV- 2 infections.52
Remdesivir
Remdesivir is an investigational phosphoramidate prodrug of adenosine analog developed by Gilead previously evaluated to treat Ebola and now being studied in patients with COVID-19 disease. Remdesivir is a Viral RNA-dependent RNA polymerases inhibitor. It inhibits RNA synthesis by replacing ATP in the polymerization and thus is a well-known premature chain terminator drug.53 In vitro studies showed that Remdesivir is a promising antiviral agent against different viruses including, the measles virus, Ebola virus, MERS-COV, Marburg virus, Nipah virus, Hendra virus, and respiratory syncytial virus infections in cultured cells, mice, and primate models. Besides, remdesivir is active against several Covs like HCoV-NL63, HCoVOC43, HCoV-229E, SARS-CoV, and MERS-CoV.53,54 A recent study showed that Remdesivir inhibits viral replication of SARS-CoV-2 (EC50 = 0.77 μM in Vero E6 cells).55,56 Wilt et al conducted a systematic review of 4 published randomized clinical trials of hospitalized COVID-19 patients compared remdesivir use for 10 days with placebo. The result showed a small reduction in mortality and a better recovery and decrease time to recovery but may have little to no effect on hospital length of stay. Remdesivir may reduce serious adverse events moderately and may reduce any adverse event by a small level.57 In another meta-analysis conducted by Elsawah HK et al included four clinical trials and one observational study. The result showed that Remdesivir increases the recovery rate of moderate and severe hospitalized COVID-19 patients, but mortality reduction has not proven although short-term benefits observed. The efficacy of remdesivir showed in severe COVID-19 patients who do not need mechanical ventilation; these patients can tailor the treatment course from 5 to 10 days without loss of the drug efficacy.58 Sarfraz A et al conducted a systematic review and meta-analysis on four studies that have 3013 participants with 46.3% (n=1395) in the remdesivir group and 53.7% (n=1618) in the placebo group. This review showed Remdesivir reduced the mortality, adverse events, and oxygen support in moderate to severely ill COVID-19 patients.59 At moment, several phases 3 studies of remdesivir are ongoing to determine the efficacy and safety in hospitalized patients, ie, NCT04252664, NCT04257656, NCT04292730, NCT04292899, NCT04280705, and NCT04315948.
Favipiravir
Favipiravir is an orally effective guanine analog approved in 2014 for the management of influenza infection. It differs from the neuraminidase inhibitors like zanamivir in that it inhibits the viral RNA-dependent RNA polymerase. Favipiravir inhibits replication of several RNA viruses, including Lassa virus, yellow fever virus, Ebola virus, chikungunya, norovirus, and enterovirus.60 A recent in vitro study displayed inhibition of SARS-CoV-2 (EC50 = 61.88 μM in Vero E6 cells).61 Recently, patients recruited to evaluate the efficacy of favipiravir (ChiCTR2000030254, ChiCTR2000029600), and favipiravir plus interferon (ChiCTR2000029600) and favipiravir plus baloxavir marboxil (ant influenza drug) (ChiCTR2000029544), Favipiravir plus Tocilizumab (ChiCTR2000030894) based on their synergistic effect. Indeed, in March 2020, favipiravir approved for experimental use in COVID-19 disease in Russia, Italy, China, and other countries.62 But, a study in Japan, Favipiravir did not display significant benefit in mild and moderate COVID-19 diseases.63 Galidesivir (BCX4430) is an adenosine derivative developed for HCV. It showed antiviral activities in preclinical studies against several RNA viruses including, SARS and MERS.
