Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
An Overview of Soft Tissue Fillers for Cosmetic Dermatology: From Filling to Regenerative Medicine
Authors Cassuto D , Bellia G , Schiraldi C
Received 25 August 2021
Accepted for publication 10 December 2021
Published 22 December 2021 Volume 2021:14 Pages 1857—1866
DOI https://doi.org/10.2147/CCID.S276676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Daniel Cassuto,1,2 Gilberto Bellia,3 Chiara Schiraldi4
1Private Practice, Tel Aviv, Israel; 2Private Practice, Milan, Italy; 3IBSA Farmaceutici Italia Srl, Lodi, Italy; 4Department of Experimental Medicine, School of Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy
Correspondence: Daniel Cassuto Email [email protected]
Abstract: Hyaluronic acid (HA)-based injectable filling agents are at the forefront of the current demand for noninvasive dermatological procedures for the correction of age-related soft tissue defects. The present review aims to summarize currently available HA-based products and critically appraise their differences in rheological nature and clinical application. Linear HA (LHA) gels may be supplemented with amino acids, lipoic acid, vitamins, nucleosides, or minerals for synergistic antiaging and antioxidant benefits (polycomponent LHA). HA hydrogels can be generated via chemical or physical crosslinking, which increases their elasticity and decreases viscosity. The performance of crosslinked fillers depends on HA concentration, degree of crosslinking, elastic modulus, cohesivity, and type of crosslinking agent employed. PEG-crosslinked LHA displays improved elasticity and resistance to degradation, and lower swelling rates as compared to BDDE-crosslinked LHA. Physical crosslinking stabilizes HA hydrogels without employing exogenous chemical compounds or altering hyaluronans’ natural molecular structure. Thermally stabilized hybrid cooperative HA complexes (HCC) are a formulation of high- and low-molecular-weight (H-HA and L-HA) hyaluronans, achieving high HA concentration, low viscosity with optimal tissue diffusion, and a duration comparable to weakly cross-linked gel. Our critical analysis evidences the importance of understanding different fillers’ properties to assist physicians in selecting the most appropriate filler for specific uses and for predictable and sustainable results.
Keywords: hyaluronic acid, filler, hydrogel, crosslinking
Background: Soft Tissue Fillers for Cosmetic Dermatology
Driven by exacting beauty standards, the demand for noninvasive dermatological procedures for cutaneous rejuvenation is constantly on the rise. In recent decades, a large volume of research has been made into developing techniques aimed at restoring tissue volume, minimizing wrinkles and fine lines, and treating cutaneous photodamage.1,2
Cutaneous aging is known to be the result of an interplay between extrinsic, environmental aggressors such as solar ultraviolet (UV) radiation, and genetically driven, para-physiologic intrinsic changes.1,2 Both the aforementioned processes share common molecular and cellular pathways of tissue damage, namely the generation of reactive oxygen species (ROS) resulting from oxidative cell metabolism.3 Furthermore, aging affects the ability of the extracellular matrix (ECM) to synthesize and catabolize components of the dermis, namely collagen, elastin and glycosaminoglycans (GAG). The end result of such processes is a reduction in the volume and richness of the ECM, in turn making it more vulnerable to increased enzymatic degradation on behalf of metalloproteinases and collagenases.4,5
Injectable filling agents have been employed for decades for the correction of age-related soft tissue defects.6 The ideal injectable product is highly biocompatible, easily injectable thanks to its favorable rheology, and produce an acceptably long-lasting effect. Xenogeneic and allogeneic collagen materials have an extensive history of successful use, offering the potential for long-lasting results.7 Notwithstanding, long-term outcomes of animal or human-derived collagen injection materials are not fully understood, with few prospective controlled clinical trials available.
Hyaluronic acid (HA) is proving increasingly popular as the standard intradermal injection material for skin rejuvenation.8 HA products’ success stems from several characteristics: extensive experience with natural HA testifies to its negligible allergic risk, and the injective procedure is quick to perform.9 Results are acceptably long-lasting (average duration of action is 6 months), while easy to correct and even fully reversible in the event of adverse effects thanks to the products’ biodegradability.9 HA injections enhance tissue hydration and enrich the dermis for one of the main ECM constituents; they have also been seen to increase the biosynthetic capacity of fibroblasts and stimulate synthesis of new extracellular compounds.10 Currently available HA-based injectables differ not only in the source and concentration of HA but also for in the modification/stabilization method and, more interesting still, in their rheology. This review presents the variety of HA formulations both alone and in combination with other molecules, in its linear or crosslinked form, and finally as hybrid low- and high-molecular-weight complexes.
