Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 13
An Overview on Mesenchymal Stem Cells Derived from Extraembryonic Tissues: Supplement Sources and Isolation Methods
Authors Salehinejad P, Moshrefi M, Eslaminejad T
Received 5 February 2020
Accepted for publication 25 June 2020
Published 7 July 2020 Volume 2020:13 Pages 57—65
DOI https://doi.org/10.2147/SCCAA.S248519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Parvin Salehinejad,1 Mojgan Moshrefi,2,3 Touba Eslaminejad4
1Neuroscience Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran; 2Medical Nanotechnology and Tissue Engineering Research Center, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran; 3Research and Clinical Center for Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran; 4Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran
Correspondence: Touba Eslaminejad
Kerman University of Medical Sciences, Kerman, Iran
Tel +9834331325245
Fax +9834331325215
Email [email protected]
Purpose: The main aim of this review was to provide an updated comprehensive report regarding isolation methods of MSCs from human extra embryonic tissues, including cord blood, amniotic fluid, and different parts of the placenta and umbilical cord, with respect to the efficacy of these methods.
Results: Extra embryonic tissues are the most available source for harvesting of mesenchymal stem cells (MSCs). They make a large number of cells accessible using non-invasive methods of isolation and the least immune-rejection reactions. A successful culture of primary cells requires obtaining a maximum yield of functional and viable cells from the tissues. In addition, there are many reports associated with their differentiation into various kinds of cells, and there are some clinical trials regarding their utilization for patients.
Conclusion: Currently, cord blood-MSCs have been tested for cartilage and lung diseases. Umbilical cord-MSCs were tested for liver and neural disorders. However, these MSCs can be isolated, expanded, and cryopreserved in a cell bank for patients in need.
Keywords: cord blood-MSCs, umbilical cord-MSCs, amniotic fluid, placenta, embryonic tissues, immune-rejection
Introduction
Mesenchymal stem cells as connective tissue cells are located in the extracellular matrix (ECM);1 the non-living viscous substance in which cells and fibers belong to the ECM. ECM is a collection of glycoproteins, collagens, laminins, fibronectin, elastin, and proteoglycans, chondroitin, heparan, keratan sulfates, and hyaluronic acid.2 Mesenchymal stem cells are an important cell population, which can possess stem cell-like characteristics.3,4 They are multi-potent, and found in nearly all tissues, mostly located in perivascular niches (Figure 1).5 Perivascular location of the mesenchymal stem cells (MSCs) correlates them to pericytes.6 Pericytes exist in the wall of the blood vessels, and closely encircle endothelial cells in capillaries, and micro vessels in multiple organs.7 According to this, MSCs can stabilize blood vessels, and contribute to tissue, and immune system homeostasis under physiological conditions. They play a more active role in the repair of damage of the local tissue.8 Since the MSCs have migratory abilities, they secrete protective factors and act as a primary matrix for tissue regeneration during inflammation, tissue injuries, and certain cancers.5 MSCs can modulate the function of different cells of the immune system, eg, T cells, B cells, natural killer cells, and dendritic cells. The in vitro immunomodulatory properties of MSCs cause more interest on the potential applications of them in in vivo assay (as an immunosuppressive cellular therapy).9 MSCs exhibit a broad differentiation capacity to osteoblasts, adipocytes, and chondrocytes, hepatocyte-like cells, neuronal, and neuroglial cells.10 They expressed mesenchymal markers such as CD105 and CD90 positively.11 Also MSCs are isolated from a variety of adult tissues, eg, peripheral blood tissues, adipose tissues, compact bone, dental pulp, and bone marrow, dermis, pancreatic islets, adult brain tissues, skeletal muscle tissues, and synovium, circulatory system tissues,7 lung, heart, and hair follicles.8 Foetal tissues such as liver, lung, and neonatal tissues including placenta, amnion and different parts of the umbilical cord,12 and cord blood5 are used to isolate the MSCs. As an alternative, stem cell populations could be harvested at birth.10 In this review, the isolation methods of MSCs from different supplement sources were discussed, and also their utilizations in medical application were explained.
