Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
An Overview of the Diagnosis and Management of Seborrheic Dermatitis
Authors Dall'Oglio F, Nasca MR , Gerbino C , Micali G
Received 2 February 2022
Accepted for publication 3 July 2022
Published 6 August 2022 Volume 2022:15 Pages 1537—1548
DOI https://doi.org/10.2147/CCID.S284671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Federica Dall’Oglio, Maria Rita Nasca, Carlo Gerbino, Giuseppe Micali
Dermatology Clinic, University of Catania, Catania, Italy
Correspondence: Giuseppe Micali, Dermatology Clinic, University of Catania, Via S. Sofia 78, Catania, 95123, Italy, Tel + 39 095 321705, Fax + 39 095 3782425, Email [email protected]
Abstract: Seborrheic dermatitis (SD) is a common chronic inflammatory skin disorder that mostly affects young adults in areas rich in sebaceous glands (scalp, face, and trunk). In adolescents and adults, SD clinical presentation may range from mild patches to diffuse scalp scaling. In infants, it mainly occurs on the scalp as yellowish, scaly patches (“cradle cap”). In adults, several environmental triggers are likely to promote SD development, along with fungal colonization by Malassezia spp., sebaceous gland activity, as well as immunosuppression, endocrine, neurogenic and iatrogenic factors. In children, early occurrence in the first trimester suggests the role of excessive sebaceous gland activity from maternal hormones, along with cutaneous microbiome alterations. The diagnosis of SD is usually clinical, and specific laboratory and/or instrumental investigations are seldom required. Treatment is aimed at modulating sebum production, reducing skin colonization by Malassezia spp., and controlling inflammation. In adults, mild-to-moderate scalp SD forms can be managed with topical antifungals (ketoconazole, ciclopirox, miconazole) or antiinflammatory (mild-to-moderate potency corticosteroids) or keratolytic/humectant (propylene glycol) agents. Recommended topical therapeutic options for mild-to-moderate facial or body areas SD include topical ketoconazole, ciclopirox, clotrimazole, mild-to-moderate potency corticosteroids, lithium succinate/gluconate, and topical calcineurin inihibitors (off-label use). In severe and/or resistant cases, the use of systemic antifungal drugs (terbinafine, itraconazole), as well as UVB phototherapy, may be considered. In children, scant scientific evidence supports the effectiveness and safety of topical drugs, and “cradle cap” is usually successfully managed with baby shampoos enriched with emollient agents and vegetable oils. Alternatively, similarly to adult scalp SD, medical device shampoos with antiinflammatory and antifungal properties, containing piroctone olamine, bisabolol, alyglicera, telmesteine, may be used. Beyond pharmacological treatments, an appropriate cosmetic approach, if correctly prescribed, may improve therapeutic outcomes.
Keywords: seborrheic dermatitis, diagnosis, therapy, topical, systemic, cosmetics
Introduction
Seborrheic dermatitis (SD) is a common chronic inflammatory skin disorder that most commonly affects young adults, and less often children. In adolescents and adults, SD clinical presentation may range from a mild scalp scaling to diffuse white, yellowish patches in regions rich in sebaceous glands such as scalp, face and trunk.1,2 In infants, SD mainly occurs on the scalp as yellowish, scaly patches, with varying degrees of inflammation, configuring the so-called “cradle cap”.2,3
SD pathogenesis is multifactorial and still poorly delineated. Although some environmental triggers (eg, low temperature and humidity in winter) are likely to promote its development, several other factors, including fungal colonization by Malassezia spp. (formerly called Pityrosporum ovale), sebaceous gland activity, as well as immunosuppression, endocrine, neurogenic and iatrogenic factors, have been postulated.3–6 In children, common early occurrence in the first trimester suggests the role of excessive sebaceous gland activity from maternal hormones, along with cutaneous microbiome alterations, including Malassezia spp., Staphylococcus spp., Streptococcus spp., and Corynebacterium spp.7
The diagnosis of SD is usually easy and based on clinical observation, and specific investigations are rarely required.
Treatment of SD is aimed at modulating sebum production, reducing skin colonization by Malassezia spp., and controlling inflammation. In mild-to-moderate SD forms, topical treatments represent the primary therapeutic approach; in severe and/or resistant cases the off-label use of some topical and systemic drugs, as well as physical treatment with UVB phototherapy, may be considered.
The aim of our study is to provide an updated review on diagnostic investigations and therapeutic management of SD in immunocompetent subjects (adults or children) and in some conditions in which a high prevalence of SD is observed, such as HIV/AIDS, neurologic disorders, Parkinson’s disease, facial paralysis and Down’s syndrome. In addition, some drugs considered as possible contributing SD-like dermatitis factors are also considered.
Materials and Methods
We performed a revision of the literature on PubMed database in the timeframe “January 2017-April 2022” using the following matched key words: “seborrheic dermatitis” OR “diagnosis” OR “therapy” AND “adult” OR “pediatric” AND “HIV” “neurological disorder” “Parkinson’s disease” “facial paralysis “Down syndrome”, “drug”.
All articles and reviews published in English were included, while publication from non-English literature were not considered. Additional publications retrieved by the reference list of included articles were also considered.