Immunotherapy
Antiviral antibodies produced in convalescent (Immune) plasma from recovered patients from viral diseases used for the treatment of various viral infections (eg, polio, Argentine hemorrhagic fever, influenza A (H5N1), H1N1, SARS-CoV, and Ebola) and post-exposure prophylaxis (eg, hepatitis, mumps, polio, measles, rabies).64 The human convalescent plasma is a promising option for management of COVID-19 patients because it provide immediate passive Immunity for COVID-19 patient. Several reports showed that convalescent plasma COVID-19 patients displayed potent neutralizing monoclonal antibody (NAb) responses in severe SARS-CoV-2 patients. However, convalescent plasma efficacy in critically ill COVID-19 patients remains unclear.65,66
Neutralizing Monoclonal Antibody
Neutralizing monoclonal antibodies (Nabs) are another passive immunotherapeutic agent for the treatment of several diseases. Nabs specifically bind to the target site to produce the desirable effects, which can be either inhibit or activate those molecular pathways. The S protein is the main inducer of Nabs. Most NAbs specifically target RBD and inhibit the interaction of RBD with its receptor ACE2. Some NAbs recognize epitopes on the S2 unit.67 The final virus removal occurs by complement activation or antibody-dependent opsonization. Several studies identified several effective Nabs like CR3014 and CR3022 that bind to RBD to neutralize the virus.68 A recent study showed that CR3022 could be a promising therapeutic candidate for the prevention and treatment of COVID-19 pneumonia that specifically binds to RBD of SARS-CoV-2 and displayed significant prophylaxis effect in vitro and animal models.69 Thus, the use of Nabs represents a promising approach to enhance humoral defense against CoVs by inhibiting different S protein epitopes.70 Nabs also target the host ACE2 receptor to inhibit the access of the virus. NAbs that bind to the soluble version of ACE2, which binds to SAR-CoV-2, thus sequestering it away from cell surface-bound ACE2 in host cells.71
Interferon
Interferons (IFN) are broad-spectrum antiviral proteins synthesized by the host cells during viral infection. IFN is used for the management of viral infection, tumors, and autoimmune diseases. IFN-α suppresses virus infection by inhibiting viral protein synthesis, degrade viral RNA, and apoptosis by promoting both innate and adaptive immune responses to infection.72 During the SARS outbreak in 2003, a study in the Rhesus monkey model revealed that IFN-α spray can prevent SARS-CoV infection by inhibiting virus replication. Further clinical evaluation showed that IFN-α significantly reduces the infection rate of the respiratory syncytial virus, adenovirus, influenza virus, and SARS-CoV.73 SARS-CoV-2 did not significantly induce types I, II, or III interferons in ex-vivo infected human lung tissues compared with 2003 SARS-CoV. Clinical trials involving interferon have been initiated, such as a trial testing the approved anti-HCV combination of pegylated interferon plus ribavirin (ChiCTR2000029387). Thus, IFN-α is a promising candidate drug for COVID-19 therapy.
Dampening Hyper Immune Activation and Harnessing Immune Response
Cytokine response is crucial for the host immune response. However, patients infected with SARS-CoV-2 experience the upregulation of cytokine, chemokine and interferon synthesis, which causes cytokine storm, followed by massive immune cell infiltration into the lungs leading to decreased lung function, and death. Cytokine storm induces a strong inflammation that contributes to Acute Respiratory Distress Syndrome (ARDS), multiple organ failure, and death. Thus, the identification of existing approved therapies to treat hyper inflammation is crucial to reduce COVI-19 associated mortality.73,74 Corticosteroids, IL-6 blocker (tocilizumab), interleukin 1 receptor antagonist (Anakinra), and JAK inhibition suppress exaggerated immune systems in different diseases. Hence, this can be important for the treatment of cytokine release syndrome and associated pathologies in COVID-19 patients.74
Steroids
Corticosteroids are extensively used for acute respiratory conditions that have similar pathological features with COVID-19 including SARS-CoV, MERS-CoV, H1N1 influenza, community-acquired pneumonia, and ARDS. But, their role in reducing mortality and improving these conditions remain controversial.75 The early observational study revealed promising results in terms of improving survival,76 but subsequent observational studies and data from a single randomized trial showed mixed results. A systematic review and meta-analysis conducted by Tlayjeh H et al included one RCT and 19 cohort studies with low to moderate risk of bias assessed the association between Corticosteroid treatment, and viral clearance, disease progression and mortality in hospitalized COVID-19 