Hyaluronans: Physicochemical Properties, Synthesis and Degradation, Biological Functions and Role in Aging
First purified in the 1930s from the vitreous humor of the eye, hyaluronic acid (HA) is the sole unsulfated- and the highest molecular weight glycosaminoglycan (GAG) occurring naturally in all mammalian species.11 This linear polysaccharide is constituted by a repeating disaccharide structure of D-glucuronic acid and N-acetyl-D-glucosamine, linked by alternating beta-1,4 and beta-1,3 glycosidic bonds (Figure 1).12 Although first isolated as an acid, at physiological pH HA is found in its salt forms, such as sodium hyaluronate. Thus, the term “hyaluronan” was introduced in the 1980s to designate the polysaccharide irrespective of its degree of dissociation.13 HA lacks sulfate substitutions, but highly charged carboxylic residues on the sugar moieties confer the molecule its notorious hydrophilic properties.14 It is furthermore devoid of protein epitopes, making the molecule non-immunogenic, as opposed to the highly allergenic collagens.15 In solution, the polymer adopts an semiflexible coil conformation thanks to internal, dynamically formed hydrogen bonds between the hydroxyl groups.16 The resulting coil structure has been estimated to trap 1000 times its weight in water, making it the ideal lubricant.16 At higher concentrations, HA solutions acquire unusual rheological properties, namely a high, shear-dependent viscosity. This confers a 1% w/v HA solution its jelly-like consistency, which however flows easily under pressure allowing for easy administration through a small-bore needle.12
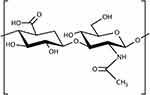 |
Figure 1 Linear hyaluronic acid is constituted by a repeating disaccharide structure of D-glucuronic acid and N-acetyl-D-glucosamine, linked by alternating beta-1,4 and beta-1,3 glycosidic bonds. |
HA is found abundantly in the extracellular matrix (ECM) in all vertebrate tissues, varying in concentrations from 0.01 to 0.1 μg/g in blood serum, to 140–338 μg/g in the vitreous body, to 1400–3600 μg/g in synovial fluid.17 HA has furthermore been identified at an intracellular level, although its functions in the cytoplasm and nucleus remain mostly unknown.18 Differently from other GAG molecules, HA is synthesized at the plasma membrane by three different hyaluronan synthase (HAS) enzymes named HAS1-3. HAS operates by alternatively adding UDP-sugar monomers to the reducing end of the growing polymer.11 Expression levels of the three HAS isoenzymes are spatio-temporally regulated, resulting in a differential distribution of HA in tissues and in different morphogenesis- and disease states.19 In its native form, hyaluronan is known as high-molecular-weight (HMW) HA, to distinguish it from the smaller, low-molecular-weight (LMW) fragments resulting from its turnover.20 HA’s half-life varies from 1 day in the epidermis to 2–3 weeks in cartilage.17
With regard to its catabolism, in mammals HA is enzymatically degraded by hyaluronidases (Hyal). Hyal-2 is expressed on the cell membrane and cleaves the HA into 20-kDa fragments, which are endocytosed and further degraded by Hyal-1 inside lysosomes.21 The size of the resulting oligomers has important implications as to their biological functions. Extracellular polymers with size of 104-kDa are known for their space-filling and hydrating properties, but furthermore exert anti-angiogenic and immunosuppressive functions. Conversely, low-size below 5-kDa polymers have been seen to be highly angiogenic, immunostimulatory and inflammatory, while small oligomers can induce heat shock proteins and play anti-apoptotic functions.21 Of note for its therapeutic applications, HA is known to play a pivotal role in tissue repair: hyaluronan synthesis is increased during tissue injury and wound healing, and HA regulates inflammatory cell activation with downstream effects on fibroblast- and epithelial cell response to injury.22
HA has been recognized as a key molecule in skin aging, given its paramount role in determining and maintaining skin moisture. One of the most notable histochemical changes occurring in senescent skin is the disappearance of epidermal HA, and a progressive reduction of the size of HA polymers in the skin.23 In the dermis, hyaluronans display increased avidity with tissue structures, with a consequent loss of HA extractability.22 The result is the characteristic dehydration, atrophy and loss of elasticity of aged skin. Photoexposed skin, and therefore extrinsic skin aging, is characterized by a significant decrease in the expression of HAS and an increased expression of Hyal; notwithstanding, the reasons for such changes in HA homeostasis with aging are still to be fully clarified.24
Linear or “Native” Hyaluronic Acid Gels
Mesotherapy consists of the stimulation of skin biorejuvenation via minimally invasive intradermal injections of biologically active substances.25 The injected ingredients are released over a prolonged period of time into the surrounding tissues, with a depotlike effect.26 Given all the above, HA was a clear natural candidate for such applications, and linear HA-based (LHA) gels are by far the most popular compounds employed for mesotherapy, both alone and in combination with other molecules.9 Mesotherapy with HA aids in restoring cutaneous metabolic function, water retention and elasticity, with an altogether improvement in the visible signs of photoaging.27 In particular, intradermal HA injection has been proven to stimulate fibroblasts to express collagen type 1 and to reduce expression of matrix metalloprotease and proinflammatory interleukins IL-1β and IL-6.28
The efficacy of linear LHA injections for the treatment of photoaged skin has been validated by numerous researchers.29 Consensus from the expert conference at the 16th Congress of the European Academy of Dermatology and Venereology (EADV) in 2007 concluded in favor of LHA’s positive effects on skin elasticity and turgor.26 Lacarrubba et al employed high-frequency ultrasound for the evaluation of the subepidermal low-echogenic band (SLEB), which is typically reduced in echogenicity with age. Multiple microinjections of LHA salts (sodium chloride, chloride sodium phosphate) of biotechnological origin on the dorsum of the hand over a 4-week period lead to a significant increase in SLEB echogenicity. Such results were confirmed by Tedeschi et al in a randomized controlled trial over a longer observation period (5 and 10 months’ treatment).30 The efficacy and patient satisfaction of linear LHA injectable products has furthermore been validated for facial rejuvenation, for instance by Di Pietro et al31 A group of 20 patients with signs of facial aging were treated with two linear HA treatments 2 weeks apart, with significant improvement in skin turgor and elasticity, and a 95% physician- and patient satisfaction rate.