Diagnosis
Blood Tests
Traditionally, extensive, severe and refractory SD forms have been considered as a possible dermatological marker of acquired immunodeficiency syndrome (AIDS), at every stage of HIV infection, in both adults and children.3,8 The incidence of SD among seropositive subjects is up to 40%, while in HIV patients, it ranges from 30% to 83% (vs 3% of the seronegative population).3,8 In these patients, a CD4 cell count drop to 450–550 cells/μL is generally observed at SD onset, with further reduction during disease worsening and increase at clinical improvement, respectively.9 Severe and recalcitrant SD forms may also be found in association with inherited (acrodermatitis enteropathica) or acquired neonatal/adult forms of zinc deficiency, as well as with alcohol addiction, alcoholic chronic pancreatitis, hepatitis C virus infection, and nutritional deficiencies (eg, riboflavin, pyridoxine, niacin, and essential fatty acids)2
Dermatoscopy
Dermatoscopy, also called “dermoscopy” or “incident light microscopy”, is a noninvasive technique that allows rapid and magnified in vivo observation of the skin, allowing detection of morphologic features that are invisible to the naked eye. It may be performed with manual devices (magnifications up to ×10) or with digital systems, requiring a video camera equipped with optic fibers and lenses (magnifications up to x1000) (videodermatoscopy). The images obtained may be visualized on a monitor and stored on a personal computer in order to check for any possible change over time.10–12 In clinical practice, dermoscopy may turn out to be crucial to differentiate between scalp SD and other common scaling disorders, such as psoriasis or tinea capitis, based on specific dermoscopy hallmarks.13 In particular, in psoriasis dermoscopy findings include the presence (100% of the cases) of bushy capillaries with twisted loops, whereas in tinea capitis “comma” hair (C-shaped hair shaft with a sharp, slanting end, and homogenous thickness), “corkscrew” hair (twisted or coiled, short, broken hair fragments), “zigzag” hair (hair shaft bent at multiple points) and “morse code” hair (presence of multiple transverse bands or gaps throughout the hair shaft) may be observed.14 Unlike psoriasis and tinea capitis, in SD dotted vessels in a patchy distribution and fine white yellowish scales, along with follicular plugs, orange-yellowish areas, whitish structureless areas and linear branching vessels may be detected.13–15 Finally, in both adults and children, some clinical trials highlight the advantages of dermoscopy as a tool for a more accurate SD severity grading as well as for its therapeutic monitoring and follow-up.16,17
Histological Examination
Histopathologic findings of SD are nonspecific, and different in acute and chronic stages. In the former, spongiosis and psoriasiform hyperplasia are often seen in the epidermis, as well as the classic finding of “shoulder parakeratosis” around follicular openings; superficial perivascular and perifollicular inflammatory infiltrates, mainly consisting of lymphocytes and histiocytes, are also evident in the dermis. On the other hand, chronic SD is characterized by marked psoriasiform epidermal hyperplasia and parakeratosis, with dilation of superficial dermal venules.3 In HIV-related SD, additional findings may characteristically be observed, such as diffuse parakeratosis and keratinocyte necrosis.
Instrumental and/or Laboratory Evaluations
In general, sebutape/sebometry investigations, stratum corneum hydration/skin surface pH measurements, along with microscopic/culture identifications, have no clinical implications, but can provide useful information to define some epidemiological and etiopathogenetic SD aspects. Several experimental in vitro and in vivo investigations have shown excessive sebum excretion (ie, hyperseborrhea) and/or altered barrier function, with a consequent increase in transepidermal water loss (TEWL) and/or skin dysbiosis in SD-affected areas.18–20
Treatment
The available topical, systemic agents and physical treatments for scalp and non-scalp SD (facial and/or body hairy areas) have been classified according to Evidence-Based Medicine (EBM) criteria from level A (strong evidence for efficacy) to E (least evidence of efficacy), based on the quality and relevance of available scientific studies supporting their use.21,22
SD of the Scalp in Adults: Topical Treatment
In adults, the use of topical agents with antifungal (ketoconazole, ciclopirox, miconazole), antiinflammatory (betamethasone valerate, clobetasol propionate) or keratolytic/humectant (propylene glycol) properties is strongly recommended (Table 1).21–24
 |
Table 1 Recommended Pharmacological Topical Agents for Scalp Seborrheic Dermatitis in Adults |
Ketoconazole (KTZ) was introduced in 1979 as the first systemic imidazole compound with activity against a wide spectrum of pathogenic fungi. Its mechanism of action is related to the inhibition of lanosterol C-14-demethylase and consequent blocking of the synthesis of ergosterol, which is an important structural component of fungal cell membranes. In addition, it exerts antiinflammatory activity by inhibiting leukotriene release.25,26 Some microscopy investigations have confirmed the fungicidal activity of KTZ when used topically by a direct necrotizing effect on internal organelles of Malassezia spp. (phenomenon known as “mummifying” effect of azoles).27 Topical KTZ (Level A) is Food and Drug Administration (FDA) approved since 1990 as 2% prescription shampoo for the treatment of moderate-to-severe scalp SD in patients 12 years and older and since 1997 as 1% over-the-counter (OTC) shampoo for scalp “dandruff”.25 In 2006 and 2007, a prescription 2% KTZ gel and foam, respectively, were approved for topical use in SD on multiple body regions including scalp in patients aged ≥12 years.25 A recent systematic review has demonstrated that 2% KTZ shampoo vs 1% KTZ shampoo vs 2.5% selenium sulfide shampoo was statistically superior in reducing mean erythema, scaling, and itch.25 The recommended frequency of use of 2% KTZ shampoo is twice weekly over 4 weeks.28 Its intermittent use (such as once weekly for 6 months), has been shown to be effective vs vehicle (19% vs 47%) in preventing SD relapse.28 Continuative use (twice daily for up to 12 months) of 2% KTZ foam has shown a high safety profile.29 A dated double-blind, controlled trial showed a fast efficacy of 2% KTZ scalp gel (twice weekly for 4 weeks) and a low rate of recurrence after its discontinuation (mean 2 weeks).30 The efficacy of 2% KTZ scalp foaming gel vs vehicle vs 0.05% betamethasone dipropionate lotion in reducing the mean score of erythema and scaling has been evaluated in a three-phase study.31