patients. This systematic review showed that Corticosteroid treatment was not associated with reduced short-term mortality but possibly with delayed viral clearance.77 A systematic review and meta-analysis conducted by Yang Z et al on a total of 5270 patients from 15 studies showed that critical patients were more likely to require corticosteroid treatment but associated with higher mortality, a longer length of stay, and a higher rate of bacterial infection.78 A recent prospective meta-analysis of 7 randomized trials conducted by Horby P et al included 1703 critically ill COVID-19 patients. This study showed that Corticosteroids were associated with lower mortality among critically ill patients at 28 days after randomization.79 Currently, WHO recommends against the routine use of steroids for COVID-19 patients, due to delayed viral clearance, vascular necrosis, and psychosis. Generally, steroids should not be used solely to treat SARS-CoV-2 infection but may be required to treat other conditions that may accompany it.80
Tocilizumab (Interleukin-6 Receptor Blocker)
In critically ill patients with COVID-19, IL-6 levels were almost tenfold higher. Tocilizumab is monoclonal antibodies that specifically bind to soluble and membrane-bound IL-6 receptors (IL-6R), thus blocking IL-6 signaling and its mediated inflammatory response. IL-6 receptors may curb fever and inflammation, but this blunts the host defense against infection. Tocilizumab has a profound immunosuppressant effect that increases the risk of sepsis, pneumonia, and hepatotoxicity. Yet, despite these severe side effects, IL-6 inhibitors are now being given to patients with COVID-19 in several countries who develop cytokine release syndrome.81
Although basic science proposes the rationale for therapeutic use of IL-6 receptor antagonists for COVID-19 patients, several studies have shown inconsistent results. A recent randomized, double-blind, placebo-controlled trial showed Tocilizumab was not effective in preventing intubation or death in moderately ill hospitalized COVID-19 patients.80,82 Another systematic review conducted by Cortegiani A et al on 28 clinical studies with 5776 COVID-19 patients (13 with a comparison group, 15 single-arm) and 3 indirect pre-clinical studies revealed no sufficient evidence on efficacy and safety of tocilizumab in patients with COVID-19.83 But, a recent non-controlled, prospective clinical trial showed tocilizumab may be a promising therapeutic agent for severe or critical COVID-19 patients, if promptly initiated during the severe stage.84 A prospective open-label uncontrolled multicenter trial showed tocilizumab may improve some clinical parameters and reduce the risk of death in COVID-19 patients, especially if used in the early stages of respiratory failure.85 Currently, several studies are undergoing on the safety and efficacy of tocilizumab to treat COVID-19 disease (ChiCTR2000029765), (ChiCTR2000030796), and combination with other drugs (ChiCTR2000030442 and ChiCTR2000030894).
JAK Inhibitors
SARS-CoV-2 enters cells through endocytosis by interacting with ACE2. AP2-associated protein kinase 1 (AAK1) is the major regulators of endocytosis. AAK1 inhibitors inhibit the entrance of the SARS-CoV2 virus into cells and may be helpful in preventing virus infections. Baricitinib, an AAK1 inhibitor and a JAK inhibitor was proposed as a candidate drug for the treatment of COVID-19. But, JAK inhibitors inhibit inflammatory cytokines including INF-a, which has a significant antiviral activity (ChiCTR2000030170), (ChiCTR2000029580).86
Conclusion
Up-to-date, there is no effective antiviral agent for the treatment and prevention of COVID-19 pandemic. Hence, authorities rely exclusively on enforcing strict preventive and control measures to reduce the risk of disease transmission. Currently, several clinical trials are ongoing to develop new drugs and vaccines. These clinical trials follow two main strategies. The first strategy is the therapeutic use of repurposed drugs including hydroxychloroquine (LPV/r) against the virus. Due to the need for urgent responses and high-quality evidence on the efficacy and safety of the drug, repurposing of existing drugs are currently the best treatment option until safe and effective vaccine development. The second approach is dampening of the proinflammatory state produced during COVID-19 with drugs such as Corticosteroids (eg, Dexamethasone), IL-6 inhibitors (eg, tocilizumab), IL-1antagonist (eg, Anakinra) and JAK inhibition which give better clinical response by reversing multi-organ failure and ARDS. Although some therapeutic agents mentioned in this review are promising, definitive evidence regarding their effectiveness remains inconclusive. In conclusion, supportive care remains the cornerstone of COVID-19 treatment. Complications should be managed according to general guidelines.