Polycomponent Linear Hyaluronic Acid-Based Gels
LHA-based injections may be supplemented with other active ingredients for additional, synergistic benefits, on condition the compounds be biocompatible and absorbable. In this, mesotherapy is extremely versatile, and allows for more complete treatment of overlapping and concomitant skin conditions, with both preventative and curative applications. Secondary ingredients classically used in such polycomponent “cocktails” include the micronutrients and biomolecules required to create a favorable environment for optimal fibroblast function.32,33 Amino acids are provided as substrates for the synthesis of dermal ECM proteins, principally collagen. Lipoic acid and vitamins such as retinol, ascorbic acid and tocopherol possess high levels of antioxidant activity, suppressing oxidative stress-induced apoptosis in skin fibroblasts. B vitamins are important coenzymes in a number of fundamental metabolic processes; nucleosides are necessary for fibroblast fission and protein synthesis. Minerals such as calcium, phosphorus and magnesium are required for cellular homeostasis, cell wall synthesis and maintaining enzymatic reactions.
Despite the extensive use of polycomponent intradermal injections in clinical practice for many years, scientific evidence on its therapeutic efficacy as cutaneous anti-aging treatments is scarce. Baspeyras et al performed non-crosslinked HA-based mesotherapy supplemented with mannitol, observing significant improvement in skin elasticity and radiance over 3 months.34 Savoia et al recorded a statistically significant improvement in facial skin brightness, texture and firmness after 2 months’ mesotherapy treatment with a HA-based formulation supplemented with HA, vitamins, amino acids, minerals, coenzymes and antioxidant substances.28 Likewise, clinical evaluation in terms of Global Aesthetic Scale (GAIS) and wrinkle severity rating demonstrated statistically significant results. Sparavigna et al tested the efficacy of injectable HA plus vitamins, mineral and amino acids for the treatment of photoaged skin.35 Middle-aged female volunteers underwent four biorevitalization sessions at 3-week intervals consisting of microinjections in the face, neck, décolletage and dorsum of hands. Treatment provided a statistically significant improvement in profilometric parameters, skin brightness, pigmentation and deep skin hydration. The study furthermore highlighted the product’s photoprotective qualities, as shown by a decrease of the visual score of UV-induced erythema.
Recent work by Stellavato et al provided strong in vitro evidence of polycomponent LHA-based hydrogels’ antiaging and antioxidant effects.36 The study aimed to compare an LHA with linear medium-molecular-weight LHA (LM-HA) with trace elements and proteins in terms of their protective ability against stressful conditions (UV radiation and H2O2 exposure) and of enhanced cell reparation (scratch test). As compared with the same LM-HA, the studied product exhibited significantly increased wound reparation rate, and greater capability of preventing UV stress and protecting from ROS damage as evidenced by antioxidant biomarker quantitation justifying the addition of trace element and protein to enhance the antiaging efficacy.
Crosslinked Hyaluronic Acid-Based Fillers
Native HA dissolved in water behaves as a fluid, with excellent biocompatibility and hydration properties, but provides poor mechanical support and is rapidly cleared from the skin. Chemical modifications have been perfected to improve both LHA’s physicochemical properties and its permanence at the implant site, while preserving its biocompatibility and biodegradability.37 Hydrogels have been generated using radiations, chemical crosslinkers, polyfunctional compounds, free radical-generating compounds, and more.38 Physical crosslinking consists of the stabilization of the naturally occurring electrostatic interactions between LHA chains such as entanglements, hydrogen bonds and can der Waals interactions.39 Conversely, in chemical crosslinking, covalent bonds are formed by chemical reaction between LHA and a crosslinking agent. HA is a suitable polymer for chemical modifications thanks to its structure: in particular, the three most commonly employed sites of covalent modification are the carboxylic groups, hydroxyl group, and amino group after deacetylation.40 Adding crosslinks between polymer chains alters the physical properties of a polymer by conferring it elasticity and decreasing its viscosity (resistance to flow).41
A wide variety of factors contribute to the performance of crosslinked fillers.37,39 The main parameter influencing crosslinked HA (CLHA) product duration is HA concentration, specifically in terms of the amount of CLHA contained in the gel. A second important parameter is the degree of crosslinking, defined as the ratio of percentage of crosslinking to the percentage of total modification. Such ratio is determined by the reaction conditions employed to crosslink the products (eg covalent bonds yield a higher ratio than physical entanglement), and a higher ratio provides better resistance to degradation and deformation.