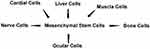 |
Figure 1 Schematic illustration of mesenchymal stem cells extracted from umbilical cord blood as source multi-potent and found in nearly all tissues. |
Cell Isolation Protocols
Dissociation of the animal cells depends on the age, species, tissue origin, and the type of dissociation medium. Tissue and species factors are not controllable, but mediums and conditions associated to tissue dissociation could be controlled to get the best results.13 Generally, two methods, eg, enzymatic and non-enzymatic are used for cell isolation. Enzymes are chosen according to the connective tissue matrix of the particular organ.14 Enzymes show different impacts on the morphological characteristics of the isolated cells.12 They are applied in simple or cocktail form.15,16 Trypsin and collagenase are common enzymes, which are used in cell separation. Furthermore, some characteristics, eg, kinds of enzymes, amount of impurities in any stock preparation, enzymes’ concentration, and temperature, have a successful influence.13,15,16
Non-enzymatic methods showed advantages over enzymatic methods, eg, preventing damage to the cell membrane, and intracellular structures. They are divided into mechanical and chemical methods. Mechanical methods include homogenizing, sieving, mincing, and affinity. Some of them are traumatic for tissue. This showed that the combination of two methods could be more effective if the mechanical methods are applied immediately before placing into the enzyme. Chemical methods are mostly mild, but they have not been used for the isolation of the cells on a large scale. Chemical methods interact via destroying cell-to-cell attachment by removal of calcium, and magnesium, separating cell layers by affecting glycine, and changing the pH through suspension in a buffer solution. Growth factors, eg, epidermal growth factor (EGF), Vascular endothelial growth factor (VEGF), or fibroblast growth factors (FGF), have been used as supplements for the in vitro expansion of isolated MSCs.17
Isolation Methods of Mesenchymal Stem Cells
Human Umbilical Cord Blood
Umbilical cord blood (UCB) is indicated as a source of hematopoietic stem cells which are transplantable for clinical practice for a decade.18 Because of the small amount of MSCs, freshly donated UCB does not qualify for transplantation.19 The isolated amounts of MSCs are affected by the volume of a UCB sample as a criterion.20 The volume of the UCB unit and the mononuclear cell count are predictive of the isolation rate of MSCs. The mean volumes of the UCB unit and number of nucleated cells per unit are 40 mL and 5.39×108, respectively. But, MSCs can successfully be isolated from UCB units with a volume of ≥54 mL containing ≥1.28×108 mononuclear cells, which results in a MSC isolation rate of >70%.19 In addition, the ratio of cord blood volume/number of nucleated cells is greater for boy neonates than for girls: 78 mL/12.4×106 cells from boys compared with the 66.6 mL/10.2×106 harvested cells from the girls.21 Another parameter for successful isolation of the cells is a transporting time of less than 15 hours from collection to isolation. However, some researchers believe that the MSC isolation rate is not affected by the interval between delivery and cell processing.19 To date, at least three different kinds of stem cells have been isolated from UCB, including hematopoietic stem cells, endothelial progenitor cells, and MSCs.18 MSCs revolve in cord blood but at much lower frequencies than hematopoietic stem cells,10 which are 0.002±0.004×106 initially plated cells while the number of MSCs from BM is 83±61×106 initially plated cells.17 Thus, their isolation and culture are more difficult.10 Generally, MSCs are successfully isolated from only 30% to 75% of UCB.8,17,19
The UCB is aspirated from the umbilical vein directly after the child’s birth while the placenta is in utero and collected in a sterile bag containing anti-coagulant; citrate phosphate dextrose (CPD) or Acid citrate dextrose formula A (ACD-A) solution.17 Any blood clot in the bag contributes to the exclusion of the bag.19 Also, in the second trimester of pregnancy, the UCB can be collected transcervical after dilatation by using a cordocentesis with a 23-gauge needle, and kept in heparin.22 Collected samples should be subjected to the following tests: complete blood count (CBC), bacteriological anaerobic and aerobic tests and immunological tests of antibodies for Herpes simplex virus type 1, 2 (HSV1, 2), Toxoplasmosis, hepatitis C virus (HCV), Hepatitis B surface antigen (HBsAg), and cytomegalovirus (CMV). Therefore, the following immunological tests were used for quality control of the samples including: Ac-HSV1+2 (IgM+IgG), HBsAg, Ac-HCV (IgM+IgG), Ac-anti Toxoplasmosis (IgM+IgG), and Ac anti-CMV (IgM+IgG).21 By incubation in NH4Cl (8.4 g/L)/KHO3 (1 g/L) buffer for 10 minutes at 4°C, then UCB is depleted from red blood cells. The most widely used cryoprotectant for banking of hematopoietic stem cells (HSC) and cord blood samples is DMSO. A cooling rate of 1–2ºC/min is suitable for most human cells, including HSC of UCB. It can be achieved by two conventional freezing methods including: controlled-rate freezing and uncontrolled-rate freezing or passive cooling. Recently, verification is used as an alternative technique for conventional methods. Viability of HSC, storage in −80ºC is decreased with the length of storage. Therefore, an ultra-low temperature is applied by direct immersion of sample bags in liquid nitrogen which has a constant temperature of −196ºC23 or are freshly processed to obtain the mesenchymal stem cells.17 Some researchers believe that the MSC isolation rate is not affected by cell processing protocol.19 Ficoll,19,24,25 hydroxyethyl starch,19 and dextran21 are the materials added to cord blood mononuclear cells to purify mononuclear cells.