Abbreviations
ACE2, angiotensin-converting enzyme 2; 3CLpro, 3C-like protease; IL-6R, IL-6 receptors; IFN, interferons; LPV/r, lopinavir–ritonavir; MERS-CoV, Middle East respiratory syndrome coronavirus; nsps, non-structural proteins; Nabs, neutralizing monoclonal antibodies; ORFs, open reading frames; PLpro, papain-like protease; RdRp, RNA-dependent RNA polymerase; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TMPRSS2, transmembrane protease serine 2.
Data Sharing Statement
All data are provided in the manuscript or found from published papers as cited.
Acknowledgment
I would like to acknowledge Mrs Fasika Abu for editing the manuscript for English Style.
Funding
There is no funding to report.
Disclosure
The author declares no conflicts of interest for this work.
References
1. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. doi:10.3390/pathogens9030231
2. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (covid-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi:10.1016/J.Ijantimicag.2020.105924
3. Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor grp78 binding site prediction. J Infect. 2020;80(5):554–562. doi:10.1016/j.jinf.2020.02.026
4. Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype of SARS-CoV-2-specific t-cells in covid-19 patients with acute respiratory distress syndrome. Medrxiv. 2020.
5. Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi:10.1016/S0140-6736(20)30920-X
6. Coronavirus 2019-Ncov, CSSE. Coronavirus 2019-ncov global cases by johns hopkins CSSE; 2020. Available from: https://Gisanddata.Maps.Arcgis.Com/Apps/Opsdashboard/Index.Html#/Bda7594740fd402994267b48e9ecf6.
7. Bhattacharjee B, Pandit B. Phylogenetic clustering of the Indian SARS-CoV-2 genomes reveals the presence of distinct clades of viral haplotypes among states. bioRxiv. 2020.
8. Al-Qahtani AA. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): emergence, history, basic and clinical aspects. Saudi J Biol Sci. 2020;27(10):2531–2538. doi:10.1016/j.sjbs.2020.04.033
9. Vellingiri B, Jayaramayya K, Iyer M, et al. COVID-19: A promising cure for the global panic. Sci Total Environ. 2020;725:138277. doi:10.1016/j.scitotenv.2020.138277
10. Fehr AR, Perlman S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses 2015 (Pp. 1-23). New York, NY: Humana Press; 2015.
11. Dhama K, Sharun K, Tiwari R, et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;1–7.
12. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ace2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92(6):595–601. doi:10.1002/jmv.25726
13. Mousavizadeh L, Ghasemi S. Genotype and phenotype of covid-19: their roles in pathogenesis. J Microbiol Immunol Infection. 2020. doi:10.1016/j.jmii.2020.03.022
14. Dhama K, Sharun K, Tiwari R. Coronavirus Disease 2019–COVID-19. Clin Microbiol Rev. 2020;33(4):e00028.
15. Shi Y, Zhang X, Mu K, et al. D3Targets-2019-ncov: a webserver for predicting drug targets and for multi-target and multi-site based virtual screening against COVID-19. Acta Pharm Sin B. 2020;10(7):1239–1248. doi:10.1016/j.apsb.2020.04.006
16. Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for covid-19 and related human coronavirus diseases. J Med Virol. 2020;34(2):315–331.
17. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1–34.