In terms of determining a gel’s clinical application, the key rheological property is elastic modulus (G’).39 G’ captures the sum of a number of factors affecting gel strength, namely CLHA concentration, starting molecular weight, type of crosslinking and the presence of unmodified HA. G’ is the primary determinant of tissue projection, as it is a measurement of firmness and a gel’s resistance to force (be it muscular or gravitational). In terms of rheologic tailoring, a firmer gel with a higher G’ is more resistant to deformation, but may feel lumpier upon implant and potentially cause more pain, inflammation and edema. Higher-G’ products are therefore indicated for deeper planes of injection and in areas such as the malar cheek, chin and jawline. Conversely, a lower-G’ gel will be softer and provide a more natural feel upon implant, and may thus be better suited for the treatment of soft tissues such as the lips and periorbital area, and of less-dynamic wrinkles. Intermediate G’ products are an effective solution for dynamic wrinkle correction, support and contouring in areas of facial animation, such as the midface. A second important parameter to be considered in treatment rheological tailoring is cohesivity, defined as the capacity of a material not to dissociate, due to the affinity of its molecules for each other.42 A high-viscosity, low-cohesivity product will disperse in the dermis as microboluses, providing more tissue projection than expansion. Conversely, a low-viscosity, high-cohesivity product will distribute homogeneously within the dermis, providing tissue expansion along a predominantly horizontal vector.
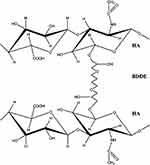
Crosslinking agents are one of the most successful chemical modifications of HA, and act as chemical species connecting two sections of LHA chains in a bridge-like fashion. The main crosslinking species currently employed are 1,4-butanediol diglycidyl ether (BDDE) and polyethylene glycol diglycidyl ether (PEG). With regard to fillers, the preferred reaction between the crosslinking agent and LHA is the formation of ether bonds, as they are stable in physiological conditions in the dermis.
The crosslinking agent employed in the majority of currently available CLHA-based dermal fillers is BDDE.43 BDDE’s crosslinking capacity is conferred by the reactivity of the epoxide groups located at the two ends of the molecule, which preferentially create an ether bond with the most accessible primary alcohol in the LHA backbone (Figure 2). Ether bonds are more stable than ester or amide bonds, making BDDE-crosslinked HA fillers durable up to 1 year. BDDE is furthermore less toxic than other ether-bond crosslinking agents such as divinyl sulfone and is biodegradable. Given concerns over its mutagenic potential, unreacted BDDE is present in dermal fillers only in trace amounts, thanks to complex purification processes.43,44 The carbohydrate backbone of HA is unaffected by the ether bonds formed in the BDDE-based crosslinking reaction; BDDE-CLHA is therefore susceptible to enzymatic degradation via the same enzymatic and oxidative degradation processes occurring on LHA and described above.45 Upon degradation, both unreacted BDDE and CLHA break down into harmless or naturally occurring substances and byproducts. The favorable clinical safety profile of BDDE-CLHA is supported by more than 20 years’ worth of clinical and biocompatibility data.43
PEG is a linear or branched polyether, with a wide range of applications in biomedicine thanks to its nontoxicity and non-immunogenicity.46 Similar to BDDE, LHA crosslinking with PEG is based on the formation of ether bonds with PEG’s epoxide groups: the reaction consists of the deprotonation of a hydroxyl group on LHA, the epoxide ring opening, and the formation of a stable C-O-C bond (Figure 3).47 As a crosslinking agent, PEG consists of a mixture of oligomers of different lengths; consequently, it introduces unequal spacers between the LHA chains, as opposed to simpler molecules such as BDDE. As assessed by Monticelli et al, the effective crosslinker ratio for PEG appears lower than that of BDDE, which may be responsible for differences in rheological properties and swelling rate of the two compounds.48 With regard to rheological behavior, PEG-CLHA hydrogels display improved elasticity (higher G’) as compared to BDDE-CLHA at the same molar concentrations.49 Concerning swelling ratios (speed of water incorporation into the hydrogel network), lower swelling rates have been reported for PEG as compared with BDDE-based formulations both in vitro and in vivo.48,49 Lastly, PEG-CLHA has been seen to possess a greater resistance to degradation by hyaluronidase than that of BDDE-CLHA.