However, density gradient centrifugation of 1.073 g/mL density compared to 1.077 g/mL density causes the greatest concentration of MSCs to 1.8-fold.17 Ficoll and hydroxyethyl starch lead to 40–60%10,26 and 50%19 of purified mononuclear cells, respectively. In addition, flow cytometry (immunoselection) for sorting of CD34 cells, with a 32% success rate,19 and the osmotic selection method are the other methods for MSC isolation from UCB.17
Amniotic Fluid
Amniotic fluid (AF) MSCs express Oct-4, the pluripotent marker, in almost 90% of the favorable condition, also they have multiple differentiation capacities like the other mesenchymal stem cells.11 Three types of adherent cells are characterized in AF. They are categorized based on their morphological growth and biochemical characteristics, which are as follows: 1) Epithelioid (E-type) cells are cuboidal to columnar cells derived from the foetal skin and urine; 2) Amniotic fluid (AF-type) cells originate from foetal membranes; and 3) Fibroblastic (F-type) cells are generated mainly from fibrous connective tissue. Both AF- and F-type cells demonstrated fibroblastoid morphology.
Human amniotic fluid stem cells (AFSCs) can be easily isolated from a small amount of sample during routine amniocenteses in 14–16 weeks of pregnancy,27 or they can be collected by puncture of the embryonic membranes during a caesarean section after opening the uterine wall. Amnion fluid should be processed within 4 h.23 Filtering of AF through a cell strainer (40 µm strainer)28,29 is the most common method for isolating MSCs, with a 25% success rate.10,30 Also, immunoselection (a kind of cell sorting) with an antibody specific for c-Kit (CD117) antigen gives the total cell count of approximately 104 to 106 of cells per 5 mL of AF.31,32
Human Placenta Tissues
Human placenta is made up of both foetal and maternal tissues, which are composed of amnion, chorion, and decidua.33 The amnion and chorion have foetal origin, while the other parts of the decidua have maternal origin.23 After fertilization the human blastocyst, composed of trophoblast and embryoblast, is embedded in the endometrium by invasion of the trophoblast cells.33
MSCs can be isolated approximately from all of the sections of the placenta.34 It is shown that MSCs obtained from the first trimester of a preterm human placenta have higher proliferation potential than term human placenta cells and adult bone marrow. These findings may be due to the fact that the cells of early gestational placenta are closer to the embryo stage than the cells of late full-term placentas or adult bone marrow.35
Amniotic Membrane
Amniotic membrane includes mesenchymal and epithelial cells. Human amniotic mesenchymal stem cells (hAMSCs) are derived from the extra embryonic mesoderm and are dispersed in the collagenous stroma, which is beneath the epithelial monolayer of the amniotic membrane.36 The amniotic cavity develops of endoderm, mesoderm, and ectoderm germ layers. The amniotic membrane lacks any vascular tissue and forms the majority of the inner layer of the foetal membrane, and is composed of three layers: 1) an outer mesenchymal cell layer; 2) an acellular intermediate basement layer, and 3) an epithelial monolayer consisting of epithelial cells. The outer layer is placed in close proximity to the chorion and has a lot of stem cells. Amniotic membrane stem cells (AMSCs) have two types, the amniotic epithelial stem cells and the amniotic MSCs, which are derived from the amniotic epithelial and the amniotic mesenchymal layers, respectively.27
Firstly, the amniotic membrane is mechanically separated from the chorion by detachment, it is rinsed in PBS or medium, minced with scissors, and then flushed via a 100-μm nylon filter,23 or the minced pieces (1–2 mm3) are transferred to a plate for explant culture for 10 days to allow the isolation and migration of the mesenchymal stem cells.33 The minced amnion tissues are incubated with 0.25% trypsin at 37°C for 5 minutes to remove the epithelial cells (EC). Then, by various types and concentrations of collagenase with or without adding DNase, the remnant tissues are digested and the mesenchymal stem cells are released.36 It was reported that using 1 mg/mL collagenase type I or IV26,37 with 20–75 µg/mL DNAse,36 or 4 mg/mL collagenase type II with 100 µg/mL DNAse, is sufficient for cell extraction.38 Nevertheless, the incubation times can vary from 30 minutes to 3 hours.36 It was shown that isolation efficacy or harvesting rate with collagenase is 62.5–100%.10 The yield from term amnion is about 1 million MSCs per gram of amnion tissue;39 approximately 1×106 cell/g of tissue.10 But some groups have treated cells with dispase and papain instead of DNase or pure MSC without the previous isolation of EC by treatment with trypsin.36
Chorionic Membrane