Ciclopirox (Level A) (also called ciclopirox olamine) is a synthetic, hydroxypyridone-derivative with broad antifungal spectrum, FDA approved for scalp adult SD as prescription 0.77% gel and 1% shampoo in 1997 and 2003, respectively.32 Stronger concentrations of ciclopirox (1.5%) are also available in other countries (ie, Canada, Ireland, Europe) as prescriptions or OTC products.32 Its mechanism of action is mainly related to the inhibition of fungal metal-dependent enzymes, with consequent alteration and damage to mitochondria and cell membranes.33,34 In addition, ciclopirox has anti-inflammatory properties related to the inhibition of prostaglandins and leukotrienes release and activity against Gram-positive and Gram-negative microorganisms.34 The clinical efficacy of ciclopirox 0.77% gel/1-1.5% shampoo vs vehicle in moderate-to-severe scalp SD has been evaluated in several double-blind, randomized, controlled trials, showing no statistically significant difference in clinical response for higher vs lower concentration.23,33 Twice-weekly use for 4 weeks appears to be an optimal treatment schedule.28 Miconazole (Level A) is an imidazole antifungal agent able to inhibit the biosynthesis of ergosterol, a major component of fungal cell membranes. A dated double-blind, controlled trial showed greater efficacy of a combination based on 2% miconazole/1% hydrocortisone solution, either when applied once daily for 3 weeks vs 2% miconazole or 1% hydrocortisone alone or when used twice monthly as prophylactic agent vs 1% hydrocortisone alone.35 The use of topical potent (betamethasone valerate) to super potent (clobetasol propionate) corticosteroids (Level A) (class III–IV according to Anatomical Therapeutic Chemical [ATC] classification) with anti-inflammatory, immunosuppressive and antiproliferative actions allows to achieve quick control of erythema, scaling and pruritus.24,36 However, prolonged use is not recommended, due to their possible side effects, such as atrophy and telangiectasias.24 Propylene glycol (PG) (Level A) is an aliphatic organic compound used for dermatological topical products due to its keratolytic and humectant properties, along with its antimicrobial action.37 A topical solution formulated for scalp use based on 15% PG + 50% ethanol + 35% water solution has been demonstrated to be an effective alternative option in the treatment of scalp SD when compared to vehicle (50% ethanol + 50% water).38 Although effective, PG is well known for its potential local irritative and allergenic effects, especially when it is used at high concentrations.39
Miscellaneous
Limited clinical evidence supports the use of the following products: 2.5% selenium sulfide (Level C) shampoo,40 1% bifonazole shampoo/lotion (used as monotherapy for 3 times/week for 6 weeks or in combination with 40% urea in case of severe dandruff),6,23,41 2% fluconazole shampoo (twice weekly for 4 weeks),42 1% naftifine hydrochloride gel (twice weekly for 4 weeks),43 0.5% piroctone olamine + 0.45% climbazole shampoo (three times a week for 4 weeks),44 0.1% lipohydroxy acid + 1.3% salicylic acid (every 2 days for 4 weeks),45 and urea + lipohydroxy acid + propylene glycol scalp solution (once daily for 4 weeks).46
In moderate-to-severe SD scalp forms, especially if itchy, there has recently been a growing interest in some FDA-approved shampoos as medical devices. These products contain non-steroidal anti-inflammatory (glycyrrhetinic acid, bisabolol) and antifungal (1% piroctone olamine, lactoferrin) (AIAF) agents able to manage and relieve scalp SD symptoms, as reported in the Cochrane Database Review and in some clinical trials.17,24 In particular, a randomized, investigator single-blind trial using clinical and trichoscopic evaluation has demonstrated a similar significant efficacy of AIAF shampoo vs 1% KTZ shampoo in reducing mean score of erythema and scaling of scalp SD at 4 and 8 weeks of treatment compared to baseline, and a significant reduction of pruritus only for AIAF shampoo at 4 weeks.17 The suggested use of AIAF products is once or twice weekly for both acute and maintenance therapy.17
SD of the Scalp in Children: Topical Treatment
In pediatric age (from birth up to 24 months), scant scientific evidence supports the effectiveness and safety of topical antifungals (1% ciclopirox shampoo, 1% KTZ cream/shampoo, 2.5% SS shampoo), anti-inflammatory (1% hydrocortisone cream/lotion) or keratolytic agents (3% salicylic acid in combination with 1.5% ciclopirox shampoo, lactamide monoethanolamine shampoo).47,48 Generally, the mainstay treatments of “cradle cap” include the use of baby shampoos enriched with emollient agents (ie, shea butter, glycerin) and vegetable oils (ie, olive oil, borage oil, almond oil), followed by gentle mechanical removal of loosen scales. Similar to adult scalp SD, AIAF medical device shampoos, as confirmed by Cochrane Review and by some clinical trials, may be used.16,47–49
Non-Scalp Seborrheic Dermatitis
The use of topical antifungal (KTZ, ciclopirox, clotrimazole) and antiinflammatory (desonide, hydrocortisone, lithium succinate/gluconate, topical pimecrolimus/tacrolimus) agents is strongly recommended for mild-to-moderate SD on face and/or body areas (Table 2).28,50–52
 |
Table 2 Recommended Pharmacological Topical Agents for Facial Seborrheic Dermatitis in Adults |
SD of the Non-Scalp Area in Adults: Topical Treatment
Recommended topical therapeutic options for mild-to-moderate facial and/or body areas SD include 2% KTZ (Level A), FDA approved in patients aged 12 years or older as cream or gel in 2002 and 2006, respectively.25 In a double-blind, randomized comparative study, the efficacy and tolerability of 2% KTZ cream vs 1% hydrocortisone cream, applied once daily for 4 weeks, showed no significant statistical differences in both groups (80.5% vs 94.4%)51,53 Other topical formulations include 2% foam or gel, the latter being superior vs vehicle vs 0.05% betamethasone dipropionate lotion in reducing mean erythema and scaling scores in a three-phase study.51,54 Recently, an in vivo and in vitro study has shown significant reduction of Malassezia count by KTZ using Polymerase Chain Reaction (PCR)-based investigations.55