Physical crosslinking stabilizes HA hydrogels by exploiting the natural electrostatic interactions between chains without the need for exogenous chemical compounds and alterations of hyaluronans’ natural molecular structure.50 The non-covalent bonds and supramolecular interactions at play in physical hydrogels are reversible, allowing for the creation of hydrogels with turnable properties and responsivity to pH, temperature or other cues.51,52 Furthermore, while physical crosslinking yields less mechanically- and chemically stable hydrogels compared to covalent crosslinking, this characteristic may be exploited to obtain hydrogels with shear-thinning and self-healing properties.53 Self-thinning hydrogels allow direct injection into tissues without the risk of premature gel formation and delivery failure, and near-instantaneous reassembly for material retention at the target site.54
Physical hydrogels can be synthesized by heating or cooling the polymer solution, by mixing polyanion and polycations, by combining polyelectrolyte with multivalent ions with an opposite charge, etc.50 One of the most employed strategies is inclusion complexation, which exploits supramolecular interactions and structural complementarity between “host” and “guest” molecules. For example, Rodell et al developed a self-assembling HA hydrogel based on the guest–host interactions of adamantane-modified HA (guest macromer) and the hydrophobic cavities of cyclodextrin-modified HA (host macromer).54 However, such synthetic strategies fail to preserve the natural chemical structure of HA, and, similarly to chemical crosslinking, require additional exogenous molecules to achieve acceptable tissue longevity.
Stabilized Hybrid Cooperative Hyaluronic Acid Complexes
Thermal production processes, such as NAHYCO® Hybrid Technology patented by IBSA, are a recent innovation in physical hydrogel synthesis, and are currently employed in a range of products including IBSA’s Profhilo®. Starting with a mixture of high-MW (110–1400 kDa) and low-MW (80–110 kDa) HA (H-HA and L-HA, respectively), the process consists of a high-temperature step followed by a low-temperature step, and yields thermally stabilized HA (HCC) with a duration similar to that of weakly chemically crosslinked gel. HCC thus achieves an in situ longevity of around 4–5 weeks, while benefiting from the rheological advantages of physically crosslinked hydrogels, namely shear-thinning and in situ reassembly. HCC low viscosity and optimal tissue diffusion, combined with one of the highest HA concentrations available on the market (64 mg/2 mL), account for excellent manageability in the treatment of skin laxity in even the most challenging areas.55
The rationale behind the combination of different-MW of HA, hereafter hybrid cooperative complexes (HCC), lies in their synergistic contribution to tissue regeneration. Indeed, both H-HA and L-HA are known to play a pivotal role in wound repair, and simultaneously occur in vivo at an injury site. D’Agostino et al proved HCC promotes wound healing in human keratinocyte monolayers at twice the speed of H-HA and L-HA alone.56 In terms of biological activity, Stellavato et al found HCC boosts ECM remodeling compared with H-HA and L-HA alone, as evidenced by an increase in the expression levels of collagen and elastin in in vitro assays on keratinocytes and fibroblasts.10 The same group furthermore studied the effect of HCC on adipose-derived stem cells (ASC) and observed increased ASC differentiation and proliferation via upregulation of adipogenic genes and related proteins.57 Conversely, differentiation was significantly lower for H-HA- and L-HA-treated ASCs, and was not induced by chemically-CLHA. Further studies by Alessio et al observed HCC’s efficacy in delaying senescence in mesenchymal stromal cells subjected to stressful conditions.58 Building on this data, Stellavato et al recently analyzed in vitro the beneficial effect of HCC on cells subjected to oxidative stress and on the recovery of muscle atrophy.59 In particular, HCC were proven to have a greater potential than LHA in promoting cell proliferation, reducing ROS damage and atrophic biomarkers, and in preserving the muscle phenotype and viability in a skeletal muscle disorder model. Taken collectively, the above in vitro evidence supports HCC’s potential as a medical device in both aesthetic and regenerative medicine.
In terms of clinical indications, HCC’s efficacy for facial rejuvenation is backed up by extensive in vivo evidence and has been evaluated over the course of six independent published studies.60–65 A monocentric retrospective observational study by Laurino et al assessed facial skin hydration, elasticity and transepidermal water loss (TEWL) 3 months after two HCC injection sittings.60 Post-treatment ecographic evaluation was consistent with widening of the subdermal area, and results in terms of skin hydration, elasticity and TEWL were statistically significant. Abascal et al likewise observed an improvement in skin viscoelasticy and hydration 8 weeks after two HCC injection sittings.61 Sparavigna et al reported a statistically significant improvement in a number of facial aging parameters 16 weeks after 2 HCC injection sittings.63 Specifically, an improvement was recorded in skin surface microrelief and profilometry, wrinkle severity and skin hydration (assessed via skin electrical capacitance), as was an overall increase in face volume (assessed via 3D volume image analysis). Satardinova et al tested HCC’s efficacy in the Oriental face via photographical evidence and 3D assessment systems, reporting a significant amelioration in skin hydration and overall wrinkle size, and a macroscopic improvement in wrinkles, fine lines and skin brightness and tone on visual comparison.64 Goltsova et al performed corneometry, cutometry and 3D complexion analysis 1 month after 3 HCC injection sittings, observing a clear reduction in wrinkle depth and smoothing of skin texture, and a statistically significant improvement in skin hydration, compliance and elasticity.65 Of note, both patient and doctor satisfaction levels were high in all the aforementioned studies. Furthermore, a recent safety assessment of HCCs as derived from worldwide post-marketing data for Profhilo® confirmed the high tolerability and safety of the product.66
Of relevance for the scope of the present review, HCC’s formulation is uniquely tailored to the cutting-edge concept of “bioremodeling”. This innovative approach aims at restoring hydration, elasticity and skin tone by synergistically associating deep tissue hydration with a mechanical lifting action. Indeed, HCC provides a stable architecture and water-retention scaffold in the dermis, with a volumetric effect similar to that of traditional fillers.