Chorionic membrane (plate) contains chorionic trophoblastic cells and MSCs.10 Chorionic MSCs are derived from the reticular layer of the chorion. So, for obtaining them, chorion is separated from the amnion by peeling it apart. As collagen is the major part of the ECM of chorion layers, so collagenase mediated chorion dissociation is a crucial step in all types of cell isolation procedures. The minced chorion fragments were exposed to 0.25% or 0.5% trypsin-EDTA, and then to complete the tissue digestion 0.5–3 mg/mL collagenase (I) was applied for 20–30 minutes.35,40 Also, it was reported that combinations of mechanical and enzymatic treatments with dispase and/or collagenase IV can be used for cell isolation from the chorionic membrane.10,41
Decidua
The decidua is composed of a thin layer of maternal endometrial tissue which is reformed by structural and functional transformation during early pregnancy.42 Decidua basalis is the attachment site of the placenta and endometrium, while decidua parietalis refers to the other parts except the attachment site of the placenta. Cells originating from these parts are very different in their function.43
Decidua basalis and decidua parietalis can be collected by scraping them from the chorion. Tissue parts are then chopped and minced before being passed through a filter23 or explanted in dish culture for 10 days until the cells migrate from the decidua.33 In addition to mechanical methods, enzymatic methods are also used for isolating the cells from the decidua, based on collagenase usage. Until now, collagenase type I and IV with trypsin, DNase, and dispase have been successfully used for the digestion of the decidua. In enzymatic method, decidua basalis is dissected from the central region of the placenta and mechanically minced into 1–2 cm3 pieces then digested with 0.25% trypsin and 1 mg/mL collagenase IV incubated for 30 minutes at 37°C.23,42 Also, the minced tissues can be digested using a solution containing 3 mg/mL collagenase type I and 271 units/mL DNase I at 37°C for 1 hour.43 The tissues can also be digested in 0.25% trypsin and 50 mg/mL DNAse 1 at 4°C overnight. Then, after inactivating the trypsin with FBS, the remaining tissues are treated with 10 mg/mL type 1 collagenase and 50 mg/mL DNAse I for 30 minutes at 37°C. In this way, at the first passage when the population displays a homogeneous fibroblast-like morphology, about 5×105 cells from an initial 8 g of tissues were obtained.44 Also, a combination of 100 U/mL collagenase type I, 1.5 μg/mL DNase I, and 2.4 U/mL dispase in serum-free DMEM can be used for 1.5 to 2 hours, while every 30 minutes the cocktail is gently agitated for 10 seconds.45
Chorionic Villi
The number of cells that can be obtained from chorionic villi specimens is limited due to their small amount of tissue and short-time culture.46 For this purpose, amnion and chorionic membranes are removed from the placenta then white terminal chorionic villi from a central cotyledon of the villous vascular floor are cut into small pieces and explant culture continues for 2 or 3 weeks until the cells are observed.47 Nevertheless, in the explant culture, contamination with maternal tissues is more likely.48 Also, in another method, the chorionic villi pieces can be hemolyzed in red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 8.0), centrifuged at 550 g for 5 minutes at room temperature, and then treated twice with 0.25% or 0.05% trypsin-EDTA in a magnetic stirrer at 37°C for 10 minutes.49,50 Besides, the chorionic villi can be digested in 2.5% trypsin and 271 unit/mL DNase, with gentle rotation at 4°C overnight. Then, the tissues are allowed to adhere to the plastic floor of six-well plates for 60 minutes. Supplemented medium is then gently added to the tissues and cultured for 14 days, with refreshing every 3 days. The MSCs migrate out of trypsin-treated tissues due to digestion of the ECM. Results showed that trypsin significantly increases the number of cells produced by the explanted chronic villi as compared to untreated villi. It is reported that there are 11.55×103 and 24.66×103 cells produced per 40 mg of untreated- and treated tissues, respectively.51 Also, it showed that 1 mg/mL collagenase type I at 37°C for 1 hour was able to separate enough cells.46
Umbilical Cord (UC)
The UC contains two arteries and one vein surrounded by Wharton’s jelly (WJ), matrix, and connective tissue of the UC, which is rich in proteoglycans and hyaluronic acid. It is revealed that MSCs from UC contain two subpopulations of 11 and 19 μm average diameter. The small-sized subpopulation expresses a higher level of CD73 and CD90 and exhibits higher proliferative capacity.17
UC can even be freshly processed for as long as 5 days for later cutting while leading to a large enough number of cells, but usually it is processed between 1–24 hours. It should be noted that 2–6 hours is the best interval time from cutting to processing of the umbilical cord.17 Also, after removing its vessels and mincing into 0.5–1 cm sections, the UC can be cryopreserved in 10% DMSO for future use.52
Wharton’s Jelly
