Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
An Overview of Hepatocellular Carcinoma After Insufficient Radiofrequency Ablation
Authors Guo Y, Ren Y, Dong X, Kan X, Zheng C
Received 15 January 2022
Accepted for publication 4 April 2022
Published 26 April 2022 Volume 2022:9 Pages 343—355
DOI https://doi.org/10.2147/JHC.S358539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Laura A. Dawson
Yusheng Guo,1,2,* Yanqiao Ren,1,2,* Xiangjun Dong,1,2 Xuefeng Kan,1,2 Chuansheng Zheng1,2
1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan, 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuansheng Zheng, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China, Tel/Fax +86-27-85726290, Email [email protected]
Abstract: Radiofrequency ablation (RFA) is a commonly used treatment for hepatocellular carcinoma (HCC), however, various complex conditions in clinical practice may lead to insufficient radiofrequency ablation (IRFA), allowing residual HCC to survive. In clinical practice and laboratory models, IRFA plays an important role in rapid tumor progression. Therefore, targeting the residual HCC and avoiding IRFA were worthwhile methods. A deeper understanding of IRFA is required; IRFA contributes to the improvement of proliferative activity, migration rates, and invasive capacity, and this may be due to the involvement of multiple complex processes or proteins, including epithelial mesenchymal transitions (EMTs), cancer stem cells (CSCs), autophagy, heat shock proteins (HSPs), changes of non-tumor cells and extracellular matrix, altered immune microenvironment, hypoxia-inducible factors (HIFs), growth factors, epigenetic alterations, and metabolic reprogramming. We focus on the processes of the above mechanisms and possible therapeutic approach, with a review of the literature. Additionally, we recapitulated the construction methods of various experimental models of IRFA (in vivo and in vitro).
Keywords: hepatocellular carcinoma, insufficient radiofrequency ablation, epithelial mesenchymal transitions, residual viable tumors
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the fifth most common cancer in the world.1,2 Patients with early-stage HCC are usually treated curatively by hepatectomy or radiofrequency ablation (RFA). RFA, a minimally invasive approach, inducing coagulative necrosis of tumor tissue through an electrode placed in the tumor, offers better safety, fewer complications and, shorter hospital stay compared to hepatectomy.3–6 Some studies have shown that RFA is inferior to surgical resection in terms of the higher risk of recurrence and metastasis,7–10 and RFA resulted in 5-year overall recurrence rates of 63.5%, much higher than that of 41.7% in patients underwent surgical resection.11 Several early clinical studies found rapid tumor progression in some patients after RFA, which may be due to residual viable tumors after RFA.12–17 It has been found that thermal ablation damage is divided into three regions, namely the central high-temperature zone, the sublethal temperature transition zone, and the surrounding normal tissue. In the transition zone, tumors suffer reversible damage and eventually survive, thus leading to rapid tumor progression in a activate state.18,19 The mechanism is complicated after IRFA and may include the following aspects: 1) IRFA causes Epithelial Mesenchymal Transitions (EMT), which is an important cause of tumor metastasis;20–36 2) IRFA leads to autophagic survival and plays a role in subsequent progression and metastasis;37–44 3) IRFA causes a significant increase in the number of cancer stem cells;21,24,31,37,45–47 4) IRFA triggers a hypoxic microenvironment that aids tumor cell survival and proliferation;24,34,38,44,48–51 5) IRFA causes sustained local inflammation with predominant myeloid suppressor cells, which inhibits the function of T cells in tumors;52 6) Heat shock response aids tumor cell survival after IRFA;21,25,39,43,47,53 7) IRFA stimulates the expression of several growth factors and receptors;24,36,42,46,48,49,54–57 8) IRFA activates non-tumor cells such as hepatic stellate cells (HSCs), tumor-associated endothelial cells, and platelets to help tumor cell survival and metastasis;10,27,30,42,45,58 9) IRFA causes epigenetic changes;26,31,32,34 10) IRFA enhances metabolic reprogramming of HCC.30,34 The above mechanisms do not exist independently, but contribute to each other and together increase the malignancy of residual viable tumors.
In this review, we first briefly describe the experimental models currently in use. Next, we describe how IRFA induces cell survival, proliferation, metastasis, and invasion through EMT, cancer stem cells, autophagy, heat shock response, immune microenvironment, and hypoxia-inducible factors. In addition, the coping strategies for IRFA are briefly discussed.