18. Hamid S, Mir MY, Rohela GK. Noval coronavirus disease (covid-19): a pandemic (epidemiology, pathogenesis and potential therapeutics). New Microbes New Infect. 2020;35:100679. doi:10.1016/j.nmni.2020.100679
19. Lythgoe MP, Middleton P. Ongoing clinical trials for the management of the COVID-19 Pandemic. Trends Pharmacol Sci. 2020;41(6):363–382. doi:10.1016/j.tips.2020.03.006
20. Yavuz S, Ünal S. Antiviral Treatment of COVID-19. Turkish J Med Sci. 2020;50(SI–1):611–619. doi:10.3906/sag-2004-145
21. Chen Y, Liu Q, Emerging Coronaviruses: GD, Structure G. Replication, and Pathogenesis. J Med Virol. 2020;92(4):418–423. doi:10.1002/jmv.25681
22. Kumar S, Zhi K, Mukherji A. Repurposing antiviral protease inhibitors using extracellular vesicle for potential therapy of COVID-19. Viruses. 2020;12(5):486. doi:10.3390/v12050486
23. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery. 2020;6(1):1–8.
24. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;1:1–5.
25. Hoffmann M, Kleine-Weber H, Schroeder S, et al. Müller MA. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052
26. Speth RC. Response to recent commentaries regarding the involvement of a ngiotensin-converting enzyme 2 (ACE2) and renin-angiotensin system blockers in SARS-CoV −2 infections. Drug Dev Res. 2020;81(6):643–646. doi:10.1002/ddr.21672
27. Roshanravan N, Ghaffari S, Hedayati M. Angiotensin converting enzyme-2 as therapeutic target in COVID-19. Diabetes Metab Syndrome. 2020;14(4):637–639. doi:10.1016/j.dsx.2020.05.022
28. Pooladanda V, Thatikonda S, Godugu C. The current understanding and potential therapeutic options to combat COVID-19. Life Sci. 2020;254(1):117765. doi:10.1016/j.lfs.2020.117765
29. Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55(5):105938. doi:10.1016/j.ijantimicag.2020.105938
30. Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. doi:10.1016/j.ijantimicag.2020.105955
31. Roldan EQ, Biasiotto G, Magro P, Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): A role for iron homeostasis? Pharm Res. 2020;158:104904. doi:10.1016/j.phrs.2020.104904
32. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020.
33. Gao J, Tian Z, Yang X. breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of covId-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi:10.5582/bst.2020.01047
34. Cortegiani A, Ippolito M, Ingoglia G, Iozzo P, Giarratano A. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care. 2020.
35. Axfors C, Schmitt AM, Janiaud P. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19: an international collaborative meta-analysis of randomized trials. MedRxiv. 2020.
36. Gallus S, Clavenna A. Does hydroxychloroquine reduce mortality for COVID-19? Eur J Intern Med. 2020. doi:10.1016/j.ejim.2020.10.015
37. Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi:10.1016/j.antiviral.2014.04.006
38. Teissier E, Zandomeneghi G, Loquet A, et al. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS One. 2011;6(1):e15874. doi:10.1371/journal.pone.0015874
39. Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking the trimerization of viral spike glycoprotein? Int J Antimicrob Agents. 2020;56(2):105998. doi:10.1016/j.ijantimicag.2020.105998
40. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020.
41. Zhang JN, Wang WJ, Peng B, et al. Potential of arbidol for post-exposure prophylaxis of covid-19 transmission—a preliminary report of a retrospective cohort study. Curr Med Sci. 2020;1.
42. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020;81(1):e1–e5. doi:10.1016/j.jinf.2020.03.002
43. Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Med Virol. 2020. doi:10.1002/jmv.26256
44. Wang Y, Zhu LQ. Pharmaceutical care recommendations for antiviral treatments in children with coronavirus disease 2019. World J Pediatrics. 2020;12:1–4.
45. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020;11.
46. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Eng J Med. 2020;382(10):929–936. doi:10.1056/NEJMoa2001191
47. Shetty R, Ghosh A, Honavar SG, Khamar P, Sethu S. Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: present and future. Indian J Ophthalmol. 2020;68(5):693. doi:10.4103/ijo.IJO_639_20
48. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58–60. doi:10.5582/ddt.2020.01012
49. Hoffmann M, Kleine-Weber H, Krüger N, Mueller MA, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020.
50. Inoue J, Yamamoto M. Identification of an existing Japanese pancreatitis drug, Nafamostat, which is expected to prevent the transmission of new coronavirus infection (COVID-19). bioRxiv. 2020.