Wharton’s jelly (WJ) is a mucoid connective tissue located around the vessels of the UC.36 There are several methods to isolate the cells from Wharton’s jelly. In an easy method, UC vessels are separated and Wharton’s jelly tissue is minced and placed in PBS for 1 hour with stirring. After discarding the remnant tissues, the remaining suspension is centrifuged and the pellet is cultured.53 Also, the minced pieces are placed into the dish during 14 days for explant culture.4,54 WJ pieces can be digested by 2 mg/mL collagenase for 16 hours and then suspended in 2.5% trypsin for 30 minutes,55 or in 75 µg/mL (0.075%) collagenase type II for 30 minutes then in 0.125% trypsin for 30 minutes56 and 1 mg/mL collagenase type B for 3 hours then 0.25% trypsin for 15 minutes with gentle agitation at 37ºC. To date, different enzymatic dosages, types, and times have been examined for obtaining MSCs from WJ. By these methods, the initial cell number is 0.5–1×104 cells per cm of UC and 1×105 cells per gram of WJ.57 Furthermore, WJ can be digested in 1 mg/mL collagenase type B and 0.3 mg/mL hyaluronidase for 2 hours, followed by 0.25% trypsin-EDTA for 15 minutes at 37°C.57 This method leads to the initial cell number of 0.25–5×104 cells/cm of UC and 0.5×105 cells/gram of WJ.10,57 Also, solution containing collagenase type I, collagenase type IV, and 100 IU of hyaluronidase at 37ºC for 45 minutes is used for digestion of the WJ. Through the enzymatic method, it should be separated by the blunt surface of forceps and passed through an 18 G needle for better digestion. The number of isolated cells by this method was 4.7×106 live cells/cm2.56,58,59 The combination of enzymatic and explant methods can lead to good results. The dissected WJ are digested in 4 mg/mL collagenase type I and 0.3–1 mg/mL hyaluronidase for 1 h, followed by 0.1% trypsin-EDTA for 30 minutes at 37°C. Then, WJ pieces are centrifuged and in addition to the cell pellets, the undigested WJ pieces are also cultured in supplemented medium until migration of MSCs.60
Umbilical Cord Vein
UC vessels have both endothelial and mast cells (MCs). For separating MCs, after immersing the whole cord in 70% ethanol for 30 seconds and washing it in PBS, it is dissected to obtain a vein. The vein is minced into small pieces (2 mm3) and is explanted with supplemented medium. Tissue explants are removed after 21 days of culture.61 For enzymatic method, the umbilical vein is cannulated with a catheter and ligated with a cardiac cotton tape and washed internally with PBS or PBS containing 100 µg/mL heparin (PBS-H). Then it is clamped at one end and perfused with 10 mL of 1–4 mg/mL collagenase type I or IV at 37°C for 20–60 minutes. During incubation, cord walls are occasionally massaged to promote enzymatic action via the pressure on underlying UC tissues. Then the clamps are released and the collagenase-containing solution from inside the vein is collected. After centrifugation, the cell pellet is cultivable.62,64 It should be noted that with using 0.25% trypsin for 15 minutes at 37°C only, endothelial cells will be separated from the umbilical vein.65
Umbilical Cord Arteries
The whole cord is immersed in 70% ethanol for 30 seconds, then immediately washed in PBS. After obtaining the arteries and mincing them into small pieces (1–2 mm3), they are explanted for 21 days until the migration of the fibroblast-like adherent cells from the fragments,61,66 or a 4–5 cm length of the artery is isolated from the surrounding matrix and the two ends are ligated with a surgical suture to form a loop. Then, it is filled with 1 mg/mL collagenase and incubated at 37°C for 15 minutes62,67 or even for 18–24 hours.59 Some studies have reported that with open-ended vessels, the cells can be isolated so it may not be necessary to seal the two ends of the blood vessel, and no signs of contamination are observed in the isolated cells.17
Umbilical Cord Lining Membrane
In fact, UC lining membrane is sub-amniotic of the cord. It has two types of cells, MSCs, and epithelial cells. The MSCs can be separated from the sub-amniotic substrate and epithelial cells can be isolated from the amniotic layer. Also MSCs can be isolated by the explant method, as explained before for the other cells.15
Whole Umbilical Cord
In general, MSCs can be isolated from the sub-endothelium, the WJ, the perivascular region, and the vessels.36 But, when the whole UC is digested by enzymes, the cell population obtained by this method may contain endothelial, epithelial, and MCs.17
After being dissected and rinsed in 75% ethanol, the whole cord is explanted.61,68 If the enzyme should be used, small pieces of the cord are digested by 1.25 mg/mL collagenase XI for 1.5 hours,69 or 1 mg/mL collagenase I for 1 hour, and then incubated in the incubator-shaker at 37°C.61
Conclusion and Remark
Taken together, due to the foetal source of extra embryonic tissues and their isolated MSCs, they have specific properties including high proliferative capacity and expansion potential. Thus, the suitable isolation process can reduce the time and passage number. In this way, a commonly used method for MSC isolation from extra embryonic sources is enzymatic digestion employing collagenase, DNase, dispase, and trypsin. However, each tissue has special characteristics that should be taken into consideration during the isolation procedure to get a considerable number of cells. Table 1 shows briefly the conclusions of this review.