Experimental Modeling of Insufficient Radiofrequency Ablation
To construct as realistic a model of IRFA as possible, researchers have used several different methods to create suitable models. Obara et al heated HCC cells in water baths at different temperatures from 37 degrees to 55 degrees for ten minutes and first proposed sublethal temperatures for cell models of HCC cells between 47 degrees and 49 degrees.59 Hence, 47 or 48 degrees eventually became the sublethal temperature of cells for the vast majority of studies. The duration of heat treatment varies between institutions, but a water bath for ten minutes is the simplest and most commonly used method. A considerable number of studies simulate IRFA through the gradient of heating time of 5min, 10min, 15min, 20min, and 25min. In addition to this, considering that RFA can lead to the formation of a transition zone between normal liver tissue and necrotic coagulation, blood stagnation, and thrombosis exposing residual tumor cells to a hypoxic microenvironment,60 Tong et al performed incubation of cells in a hypoxic incubator (1%O2) after heat treatment at 47 degrees to simulate the hypoxic environment to which tumor cells are exposed after thermal ablation.24 In general, compared to unheated HCC cells, HCC cells after sublethal heat treatment exhibit higher proliferative activity, higher migration rates, and greater invasive capacity. Microscopically, heated cells often exhibited a spindle cell-like morphology with less intercellular adhesion and loss of polarity.
In studies with animal models, BALB/c mice have become the most frequently used animals, and New Zealand rabbits have also been used in some studies. IRFA models are usually constructed by the following methods. In mice, for example, the cells after heat treatment in vitro were directly transplanted into the liver or subcutaneous, and the follow-up experiments were carried out after tumor formation. Some studies transplant untreated tumor cells into the liver or subcutaneous for subsequent ablation, while other studies first implant heat-treated cells or IRFA tumor blocks into the subcutis or liver of tumor-bearing mice, and then cut up the blocks for “replantation” after tumor formation, which may facilitate stable tumor growth. In vivo, To partially ablate the tumor, the power of the ablation needle will be lower than that used clinically, and a considerable number of studies chose to ablate for 30s with a power output of 5 watts. The temperature range of the incompletely ablated zone as seen by infrared imaging was 41 degrees to 50 degrees, with the temperature in the center of the ablation greater than 50 degrees and the surrounding normal tissue temperature less than 40 degrees. Similar to heated liver cancer cells, incompletely ablated tumor tissue often exhibits faster growth rates, larger final tumor volumes and tumor specimens show that IRFA can cause more tumor metastases than tumor tissue with no ablation. Supplementary Table S1 summarizes the model construction methods that have been used.
Epithelial Mesenchymal Transition
EMT is closely associated with embryonic development, organogenesis, repair of damage, and migration and invasion of malignant tumors.61,62 The main manifestation is that tumor epithelial cells undergo phenotypic transformation after receiving some stimulation and turn into mesenchymal cells with migration ability. The main molecular markers are E-cadherin, which is down-regulated, and N-cadherin, vimentin, Snail, and Twist, which are up-regulated.63,64 Dong et al conducted research related to EMT on SMMC7721 and Huh7 cells after IRFA for the first time in 2013, demonstrating that EMT markers were significantly increased in surviving cells after heat treatment. Other studies have confirmed the occurrence of heat-induced EMT.20 Yoshida et al found that this process was reversible. Expression of EMT markers (Snail, TWIST1, CHD1L, and COL1A1) showed an upward trend 5 days after treatment at 50°C, and returned to baseline level on day 12 after IRFA. Su et al found that IRFA could lead to epigenetic alterations in tumor cells, which subsequently led to EMT, but the relevant indicators decreased gradually over two days, suggesting its reversibility and plasticity.21 This heat-induced EMT is likely to occur within a short period, suggesting that drugs should be applied early after RFA to inhibit the activation of EMT.