51. Liu W, Morse JS, Lalonde T, Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;2.
52. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). bioRxiv. 2020;149–150.
53. Amirian ES, Levy JK. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi:10.1016/j.onehlt.2020.100128
54. Brown AJ, Won JJ, Graham RL, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi:10.1016/j.antiviral.2019.104541
55. Choy KT, Wong AY, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi:10.1016/j.antiviral.2020.104786
56. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0
57. Wilt TJ, Kaka AS, MacDonald R, Greer N, Obley A, Duan-Porter W. Remdesivir for Adults With COVID-19: A Living Systematic Review for an American College of Physicians Practice Points. Ann Intern Med. 2020. doi:10.7326/M20-5752
58. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. 2020;e2187.
59. Sarfraz A, Sarfraz Z, Sanchez-Gonzalez M, et al. Randomized Controlled Trials of Remdesivir in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. medRxiv. 2020.
60. Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;108(2):242–247. doi:10.1002/cpt.1844
61. Lu CC, Chen MY, Chang YL. Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc. 2020.
62. Favipiravir: YH, Possible Pharmaceutical A. Treatment for COVID-19. J Endocrinol Metab. 2020;10(2):33–34. doi:10.14740/jem645
63. Chakraborty R, Parvez S. COVID-19: an overview of the current pharmacological interventions, vaccines, and clinical trials. Biochem Pharmacol. 2020;180:114184. doi:10.1016/j.bcp.2020.114184
64. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi:10.1016/S0140-6736(20)30154-9
65. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi:10.1001/jama.2020.4783
66. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi:10.1016/S1473-3099(20)30141-9
67. Zhou G, Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int J Biol Sci. 2020;16(10):1718.
68. Hussain A, Hasan A, Babadaei MM, et al. Targeting SARS-CoV2 spike protein receptor binding domain by therapeutic antibodies. Biomed Pharm. 2020;130:110559. doi:10.1016/j.biopha.2020.110559
69. Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspective. Int J Antimicrob Agents. 2020;105951.
70. Tian X, Li C, Huang A, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Em Microbes Infect. 2020;9(1):382–385. doi:10.1080/22221751.2020.1729069
71. Iwanaga N, Cooper L, Rong L, et al. Novel ACE2-IgG1 fusions with improved activity against SARS-CoV2. bioRxiv. 2020.
72. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N, Florence AD. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi:10.1016/j.antiviral.2020.104791
73. Shen K-L, Yang Y-H. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. bioRxiv. 2020;1–3.
74. Nile SH, Nile A, Qiu J, Li L, Jia X, Kai GCOVID-19. Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi:10.1016/j.cytogfr.2020.05.002
75. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi:10.1016/S0140-6736(20)30317-2
76. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi:10.1001/jamainternmed.2020.0994
77. Tlayjeh H, Mhish OH, Enani MA, et al. Association of corticosteroids use and outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect Public Health. 2020;13(11):1652–1663. doi:10.1016/j.jiph.2020.09.008
78. Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi:10.1016/j.jinf.2020.03.062
79. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19-preliminary report. N Engl J Med. 2020.
80. Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am J Emerg Med. 2020.
81. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18(1):1–5. doi:10.1186/s12967-020-02339-3
82. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Eng J Med. 2020. doi:10.1056/NEJMoa2028836
83. Cortegiani A, Ippolito M, Greco M, et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2020.
84. Dastan F, Saffaei A, Haseli S, et al. Promising effects of tocilizumab in COVID-19: a non-controlled, prospective clinical trial. Int Immunopharmacol. 2020;88:106869. doi:10.1016/j.intimp.2020.106869
85. Malekzadeh R, Abedini A, Mohsenpour B, et al. Subcutaneous tocilizumab in adults with severe and critical COVID-19: A prospective open-label uncontrolled multicenter trial. Int Immunopharmacol. 2020;89:107102. doi:10.1016/j.intimp.2020.107102
86. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30. doi:10.1016/S0140-6736(20)30304-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