 |
Table 1 Brief Conclusions of This Review |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tavassoli A, Matin MM, Niaki MA, Mahdavi-Shahri N, Shahabipour F. Mesenchymal stem cells can survive on the extracellular matrix-derived decellularized bovine articular cartilage scaffold. Iran J Basic Med Sci. 2015;18(12):1221–1227.
2. Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23(8 Suppl 1):S20–S23. doi:10.1097/IJG.0000000000000108
3. Salehinejad P, Alitheen NB, Ali AM, et al. Neural differentiation of human umbilical cord matrix-derived mesenchymal cells under special culture conditions. Cytotechnology. 2015;67(3):449–460. doi:10.1007/s10616-014-9703-6
4. Salehinejad P, Alitheen NB, Mandegary A, Nematollahi-Mahani SN, Janzamin E. Effect of EGF and FGF on the expansion properties of human umbilical cord mesenchymal cells. In Vitro Cell Dev Biol Anim. 2013;49(7):515–523. doi:10.1007/s11626-013-9631-3
5. Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9(1):12. doi:10.1186/1478-811X-9-12
6. Mills SJ, Cowin AJ, Kaur P. Pericytes, mesenchymal stem cells and the wound healing process. Cells. 2013;2(3):621–634. doi:10.3390/cells2030621
7. Wagey R. MSCs in the Bone Marrow Stroma. Stemcell Technologies; 2015.
8. Sandhaanam DS, Pathalam G, Dorairaj S, Savariar V. Mesenchymal Stem Cells (MSC): Identification, Proliferation and Differentiation – A Review Article. PeerJ PrePrints; 2013.
9. Rossi D, Pianta S, Magatti M, Sedlmayr P, Parolini O, Rojas M. Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One. 2012;7(10):e46956. doi:10.1371/journal.pone.0046956
10. Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: from biology to cell therapy. World J Stem Cells. 2010;2(4):81–92. doi:10.4252/wjsc.v2.i4.81
11. Kim EY, Lee KB, Kim MK. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014;47(3):135–140. doi:10.5483/BMBRep.2014.47.3.289
12. Salehinejad P, Alitheen NB, Nematollahi-Mahani SN, et al. Effect of culture media on expansion properties of human umbilical cord matrix-derived mesenchymal cells. Cytotherapy. 2012;14(8):948–953. doi:10.3109/14653249.2012.684377
13. Karasarides M, Chi LA. Handbook of Primary Cell Culture a Practical Manual to the Labtoratory Standard. CHI Scientific; 2007.
14. Vellacott Johnson RP. A Study of the Factors Influencing the Collagenase Digestion Phase of Human and Porcine Islet Isolation. Leicester: Department of Surgery; 2001.
15. Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. doi:10.3727/096368915X686841
16. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202. doi:10.4252/wjsc.v6.i2.195
17. Lv F, Lu M, Cheung KM, Leung VY, Zhou G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Ther. 2012;7(6):389–399. doi:10.2174/157488812804484611
18. Van Pham P, Truong NC, Le PT, et al. Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell Tissue Bank. 2016;17(2):289–302. doi:10.1007/s10561-015-9541-6
19. Yoshioka S, Miura Y, Iwasa M, et al. Isolation of mesenchymal stromal/stem cells from small-volume umbilical cord blood units that do not qualify for the banking system. Int J Hematol. 2015;102(2):218–229. doi:10.1007/s12185-015-1828-7
20. Vasaghi A, Dehghani A, Khademalhosseini Z, Khosravi Maharlooei M, Monabati A, Attar A. Parameters that influence the isolation of multipotent mesenchymal stromal cells from human umbilical cord blood. Hematol Oncol Stem Cell Ther. 2013;6(1):1–8. doi:10.1016/j.hemonc.2013.02.002
21. Revencu T, Trifan V, Nacu L, et al. Collection, isolation and characterization of the stem cells of umbilical cord blood. Rom J Morphol Embryol. 2013;54(2):291–297.