Ciclopirox 1% cream (Level A) showed significantly higher clinical efficacy vs vehicle when applied twice daily for 29 days and no significant difference vs 2% KTZ foaming gel when applied twice daily for 28 days followed by once-daily for another 28 days (maintenance phase).56,57 The antiinflammatory properties in facial SD of 1% clotrimazole cream (Level A) vs corticosteroids vs Emu oil (a natural antiinflammatory/antioxidant agent) has been evaluated in a randomized intra-patient controlled study, showing a significant improvement of scaling in both groups.23,58 As regards to the use of mild (hydrocortisone) to moderate potency (desonide) corticosteroids (class I–II according to ATC classification), the superior clinical efficacy of 1% hydrocortisone cream (Level A) vs 2% KTZ cream, once daily for 4 weeks, has been demonstrated in a double-blind clinical trial (94.4% vs 80.5%) that has also shown a low incidence of side effects in both groups.24,53 Although effective, especially in acute stage SD or in secondary SD due to immunosuppression (eg, association with HIV infection),59 the prolonged use of topical corticosteroids is not recommended, because of their possible local side effects that may occur with long-term use50 Lithium succinate/gluconate (Level A) ointment has anti-inflammatory and antifungal effects by inhibiting the production of arachidonic acid, the release of the all-free fatty acids and the Toll-like receptor expression.60 Two randomized, multicenter studies on 8% lithium gluconate ointment showed its higher efficacy vs vehicle (90.9% vs 54.7%) vs 2% KTZ emulsion (52% vs 30.1%) when applied twice daily for 8 weeks.61,62 The off-label use of topical calcineurin inhibitors (TCIs) (1% pimecrolimus cream and 0.003% cream/0.1% tacrolimus ointment) (Level A), FDA-approved macrolide lactones for the treatment of atopic dermatitis in patients over 2 years of age, may represent a possible alternative to 0.1% methylprednisolone aceponate cream or 2% KTZ cream, having demonstrated, beyond anti-inflammatory properties, fungicidal activity against M. furfur.63–65 Their recommended frequency of use is twice daily over 4–8 weeks in SD acute phase or once-weekly for 12 weeks as maintenance therapy.51
Miscellaneous
Alternative topical options include 0.75% metronidazole gel (Level B) (antiinflammatory action through inhibition of release of inflammatory mediators and of lymphocyte chemotaxis, along with antioxidant activity by reducing the production of reactive oxygen species), applied twice daily for 8 weeks.66,67 One percent terbinafine cream (Level C) (antifungal agent against dermatophytes, molds, dimorphic fungi, and M. furfur, with antioxidant and anti-inflammatory properties).68 Similar to scalp SD, AIAF cream containing 1% piroctone olamine, 1.2% bisabolol, 1% alglycera, and 0.01% telmesteine may be used in adult non-scalp SD, both in acute phase and maintenance therapy, as well as in case of no improvement with conventional topical agents.1,24 The efficacy of AIAF cream has been evaluated in an open-label, prospective, unblinded, intra-patient, controlled, clinical trial that showed in the target area a significant reduction in erythema score since day 7 compared to baseline.69
SD of the Non-Scalp Area in Infants: Topical Treatment
In infants and young children with mild-to-moderate facial/body SD, limited evidence supports the use of a topical pharmacological approach, due to scarce data and to the possibility of systemic drug absorption in newborns.47,48 The use of natural antiinflammatory/antioxidants (eg, stearyl glycyrrhetinate, Aloe vera, vitamin E, Echinacea purpurea, lactoferrin) and/or emollient agents (eg, hyaluronic acid) as gel or cream, as well as AIAFp products, may be considered.47
SD in Adults: Systemic Treatment
Systemic antifungals (terbinafine, itraconazole) are mainly indicated in acute and/or severe and/or resistant adult SD forms, as well as in selected and difficult-to-treat conditions.21 In these cases, the goal of systemic approach is the prompt reduction of symptoms and the possibility to use topical agents as a maintenance therapy.
Terbinafine (Level A), is a lipophilic molecule able to create a cutaneous reservoir of effective drug concentrations even after its withdrawal.70 Its action is mainly related to its antimycotic properties against dermatophytes, molds, dimorphic fungi, as well as pathogenic yeasts, including some Malassezia furfur strains.71 When used orally, it is administered at a dosage of 250 mg/day for 4–6 weeks or 250 mg/day for the first 12 days of the month for 3 consecutive months (pulse regimen). It has shown superior efficacy vs moisturizing ointment vs placebo and a safe pharmacological profile with low incidence of side effects.72–74 Itraconazole (Level B) is a highly keratinophilic and lipophilic triazole agent secreted with sebum at the stratum corneum level where Malassezia colonies are generally present.75 It also has an anti-inflammatory effect related to inhibition of 5-hypoxygenase metabolites’ synthesis, which are involved in several inflammatory diseases, including SD.76,77 Its lipophilicity allows for a prolonged drug persistence in the skin and its appendages even after treatment discontinuation.78 A study conducted on 29 SD patients demonstrated a marked reduction in inflammation as well as an improvement in SD symptoms after 200 mg/day itraconazole for a week followed by drug administration at a resuming dosage of 200 mg/day for the first 2 days of the month for the following 2 months.79 Moreover, SD patients treated with 150–200 mg/day itraconazole once a week for a period of 2 or 3 months showed complete clearing/marked improvement at the end of the treatment in 67% of the cases.2,80
SD in Children: Systemic Treatment
In children, limited and conflicting data support the benefit of oral biotin supplementation (4 mg/day for 4 weeks) vs placebo as shown in a dated double-blind, crossover trial.47,48,81
SD in Adult: Physical Treatment
SD patients often experience improvement during the summer. An antiinflammatory and/or inhibiting effect on Malassezia yeasts cultured from the skin as a result of UVA and UVB light exposure has been demonstrated by some in vitro studies.82,83 However, limited clinical evidence supports the advantages of UVB phototherapy (Level C) in diffuse and resistant SD forms.82 There is only one prospective, open-label clinical study showing narrow-band UVB phototherapy, three times weekly up to a maximum of 8 weeks, to be effective in improving severe SD, with complete clearance in 6 patients and marked improvement in 12.83
Miscellaneous