Conclusions
Due to their negligible allergic potential, ease of performance, reasonably long-lasting and fully reversible effects, HA products are experiencing a surge in demand in the current age of noninvasive dermatological procedures for cutaneous rejuvenation. A wide variety of hyaluronan-based products has been designed developed and commercialized: in this review, we have presented the plethora of HA formulations both alone and in combination with other molecules, in linear or crosslinked form, and as hybrid low- and high-molecular-weight complexes. The importance of adequately investigating and understanding different fillers’ properties lies in helping physicians select the most appropriate filler for specific uses and for predictable and sustainable results.
Together with advances in purification and crosslinking techniques, HA injection technique has undergone a shift in consciousness regarding its use and scope, bolstered by in vitro and in vivo evidence of its biological potential. The biomechanical and biochemical effects of HA on the local microenvironment of the injected site are key to its success as a soft tissue filler. HCC have specifically been observed to possess specific potency in promoting cell proliferation, reducing ROS damage, thus combating senescence as compared with LHA. Indeed, far from being a mere filler product, HA is now known to promote healing, bioremodeling and overall possess strong bioactivity, with extensive potential in anti-aging applications. An area of potential research is investigating the crosstalk between HA gels delivered by intradermal/subdermal injection, and the surrounding tissue. Improved knowledge of the tissue–device interface will guide both aesthetic and practical evidence-based decision-making and lead to optimal outcomes for patients.
Lastly, in future, more intensive experimental and clinical studies are warranted to thoroughly investigate the diagnostic and therapeutic potential of HA. In the view of the authors, the future scope for research lies in further correlating in vitro research results and extensive available clinical evidence, to fully exploit the translational value of these “old” macromolecules with new, exciting rejuvenating capacities.
Acknowledgments
The authors are grateful to Alba Sommerschield, who helped with the writing of this manuscript. Medical writing has been sponsored by IBSA Farmaceutici Italia.
Disclosure
GB is an employee of IBSA Farmaceutici Italia. Professor Chiara Schiraldi reports non-financial support from Bioteknet, grants from Invitalia-Italian public funding to research and development, outside the submitted work. In addition, Professor Chiara Schiraldi has a patent 10266611 issued to IBSA. The authors report no other conflicts of interest related to this work.
References
1. Berneburg M, Berneburg M, Trelles M, et al. How best to halt and/or revert UV-induced skin ageing: strategies, facts and fiction. Exp Dermatol. 2008;17:228–229. doi:10.1111/j.1600-0625.2007.00665_1.x
2. Makrantonaki E, Adjaye J, Herwig R, et al. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5:331–344. doi:10.1111/j.1474-9726.2006.00223.x
3. Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi:10.1001/archderm.138.11.1462
4. Peres PS, Terra VA, Guarnier FA, Cecchini R, Cecchini AL. Photoaging and chronological aging profile: understanding oxidation of the skin. J Photochem Photobiol B Biol. 2011;103:93–97. doi:10.1016/j.jphotobiol.2011.01.019
5. Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31:65–74. doi:10.1111/phpp.12145
6. Eppley BL, Dadvand B. Injectable soft-tissue fillers: clinical overview. Plast Reconstr Surg. 2006;118:98e–106e. doi:10.1097/01.prs.0000232436.91409.30
7. Baumann L, Kaufman J, Saghari S. Collagen fillers. Dermatol Ther. 2006;19:134–140. doi:10.1111/j.1529-8019.2006.00067.x
8. Cohen JL, Dayan SH, Brandt FS, et al. Systematic review of clinical trials of small- and large-gel-particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg. 2013;39:205–231. doi:10.1111/dsu.12036
9. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plastic Surg. 2009;25:86–94. doi:10.1055/s-0029-1220647
10. Stellavato A, Corsuto L, D’Agostino A, et al. Hyaluronan hybrid cooperative complexes as a novel frontier for cellular bioprocesses re-activation. PLoS One. 2016;11:e0163510. doi:10.1371/journal.pone.0163510
11. Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–634. doi:10.1016/S0021-9258(18)75338-6
12. Necas J, Bartosikova L, Brauner P, Kolar J. Hyaluronic acid (hyaluronan): a review. Vet Med (Praha). 2008;53:397–411. doi:10.17221/1930-VETMED
13. Balazs EA, Laurent TC, Jeanloz RW. Nomenclature of hyaluronic acid. Biochem J. 1986;235:903. doi:10.1042/bj2350903
14. Maytin EV. Hyaluronan: more than just a wrinkle filler. Glycobiology. 2016;26:553–559. doi:10.1093/glycob/cww033
15. Gilbert E, Hui A, Meehan S, Waldorf HA. The basic science of dermal fillers: past and present part II: adverse effects. J Drugs Dermatol. 2012;11:1069–1076.
16. Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791–809. doi:10.1016/j.carres.2005.01.022
17. Dicker K, Gurski LA, Pradhan-Bhatt S, et al. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1–7. doi:10.1016/j.actbio.2013.12.019
18. Hascall VC, Majors AK, De La Motte CA, et al. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi:10.1016/j.bbagen.2004.02.013
19. Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi:10.1074/jbc.272.22.13997
20. Litwiniuk M, Krejner A, Grzela T, Gauto AR, Grzela T. Hyaluronic acid in inflammation and tissue regeneration | wounds research. Wounds. 2016;28:78–88.
21. Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–325. doi:10.1078/0171-9335-00392
22. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4:253–258. doi:10.4161/derm.21923
23. Meyer LJM, Stern R. Age-dependent changes of hyaluronan in human skin. J Invest Dermatol. 1994;102:385–389. doi:10.1111/1523-1747.ep12371800
24. Tzellos TG, Klagas I, Vahtsevanos K, et al. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol. 2009;18:1028–1035. doi:10.1111/j.1600-0625.2009.00889.x
25. Lee JC, Daniels MA, Roth MZ. Mesotherapy, microneedling, and chemical peels. Clin Plast Surg. 2016;43:583–595. doi:10.1016/j.cps.2016.03.004
26. Wiest L, Kerscher M. Native hyaluronic acid in dermatology – results of an expert meeting. JDDG. 2008;6:176–180. doi:10.1111/j.1610-0387.2008.06639.x
27. Lacarrubba F, Tedeschi A, Nardone B, Micali G. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol Ther. 2008;21:S1–S5. doi:10.1111/j.1529-8019.2008.00234.x
28. Savoia A, Landi S, Baldi A. A new minimally invasive mesotherapy technique for facial rejuvenation. Dermatol Ther (Heidelb). 2013;3:83–93. doi:10.1007/s13555-012-0018-2
29. Bukhari SNA, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120:1682–1695. doi:10.1016/j.ijbiomac.2018.09.188
30. Tedeschi A, Lacarrubba F, Micali G. Mesotherapy with an intradermal hyaluronic acid formulation for skin rejuvenation: an intrapatient, placebo-controlled, long-term trial using high-frequency ultrasound. Aesthetic Plast Surg. 2015;39:129–133. doi:10.1007/s00266-014-0432-1
31. Di Pietro A, Di Sante G. Recovery of skin elasticity and turgor by intradermal injection of hyaluronic acid (Lal-System®) by the cross-linked technique. G Ital Di Dermatologia e Venereol. 2001;136:187–194.
32. Prikhnenko S. Polycomponent mesotherapy formulations for the treatment of skin aging and improvement of skin quality. Clin Cosmet Investig Dermatol. 2015;8:151–157. doi:10.2147/CCID.S76721
33. Blake S. Vitamins and Minerals Demystified. Mc Graw Hill Professional; 2007.
34. Baspeyras M, Rouvrais C, Liégard L, et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013;305:673–682. doi:10.1007/s00403-013-1360-7
35. Sparavigna A, Tenconi B, De Ponti I. Antiaging, photoprotective, and brightening activity in biorevitalization: a new solution for aging skin. Clin Cosmet Investig Dermatol. 2015;8:57–65. doi:10.2147/CCID.S77742
36. Stellavato A, Pirozzi AVA, Donato S, et al. Positive effects against UV-A induced damage and oxidative stress on an in vitro cell model using a hyaluronic acid based formulation containing amino acids, vitamins, and minerals. Biomed Res Int. 2018;2018:1–11. doi:10.1155/2018/8481243
37. Kablik J, Monheit GD, Yu LP, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35:302–312. doi:10.1111/j.1524-4725.2008.01046.x
38. Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi:10.1016/S0169-409X(01)00239-3
39. Fagien S, Bertucci V, von Grote E, Mashburn JH. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg. 2019;143:707e–720e. doi:10.1097/PRS.0000000000005429
40. Khunmanee S, Jeong Y, Park H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J Tissue Eng. 2017;8:204173141772646. doi:10.1177/2041731417726464
41. Maitra J, Gautam VKS. Cross-linking in hydrogels - a review. Am J Polym Sci. 2014;4:25–31.
42. Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six US food and drug administration-approved fillers. Plast Reconstr Surg. 2015;136:678–686. doi:10.1097/PRS.0000000000001638
43. De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39:1758–1766. doi:10.1111/dsu.12301
44. National Technical Reports Library - NTIS. Cutaneous Carcinogenicity Study with mice on the diglycidyl ether of 1,4-butane diol with attachments and cover letter dated 09/ 28/1987. 1987.
45. Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010;36:804–809. doi:10.1111/j.1524-4725.2010.01550.x
46. Zerbinati N, Esposito C, Cipolla G, et al. Chemical and mechanical characterization of hyaluronic acid hydrogel cross‐linked with polyethylene glycol and its use in dermatology. Dermatol Ther. 2020;33. doi:10.1111/dth.13747
47. Zerbinati N, Lotti T, Monticelli D, et al. In vitro evaluation of the sensitivity of a hyaluronic acid PEG cross-linked to bovine testes hyaluronidase. Open Access Maced J Med Sci. 2018;6:20–24. doi:10.3889/oamjms.2018.046
48. Monticelli D, Martina V, Mocchi R, et al. Chemical characterization of hydrogels crosslinked with polyethylene glycol for soft tissue augmentation. Open Access Maced J Med Sci. 2019;7:1077–1081. doi:10.3889/oamjms.2019.279
49. Lee H-Y, Jeong S-H, Baek J-U, Song J-H, Kim H-E. Mechanical improvement of Hyaluronic Acid (HA) hydrogels and incorporation of Polyethylene Glycol (PEG). Archiv Neurol. 2001;58:1105–1109. doi:10.1001/archneur.58.7.1105
50. Trombino S, Servidio C, Curcio F, Cassano R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics. 2019;11:407. doi:10.3390/pharmaceutics11080407
51. Zheng Z, Hu J, Wang H, et al. Dynamic softening or stiffening a supramolecular hydrogel by ultraviolet or near-infrared light. ACS Appl Mater Interfaces. 2017;9:24511–24517. doi:10.1021/acsami.7b07204
52. Rombouts WH, de Kort DW, Pham TTH, et al. Reversible temperature-switching of hydrogel stiffness of coassembled, silk-collagen-like hydrogels. Biomacromolecules. 2015;16:2506–2513. doi:10.1021/acs.biomac.5b00766
53. Appel EA, Del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chem Soc Rev. 2012;41:6195–6214. doi:10.1039/c2cs35264h
54. Rodell CB, Kaminski AL, Burdick JA. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules. 2013;14:4125–4134. doi:10.1021/bm401280z
55. Agolli E, Diffidenti B, Di Zitti N, et al. Hybrid cooperative complexes of high and low molecular weight hyaluronans (Profhilo®): review of the literature and presentation of the VisionHA project. Esperienze Dermatologiche. 2021;5:20.
56. D’Agostino A, Stellavato A, Busico T, et al. In vitro analysis of the effects on wound healing of high- and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biol. 2015;16. doi:10.1186/s12860-015-0064-6
57. Stellavato A, La Noce M, Corsuto L, et al. Hybrid complexes of high and low molecular weight hyaluronans highly enhance hascs differentiation: implication for facial bioremodelling. Cell Physiol Biochem. 2017;44:1078–1092. doi:10.1159/000485414
58. Alessio N, Stellavato A, Squillaro T, et al. Hybrid complexes of high and low molecular weight hyaluronan delay in vitro replicative senescence of mesenchymal stromal cells: a pilot study for future therapeutic application. Aging (Albany NY). 2018;10:1575–1585. doi:10.18632/aging.101493
59. Stellavato A, Abate L, Vassallo V, et al. An in vitro study to assess the effect of hyaluronan-based gels on muscle-derived cells: highlighting a new perspective in regenerative medicine. PLoS One. 2020;15:e0236164. doi:10.1371/journal.pone.0236164
60. Laurino C, Palmieri B, Coacci A. Efficacy, safety, and tolerance of a new injection technique for high- and low-molecular-weight hyaluronic acid hybrid complexes. Eplasty. 2015;15:e46.
61. Rodríguez Abascal M, Saldana Fernandez M. Bio-remodelación facial mediante inyección intradérmica de un complejo híbrido estabilizado de ácido hialurónico de alto y bajo peso molecular: estudio prospectivo en 30 pacientes. Eur Aesthetic Plast Surg J. 2015;5:123–131.
62. Beatini A, Schiraldi C, Sparavigna A. Hyaluronic acid hybrid cooperative complexes and the BAP (Bio Aesthetic Points) technique: the new edge in biorejuvenation. Aesthet Med. 2016;2:45–51.
63. Sparavigna A, Tenconi B. Efficacy and tolerance of an injectable medical device containing stable hybrid cooperative complexes of high- and low-molecular-weight hyaluronic acid: a monocentric 16 weeks open-label evaluation. Clin Cosmet Investig Dermatol. 2016;9:297–305. doi:10.2147/CCID.S114450
64. Satardinova E. Hybrid cooperative complexes of high and low molecular weight hyaluronans for facial skin rejuvenation in the Oriental mongoloid face: a case series. Aesthet Med. 2019;5:14–19.
65. Goltsova E, Shemonaeva O. Hybrid cooperative complexes of H-HA and L-HA (Profhilo®) and the BAP technique for facial skin bioremodeling: a clinical experience at the NEO-Clinic (Tyumen, Russia). Esperienze Dermatologiche. 2019;21:47–53.
66. Cassuto D, Delledonne M, Zaccaria G, et al. Safety assessment of high- and low-molecular-weight hyaluronans (Profhilo®) as derived from worldwide postmarketing data. Biomed Res Int. 2020;2020:1–9. doi:10.1155/2020/8159047
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.