22. Mirabet V, Solves P. Cryopreservation of hematopoietic stem cells from umbilical cord blood for transplantation. In: Stem Cells and Cancer Stem Cells. Vol. 9. Springer; 2013:3–11.
23. In ‘T Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. doi:10.1634/stemcells.2004-0058
24. Pievani A, Scagliotti V, Russo FM, et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy. 2014;16(7):893–905. doi:10.1016/j.jcyt.2014.02.008
25. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi:10.1182/blood-2003-05-1670
26. Mihu CM, Rus Ciuca D, Soritau O, Susman S, Mihu D. Isolation and characterization of mesenchymal stem cells from the amniotic membrane. Rom J Morphol Embryol. 2009;50(1):73–77.
27. Roubelakis MG, Trohatou O, Anagnou NP. Amniotic fluid and amniotic membrane stem cells: marker discovery. Stem Cells Int. 2012;2012:107836. doi:10.1155/2012/107836
28. DeKoninck P, Toelen J, Zia S, et al. Routine isolation and expansion late mid trimester amniotic fluid derived mesenchymal stem cells in a cohort of fetuses with congenital diaphragmatic hernia. Eur J Obstet Gynecol Reprod Biol. 2014;178:157–162. doi:10.1016/j.ejogrb.2014.04.007
29. Zia S, Toelen J, Mori da Cunha M, Dekoninck P, de Coppi P, Deprest J. Routine clonal expansion of mesenchymal stem cells derived from amniotic fluid for perinatal applications. Prenat Diagn. 2013;33(10):921–928. doi:10.1002/pd.4162
30. Savickiene J, Treigyte G, Baronaite S, et al. Human amniotic fluid mesenchymal stem cells from second- and third-trimester amniocentesis: differentiation potential, molecular signature, and proteome analysis. Stem Cells Int. 2015;2015:319238. doi:10.1155/2015/319238
31. Moorefield EC, McKee EE, Solchaga L, et al. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One. 2011;6(10):e26535. doi:10.1371/journal.pone.0026535
32. Sun Q, Li F, Li H, et al. Amniotic fluid stem cells provide considerable advantages in epidermal regeneration: B7H4 creates a moderate inflammation microenvironment to promote wound repair. Sci Rep. 2015;5(1):11560. doi:10.1038/srep11560
33. Shaer A, Azarpira N, Aghdaie MH, Esfandiari E. Isolation and characterization of human mesenchymal stromal cells derived from placental decidua basalis; umbilical cord Wharton’s jelly and amniotic membrane. Pak J Med Sci. 2014;30(5):1022–1026. doi:10.12669/pjms.305.4537
34. Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30(1):2–10. doi:10.1016/j.placenta.2008.09.009
35. Sung HJ, Hong SC, Yoo JH, et al. Stemness evaluation of mesenchymal stem cells from placentas according to developmental stage: comparison to those from adult bone marrow. J Korean Med Sci. 2010;25(10):1418–1426. doi:10.3346/jkms.2010.25.10.1418
36. Lindenmair A, Hatlapatka T, Kollwig G, et al. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells. 2012;1(4):1061–1088. doi:10.3390/cells1041061
37. Kang JW, Koo HC, Hwang SY, et al. Immunomodulatory effects of human amniotic membrane-derived mesenchymal stem cells. J Vet Sci. 2012;13(1):23–31. doi:10.4142/jvs.2012.13.1.23
38. Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells. 2012;4(6):53–61. doi:10.4252/wjsc.v4.i6.53
39. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300–311. doi:10.1634/stemcells.2007-0594
40. Koo BK, Park IY, Kim J, et al. Isolation and characterization of chorionic mesenchymal stromal cells from human full term placenta. J Korean Med Sci. 2012;27(8):857–863. doi:10.3346/jkms.2012.27.8.857
41. Rus Ciuca D, Soritau O, Susman S, Pop VI, Mihu CM. Isolation and characterization of chorionic mesenchyal stem cells from the placenta. Rom J Morphol Embryol. 2011;52(3):803–808.
42. Huang YC, Yang ZM, Chen XH, et al. Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev. 2009;5(3):247–255. doi:10.1007/s12015-009-9069-x
43. Abomaray FM, Al Jumah MA, Alsaad KO, et al. Phenotypic and functional characterization of mesenchymal stem/multipotent stromal cells from decidua basalis of human term placenta. Stem Cells Int. 2016;2016:5184601. doi:10.1155/2016/5184601
44. Kusuma GD, Manuelpillai U, Abumaree MH, Pertile MD, Brennecke SP, Kalionis B. Mesenchymal stem cells reside in a vascular niche in the decidua basalis and are absent in remodelled spiral arterioles. Placenta. 2015;36(3):312–321. doi:10.1016/j.placenta.2014.12.014
45. Pelekanos RA, Sardesai VS, Futrega K, Lott WB, Kuhn M, Doran MR. Isolation and expansion of mesenchymal stem/stromal cells derived from human placenta tissue. J Vis Exp. 2016;(112). doi:10.3791/54204.