Conditions associated with a higher incidence and/or a more refractory course of SD include HIV-infection, neurological diseases, and Down’s syndrome. Additionally, in case of SD-like dermatitis, the causative role of some systemic drugs should be ruled out. In HIV-infected patients, SD is generally not only more prevalent but also more severe than usual, and frequently associated to a relapsing course (Figure 1).59,84 Several factors are likely to promote its development, including immune system failure causing an overgrowth of Malassezia yeast. Mild SD forms, in either children or adults, may be treated with topical 2% ketoconazole, two to three times per week for 4 weeks, with a once weekly maintenance treatment as needed. In case of moderate-to-severe SD, the therapy may be challenging, as these forms generally show poor response to topical and systemic antifungals and/or to mid- to high-potency corticosteroids.84 In these cases, the control of CD4+ values with antiretroviral drugs may be helpful to achieve SD improvement. The high prevalence of SD in patients with neurological disorders, such as Parkinson’s disease, pyramidal syndromes, depression, as well as traumatic spinal cord injury, cerebrovascular accidents, epilepsy, and facial nerve paralysis, has been widely acknowledged. In particular, unilateral facial SD has frequently been observed in longstanding Parkinson’s disease (“masked facies” or “hypomimia”) as well as in subjects with paralysis occurring after cerebrovascular accidents. The mechanisms underlying sebum changes in bilateral seborrhea in unilateral Parkinsonism could be regulated endocrinologically rather than neurologically.3 The increased sebum production rate, coupled with reduced mimic facial movements, may provide permissive conditions for Malassezia proliferation.85 The mainstay of SD treatment in Parkinson’s disease patients consists of topical antifungals, such as ketoconazole, and antiinflammatory agents, including corticosteroids and topical calcineurin inhibitors. Systemic antifungals, although effective, should be avoided for the risk of neurological side effects (eg, paresthesia, dysesthesia).86 Since poor hygiene has also been considered among SD predisposing factors in Parkinson subjects, daily cleaning of the affected areas with oil-free detergents is recommended. Severe facial and/or scalp SD forms are commonly observed in Down’s syndrome. In this case, the pathogenesis is still poorly delineated, although a role of immune dysregulation in promoting Malassezia proliferation seems likely.87 Mild-to-moderate SD forms can be managed with topical antifungals or antiinflammatory agents (mild-to-moderate potency corticosteroids). In severe and/or resistant cases, the use of systemic antifungals may be considered. Finally, beyond Malassezia, several drugs (such as buspirone, chlorpromazine, cimetidine, ethionamide, 5-fluorouracil, gold salts, griseofulvin, haloperidol, hydrochloride, interferon-α, interleukin-2, lithium salts, methyldopa, psoralen, stanazole, testosterone) are known to occasionally induce an SD-like dermatitis, although their precise mechanism of action still remains unknown.2 Their treatment is similar to that of SD unrelated to drug use, and discontinuation of the offending drug is recommended.
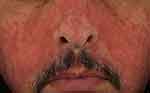 |
Figure 1 HIV-related seborrheic dermatitis. |
Discussion
Diagnosis of SD is usually easily made based on past history and typical clinical features. However, in selected cases, especially in more severe and/or recurrent forms, blood tests to rule out HIV infection, nutritional deficiencies, as well as dermatoscopy, useful to identify other dermatoses (eg, psoriasis or tinea capitis), are recommended. Nevertheless, the presence of concomitant precipitating and/or aggravating factors, including neurological/psychiatric disorders or iatrogenic factors, must always be considered. Because of its chronic relapsing course, treatment of SD should be effective, but not aggressive. The therapeutic approach should be selected according to the extension, severity, and location of the disease. In order to achieve the best clinical outcome, it is also important to consider patient’s age, immune status and response to previous therapies, taking into account any concurrent disorders.
The most commonly used drugs for SD include topical azole agents, as monotherapy or in association with corticosteroids when a fast control of inflammation is advisable. The clinical efficacy of the above agents is widely demonstrated, although their prolonged use may sometimes be related to some local adverse effects, such as irritant contact dermatitis from the use of antifungals. In addition, the use of topical corticosteroids should be discouraged, due to the risk of consistent skin atrophy, striae, telangiectasias, folliculitis, hypopigmentation, and tachyphylaxis. Nonetheless, there is a wide range of topical agents worth considering, with the exception of zinc pyrithione that has recently been withheld and classified as carcinogenic, mutagenic or toxic for reproduction by EU commission regulation law 2021/1902.88 The off-label use of systemic antifungals is burdened by several limitations, including the risk of some systemic side effects, eg, abnormal liver function or blood tests, interactions with other concomitant systemic drug. Therefore, they are recommended in selected cases only. Finally, although the mainstay treatment of SD is a pharmacologic one, the use of appropriate cosmetic products may improve both treatment outcome and patients compliance. The majority of such products contain a mix of botanic ingredients, including Sabal serrulata (sebum controlling), Tea tree oil (anti-microbial),18-β-glycyrrhetinic acid (anti-inflammatory), Aloe vera (soothing), telmesteine and shea butter (moisturizers), that are supposed to act synergistically. Although widely used, as of today, limited clinical evidence supports the use of emollients, able to improve barrier function, in SD (Level D).21,50,51 Another category of non-pharmacologic treatments includes the so-called “cosmeceuticals”, ie, cosmetic products formulated with active ingredients at lower concentrations than those used in OTC or prescription drugs based on specific regulatory directions, variable according to the different countries. They may contain nicotinamide (water-soluble amide with anti-inflammatory and sebum-controlling properties), vitamin E (antioxidant agent), and hyaluronic acid (non-sulfate anionic glycosaminoglycans with moisturizer effect). In order to optimize SD management, their choice should take into consideration the age of patients, the vehicle, the area to be treated and ongoing pharmacological therapy.2
Conclusion
SD patients should be advised that SD is a chronic disease and therefore a complete resolution after topical or systemic therapy is a difficult goal to achieve. Therefore, due to SD relapsing course, maintenance treatment will often be necessary.22 Also, SD subjects should be warned about the various precipitating conditions described above, including intake of some drugs, nutritional deficiency, and concurrent immunosuppression and/or comorbidities. Finally, the role of environmental factors (cold, low humidity, excessive sun exposure), physical/psychological stress, unhealthy lifestyle (alcohol consumption), wrong or inadequate cosmetic use, that may contribute to worsen SD, should also be considered.3 On this last regard, it is important not to let patients freely choose the cosmetic product they prefer, because of the high risk of compromising treatment outcome. For instance, the use of oil/comedogenic products may cause SD worsening, whereas aggressive overtreatment may induce xerosis, and/or irritant/allergic contact dermatitis.