46. Katsiani E, Garas A, Skentou C, et al. Chorionic villi derived mesenchymal like stem cells and expression of embryonic stem cells markers during long-term culturing. Cell Tissue Bank. 2016;17(3):517–529. doi:10.1007/s10561-016-9559-4
47. Zhang X, Mitsuru A, Igura K, et al. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006;340(3):944–952. doi:10.1016/j.bbrc.2005.12.091
48. Kmiecik G, Niklinska W, Kuc P, et al. Fetal membranes as a source of stem cells. Adv Med Sci. 2013;58(2):185–195. doi:10.2478/ams-2013-0007
49. Castrechini NM, Murthi P, Gude NM, et al. Mesenchymal stem cells in human placental chorionic villi reside in a vascular niche. Placenta. 2010;31(3):203–212. doi:10.1016/j.placenta.2009.12.006
50. Mathews S, Lakshmi Rao K, Suma Prasad K, et al. Propagation of pure fetal and maternal mesenchymal stromal cells from terminal chorionic villi of human term placenta. Sci Rep. 2015;5(1):10054. doi:10.1038/srep10054
51. Abumaree MH, Al Jumah MA, Kalionis B, et al. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013;9(1):16–31. doi:10.1007/s12015-012-9385-4
52. Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13(12):1477–1486. doi:10.1016/j.bbmt.2007.08.048
53. Beiki B, Zarrabi M, Radmanesh M. A rapid, simple and economical method for the isolationof mesenchymal stem cells from Wharton’s jellyby phosphate buffer saline. Sci J Iran Blood Transfus Organ. 2015;12(2):143–152.
54. Eftekhar-Vaghefi SH, Zahmatkesh L, Salehinejad P, Totonchi S, Shams-Ara A. Evaluation of neurogenic potential of human umbilical cord mesenchymal cells; a time- and concentration-dependent manner. Iran Biomed J. 2015;19(2):82–90. doi:10.6091/ibj.1452.2015
55. Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–1337. doi:10.1634/stemcells.2004-0013
56. Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026.
57. Salehinejad P, Alitheen NB, Ali AM, et al. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev Biol Anim. 2012;48(2):75–83. doi:10.1007/s11626-011-9480-x
58. Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007;15(6):708–718. doi:10.1016/S1472-6483(10)60539-1
59. Sarugaser R, Ennis J, Stanford WL, Davies JE. Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs). Methods Mol Biol. 2009;482:269–279.
60. Azandeh S, Orazizadeh M, Hashemitabar M, et al. Mixed enzymatic-explant protocol for isolation of mesenchymal stem cells from Wharton’s jelly and encapsulation in 3D culture system. J Biomed Sci Eng. 2012;5(10):580–586. doi:10.4236/jbise.2012.510071
61. Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. doi:10.1155/2013/916136
62. Covas DT, Siufi JL, Silva AR, Orellana MD. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36(9):1179–1183. doi:10.1590/S0100-879X2003000900006
63. Kadivar M, Khatami S, Mortazavi Y, Shokrgozar MA, Taghikhani M, Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340(2):639–647. doi:10.1016/j.bbrc.2005.12.047
64. Paladino FV, Peixoto-Cruz JS, Santacruz-Perez C, Goldberg AC. Comparison between isolation protocols highlights intrinsic variability of human umbilical cord mesenchymal cells. Cell Tissue Bank. 2016;17(1):123–136. doi:10.1007/s10561-015-9525-6
65. Du W, Li X, Chi Y, et al. VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity. Stem Cell Res Ther. 2016;7(1):49. doi:10.1186/s13287-016-0297-0
66. Ishige I, Nagamura-Inoue T, Honda MJ, et al. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int J Hematol. 2009;90(2):261–269. doi:10.1007/s12185-009-0377-3
67. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105–110. doi:10.1634/stemcells.21-1-105
68. Petsa A, Gargani S, Felesakis A, Grigoriadis N, Grigoriadis I. Effectiveness of protocol for the isolation of Wharton’s jelly stem cells in large-scale applications. In Vitro Cell Dev Biol Anim. 2009;45(10):573–576. doi:10.1007/s11626-009-9227-0
69. Islam A, Hansen AK, Mennan C, Martinez-Zubiaurre I. Mesenchymal stromal cells from human umbilical cords display poor chondrogenic potential in scaffold-free three dimensional cultures. Eur Cell Mater. 2016;31:407–424. doi:10.22203/eCM.v031a26
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