Acknowledgments
The manuscript is an original unpublished work and it is not submitted for publication elsewhere.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheong WK, Yeung CK, Torsekar RG, et al. Treatment of seborrhoeic dermatitis in Asia: a consensus guide. Skin Appendage Disorders. 2015;1(4):187–196. doi:10.1159/000444682
2. Micali G, Veraldi S, Kwong CW, Suh DH, eds. Seborrheic Dermatitis.
3. Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2). doi:10.13188/2373-1044.1000019
4. Araya M, Kulthanan K, Jiamton S. Clinical characteristics and quality of life of seborrheic dermatitis patients in a tropical country. Indian J Dermatol. 2015;60(5):519.
5. Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31(4):343–351.
6. Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18(1):13–26.
7. Schoch JJ, Monir RL, Satcher KG, Harris J, Triplett E, Neu J. The infantile cutaneous microbiome: a review. Pediatr Dermatol. 2019;36(5):574–580.
8. Cedeno-Laurent F, Gómez-Flores M, Mendez N, et al. New insights into HIV-1-primary skin disorders. J Int AIDS Soc. 2011;24(14):5.
9. Naldi L, Rebora A. Clinical practice. Seborrheic dermatitis. N Engl J Med. 2009;360(4):387–396.
10. Golińska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;228(3):412-421.
11. Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb). 2016;6(4):471–507.
12. Lacarrubba F, Micali G, Tosti A. Scalp dermoscopy or trichoscopy. Curr Probl Dermatol. 2015;47:21–32.
13. Lacarrubba F, Verzì AE, Dinotta F, Scavo S, Micali G. Dermatoscopy in inflammatory and infectious skin disorders. G Ital Dermatol Venereol. 2015;150(5):521–531.
14. Lacarrubba F. Clinical and trichoscopic aspects of scalp psoriasis: commentary to ‘Clinical and trichoscopic features in various forms of scalp psoriasis’ by F. Bruni et al. J Eur Acad Dermatol Venereol. 2021;35(9):1744–1745.
15. Lacarrubba F, Verzì AE, Micali G. Newly described features resulting from high-magnification dermoscopy of tinea capitis. JAMA Dermatol. 2015;151(3):308–310.
16. Micali G, Pulvirenti N, Dall’Oglio F, Tedeschi A, Quattrocchi E, Lacarrubba F. Treatment of cradle cap in infants with a new cosmetic non-steroidal gel cream: clinical, laboratory, and instrumental evaluation. J Cosmet Dermatol. 2021;20 Suppl 1(Suppl1):14–17.
17. Dall’Oglio F, Lacarrubba F, Verzì AE, Micali G. Noncorticosteroid combination shampoo versus 1% ketoconazole shampoo for the management of mild-to-moderate seborrheic dermatitis of the scalp: results from a randomized, investigator-single-blind trial using clinical and trichoscopic evalutation. Skin Appendage Disords. 2015;1(3):126–130.
18. Tao R, Li R, Wang R. Skin microbiome alterations in seborrheic dermatitis and dandruff: a systematic review. Exp Dermatol. 2021;30(10):1546–1553.
19. Lin Q, Panchamukhi A, Li P, et al. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst Eng. 2021;44(5):965–975.
20. Turner GA, Hoptroff M, Harding CR. Stratum corneum dysfunction in dandruff. Int J Cosmet Sci. 2012;34(4):298–306.
21. Dhawan GK, Coulson IH. Seborrheic eczema. In: Lebwohl MG, Heymann WR, Coulson IH, Murrell DF, editors. Treatment of Skin Disease: Comprehensive Therapeutic Strategies.
22. Hald M, Arendrup MC, Svejgaard EL, Lindskov R, Foged EK, Saunte DM. Danish Society of Dermatology. Evidence-based Danish guidelines for the treatment of Malassezia-related skin diseases. Acta Derm Venereol. 2015;95(1):12–19.
23. Okokon EO, Verbeek JH, Ruotsalainen JH, Ojo OA, Bakhoya VN. Topical antifungals for seborrhoeic dermatitis. Cochrane Database Syst Rev. 2015;2(5):CD008138.
24. Kastarinen H, Oksanen T, Okokon EO, et al. Topical anti-inflammatory agents for seborrhoeic dermatitis of the face or scalp. Cochrane Database Syst Rev. 2014;2014(5):CD009446.
25. Choi FD, Juhasz MLW, Atanaskova Mesinkovska N. Topical ketoconazole: a systematic review of current dermatological applications and future developments. J Dermatolog Treat. 2019;30(8):760–771.
26. Chowdhry S, Gupta S, D’souza P. Topical antifungals used for treatment of seborrheic dermatitis. J Bacteriol Mycol. 2017;4(1):1–7.
27. Faergemann J, Borgers M, Degreef H. A new ketoconazole topical gel formulation in seborrhoeic dermatitis: an updated review of the mechanism. Expert Opin Pharmacother. 2007;8(9):1365–1371.
28. Del Rosso JQ. Adult seborrheic dermatitis: a status report on practical topical management. J Clin Aesthet Dermatol. 2011;4(5):32–38.
29. Draelos ZD, Feldman SR, Butners V, Alió Saenz AB. Long-term safety of ketoconazole foam 2% in the treatment of seborrheic dermatitis: results of a Phase IV, open-label study. J Drugs Dermatol. 2013;12(1):e1–6.
30. Cauwenbergh G, De Doncker P, Schrooten P, Degreef H. Treatment of dandruff with a 2% ketoconazole scalp gel. A double-blind placebo-controlled study. Int J Dermatol. 1986;25(8):541.
31. Ortonne JP, Lacour JP, Vitetta A, Le Fichoux Y. Comparative study of ketoconazole 2% foaming gel and betamethasone dipropionate 0.05% lotion in the treatment of seborrhoeic dermatitis in adults. Dermatology. 1992;184(4):275–280.
32. Ciclopirox olamine: summary report. Available from: https://archive.hshsl.umaryland.edu/handle/10713/12089.
33. Subissi A, Monti D, Togni G, Mailland F. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70(16):2133–2152.
34. Gupta AK. Ciclopirox: an overview. Int J Dermatol. 2001;40(5):305–310.
35. Faergemann J. Seborrhoeic dermatitis and Pityrosporum orbiculare: treatment of seborrhoeic dermatitis of the scalp with miconazole-hydrocortisone (Daktacort), miconazole and hydrocortisone. Br J Dermatol. 1986;114(6):695–700.
36. Ortonne JP, Lacour JP, Vitetta A, Le Fichoux Y. Efficacious and safe management of moderate to severe scalp seborrhoeic dermatitis using clobetasol propionate shampoo 0·05% combined with ketoconazole shampoo 2%: a randomized, controlled study. Br J Dermatol. 2011;165(1):171–176.
37. Catanzaro JM, Smith JG. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24(1):90–95.
38. Faergemann J. Propylene glycol in the treatment of seborrheic dermatitis of the scalp: a double-blind study. Cutis. 1988;42(1):69–71.
39. McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29(1):6–12.
40. Danby FW, Maddin WS, Margesson LJ, Rosenthal D. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol. 1993;29(6):1008–1012.
41. Bukvić Mokos Z, Kralj M, Basta-Juzbašić A, Lakoš Jukić I. Seborrheic dermatitis: an update. Acta Dermatovenerol Croat. 2012;20(2):98–104.
42. Rigopoulos D, Katsambas A, Antoniou C, Theocharis S, Stratigos J. Facial seborrheic dermatitis treated with fluconazole 2% shampoo. Int J Dermatol. 1994;33(2):136–137.
43. Gold MH, Bridges T, Avakian EV, Plaum S, Fleischer AB, Hardas B. An open-label pilot study of naftifine 1% gel in the treatment of seborrheic dermatitis of the scalp. J Drugs Dermatol. 2012;11(4):514–518.
44. Schmidt-Rose T, Braren S, Fölster H, et al. Efficacy of a piroctone olamine/climbazole shampoo in comparison with a zinc pyrithione shampoo in subjects with moderate to severe dandruff. Int J Cosmet Sci. 2011;33(3):276–282.
45. Seite S, Rougier A, Talarico S. Randomized study comparing the efficacy and tolerance of a lipohydroxy acid shampoo to a ciclopiroxolamine shampoo in the treatment of scalp seborrheic dermatitis. J Cosmet Dermatol. 2009;8(4):249–253.
46. Emtestam L, Svensson Å, Rensfeldt K. Treatment of seborrhoeic dermatitis of the scalp with a topical solution of urea, lactic acid, and propylene glycol (K301): results of two double-blind, randomised, placebo-controlled studies. Mycoses. 2012;55(5):393–403.
47. Hassan S, Szeto MD, Sivesind TE, et al. From the Cochrane Library: interventions for infantile seborrheic dermatitis (including cradle cap). J Am Acad Dermatol. 2022;66(2):e87-88..
48. Victoire A, Magin P, Coughlan J, van Driel ML. Interventions for infantile seborrhoeic dermatitis (including cradle cap). Cochrane Database Syst Rev. 2019;3(3):CD011380.
49. David E, Tanuos H, Sullivan T, Yan A, Kircik LH. A double-blind, placebo-controlled pilot study to estimate the efficacy and tolerability of a nonsteroidal cream for the treatment of cradle cap (seborrheic dermatitis). J Drugs Dermatol. 2013;12(4):448–452.
50. Piquero-Casals J, Hexsel D, Mir-Bonafé JF, Rozas-Muñoz E. Topical non-pharmacological treatment for facial seborrheic dermatitis. Dermatol Ther (Heidelb). 2019;9(3):469–477.
51. Gupta AK, Versteeg SG. Topical treatment of facial seborrheic dermatitis: a systematic review. Am J Clin Dermatol. 2017;18(2):193–213.
52. Berk T, Scheinfeld N. Seborrheic dermatitis. P T. 2010;35(6):348–352.
53. Stratigos JD, Antoniou C, Katsambas A, et al. Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrheic dermatitis. A double-blind comparative study. J Am Acad Dermatol. 1988;19(5 Pt 1):850–853.
54. Elewski BE, Abramovits W, Kempers S, et al. A novel foam formulation of ketoconazole 2% for the treatment of seborrheic dermatitis on multiple body regions. J Drugs Dermatol. 2007;6(10):1001–1008.
55. Tao R, Wang R, Wan Z, et al. Ketoconazole 2% cream alters the skin fungal microbiome in seborrheic dermatitis: a cohort study. Clin Exp Dermatol. 2022;47(6):1068-1096.
56. Unholzer A, Varigos G, Nicholls D, et al. Ciclopiroxolamine cream for treating seborrheic dermatitis: a double-blind parallel group comparison. Infection. 2002;30(6):373–376.
57. Chosidow O, Maurette C, Dupuy P. Randomized, open-labeled, non-inferiority study between ciclopiroxolamine 1% cream and ketoconazole 2% foaming gel in mild to moderate facial seborrheic dermatitis. Dermatology. 2003;206(3):233–240.
58. Attarzadeh Y, Asilian A, Shahmoradi Z, Adibi N. Comparing the efficacy of Emu oil with clotrimazole and hydrocortisone in the treatment of seborrheic dermatitis: a clinical trial. J Res Med Sci. 2013;18(6):477–481.
59. Supanaranond W, Desakorn V, Sitakalin C, Naing N, Chirachankul P. Cutaneous manifestations in HIV positive patients. Southeast Asian J Trop Med Public Health. 2001;32(1):171–176.
60. Cuelenaere C, De Bersaques J, Kint A. Use of topical lithium succinate in the treatment of seborrhoeic dermatitis. Dermatology. 1992;184(3):194–197.
61. Dreno B, Chosidow O, Revuz J, Moyse D. Study Investigator Group. Lithium gluconate 8% vs ketoconazole 2% in the treatment of seborrhoeic dermatitis: a multicentre, randomized study. Br J Dermatol. 2003;148(6):1230–1236.
62. Dreno B, Moyse D. Lithium gluconate in the treatment of seborrhoeic dermatitis: a multicenter, randomised, double-blind study versus placebo. Eur J Dermatol. 2002;12(6):549–552.
63. Ang-Tiu CU, Meghrajani CF, Maano CC. Pimecrolimus 1% cream for the treatment of seborrheic dermatitis: a systematic review of randomized controlled trials. Expert Rev Clin Pharmacol. 2012;5(1):91–97.
64. Ozden MG, Tekin NS, Ilter N, Ankarali H. Topical pimecrolimus 1% cream for resistant seborrheic dermatitis of the face: an open-label study. Am J Clin Dermatol. 2010;11(1):51–54.
65. Cook BA, Warshaw EM. Role of topical calcineurin inhibitors in the treatment of seborrheic dermatitis: a review of pathophysiology, safety, and efficacy. Am J Clin Dermatol. 2009;10(2):103–118.
66. Ozcan H, Seyhan M, Yologlu S. Is metronidazole 0.75% gel effective in the treatment of seborrhoeic dermatitis? A double-blind, placebo controlled study. Eur J Dermatol. 2007;17(4):313–316.
67. Parsad D, Pandhi R, Negi KS, Kumar B. Topical metronidazole in seborrheic dermatitis-a double-blind study. Dermatology. 2001;202(1):35–37.
68. Gündüz K, Inanir I, Sacar H. Efficacy of terbinafine 1% cream on seborrhoeic dermatitis. J Dermatol. 2005;32(1):22–25.
69. Dall’Oglio F, Tedeschi A, Guardabasso V, Micali G. Evaluation of a topical anti-inflammatory/antifungal combination cream in mild-to-moderate facial seborrheic dermatitis: an intra-subject controlled trial examining treated vs. untreated skin utilizing clinical features and erythema-directed digital photography. J Clin Aesthet Dermatol. 2015;8(9):33–38.
70. Ryder NS. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol. 1992;126(Suppl 39):2–7.
71. Leeming JP, Sansom JE, Burton JL. Susceptibility of Malassezia furfur subgroups to terbinafine. Br J Dermatol. 1997;137(5):764–767.
72. Gupta AK, Richardson M, Paquet M. Systematic review of oral treatments for seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2014;28(1):16–26.
73. Vena GA, Micali G, Santoianni P, Cassano N, Peruzzi E. Oral terbinafine in the treatment of multi-site seborrheic dermatitis: a multicenter, double-blind placebo-controlled study. Int J Immunopathol Pharmacol. 2005;18(4):745–753.
74. Cassano N, Amoruso A, Loconsole F, Vena GA. Oral terbinafine for the treatment of seborrheic dermatitis in adults. Int J Dermatol. 2002;41(11):821–822.
75. Das J, Majumdar M, Chakraborty U, Majumdar V, Mazumdar G, Nath J. Oral itraconazole for the treatment of severe seborrhoeic dermatitis. Indian. J Dermatol. 2011;56(5):515–516.
76. Trznadel-Grodzka E, Błaszkowski M, Rotsztejn H. Investigations of seborrheic dermatitis. Part II. Influence of itraconazole on the clinical condition and the level of selected cytokines in seborrheic dermatitis. Dosw (Online). 2012;14(66):848–854.
77. Shemer A, Kaplan B, Nathansohn N, Grunwald MH, Amichai B, Trau H. Treatment of moderate to severe facial seborrheic dermatitis with itraconazole: an open non-comparative study. Isr Med Assoc J. 2008;10(6):417–418.
78. Ghodsi SZ, Abbas Z, Abedeni R. Efficacy of oral itraconazole in the treatment and relapse prevention of moderate to severe seborrheic dermatitis: a randomized, placebo-controlled trial. Am J Clin Dermatol. 2015;16(5):431–437.
79. Kose O, Erbil H, Gur AR. Oral itraconazole for the treatment of seborrhoeic dermatitis: an open, noncomparative trial. J Eur Acad Dermatol Venereol. 2005;19(2):172–175.
80. Mastaro H. Treatment of seborrhoeic dermatitis with antifungal drugs. J Clin Exp Med. 1995;173:1026–1027.
81. Keipert JA. Oral use of biotin in seborrhoeic dermatitis of infancy: a controlled trial. Med J Aust. 1976;1(16):584–585.
82. Stefanaki I, Katsambas A. Therapeutic update on seborrheic dermatitis. Skin Therapy Lett. 2010;15(5):1–4.
83. Pirkhammer D, Seeber A, Hönigsmann H, Tanew A. Narrow-band ultraviolet B (ATL-01) phototherapy is an effective and safe treatment option for patients with severe seborrhoeic dermatitis. Br J Dermatol. 2000;143(5):964–968.
84. World Health Organization. Guidelines on the treatment of skin and oral HIV-associated conditions in children and adults. Geneva:World Health Organization; 2014. Evidence and recommendations on seborrhoeic dermatitis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK305403/.
85. Niemann N, Billnitzer A, Jankovic J. Parkinson’s disease and skin. Parkinsonism Relat Disord. 2021;82:61–76.
86. Eşkut N, Gedizlioğlu M, Ünal O, Özlü C, Ergene U. Acute fluconazole toxicity: a case presenting with protean manifestations including systemic and neurologic symptoms. Postgrad Med. 2021;133(2):250–252.
87. Ryan C, Vellody K, Belazarian L, Rork JF. Dermatologic conditions in Down syndrome. Pediatr Dermatol. 2021;38(Suppl 2):49–57.
88. EUR-Lex. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32021R1902.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