P-ERK1/2 has an important role in heat-induced EMT. Four studies20,21,23,28 performed heat treatment on MHCC97H, HepG2, HuH7, and HEP3B cell lines and found that ERK was significantly phosphorylated. EMT was attenuated after inhibition of P-ERK1/2, which was similar to other ways of activating EMT.65–67 They found that P13K, P46-Shc, and Periostin were activated as upstream proteins of ERK after heat treatment, causing tumor invasion and metastasis. WNT pathway and FLOT1/FOLT2 were also found to be activated by heat induction, which together promotes nuclear translocation of β-catenin and causes EMT.22,29 Other classical signaling pathways or proteins are also activated. Zhou et al found significantly elevated IL-6 expression in heat-treated H22 cells, which in turn activated Jak2 and stat3 thereby triggering EMT.33 Consistent with previous studies, NF-κB also plays a role in heat-induced EMT.30,68,69
Epigenetics has been reported to have an important relationship with EMT.70,71 Heat-induced stress can induce a significant rise in the “writer” METTL3 and m6A, meanwhile, two “readers” IGF2BP1 and YTHDF1 are elevated, which in turn induces significant elevations in CD47 and EGFR expression or translation, ultimately inducing the occurrence of EMT.10,36 Non-coding RNAs also play a role in heat-induced EMT, and different lncRNAs may play opposite roles, with FUNDC2P4 being significantly downregulated in HCC tissue remaining after RFA,26 in contrast to ASMTL-AS1 being significantly elevated.32 The possible signaling pathways are listed in Figure 1. Notably, although ZEB1 has been reported to produce a role in EMT in HCC,72 Zeb1 is not currently reported to be activated in heat-induced EMT in HCC.
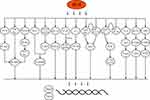 |
Figure 1 The possible signaling pathways of IRFA and EMT. |
Cancer Stem Cell
Cancer stem cells (CSC) are described as a small group of cancer cells that can self-renew and may be responsible for recurrence, metastasis, and drug resistance.73,74 In HCC, the main markers of CSCs are EpCAM, CD90, CD44, CD133, CD24, CD13, Nanog, and OCT4.75–77 Sub-lethal heat treatment or hypoxic environments can lead to the generation of tumor stem cells. Among studies involving IRFA and HCC, CD133,21,37,45–47 CD90,24 CD44,46,47 EpCAM,45,46 Nanog31,45 and OCT431 are reported to be induced. Zhang et al reported similar expression trends of CD133 with CD31 and VEGFA in the IRFA-liver cancer tissue, suggesting that IRFA may affect tumor angiogenesis through CSCs,57 and similar findings have been reported by other studies.24,46 Interestingly, activated HSCs may promote the progression of residual tumor cells after thermal ablation by enriching CSCs.45
Autophagy
In the progression of cancer, autophagy plays a dual role of inhibition or promotion depending on the type and degree of tumor progression and genetic background.78–83 Similarly, autophagy has been reported to play a paradoxical role in HCC. It protected cells from carcinogenesis at the early stage and promoted tumor progression at the advanced stage.84,85 Previous studies have shown that sublethal heat stress induces autophagy in several human cancer cell lines, such as HeLa and A549 cells.86 In this review, we summarize the effect of IRFA on autophagy in HCC. To our knowledge, Wang et al first reported that autophagy plays an important role in the elevated survival and invasiveness of Huh-7 and SMMC7721 cells after IRFA. When autophagy was inhibited (autophagy inhibitors such as 3-MA or chloroquine), the malignancy of the cells is reduced. Interestingly, this process is synchronized with the expression trend of CD133, suggesting a link between autophagy and CSC.37 In addition, the hypoxic environment may also act synergistically with autophagy. Zhao et al observed an increase in the LC3B protein region consistent with a region of high HIF-1a expression in tumor tissues.38 Xu et al knocked down the BNIP3 in heat-induced hepatocellular carcinoma cells and LC3B and HIF-1α showed a common downward trend.41 Another study came to a similar conclusion.44 As mentioned previously, HSCs are a non-negligible role in the survival and tumor progression of HCC cells. Zhang et al co-cultured surviving HCC cells after IRFA with HSCs culture medium (HSC-CM) and found that HSC-CM could increase the expression of LC3B at the early stage, suppress the cell apoptosis and promote the survival of cell autophagy. Expression of LC3B was gradually down-regulated and CyclinD1 was gradually up-regulated in the later stages.42 This dynamic change demonstrates an important role for heat-induced autophagy and again suggests an important role for HCSs in this process. Table 1 shows the potential relations or mechanisms of autophagy and IRFA.
 |
Table 1 Autophagy and IRFA |
Heat Shock Proteins
When cells are under stress (such as extreme temperature, hypoxia, and drugs), some proteins undergo denaturation or folding.87,88 Heat shock proteins (HSP) can reverse or inhibit this process, making HSP an important regulator of many important processes such as cell proliferation and differentiation.89 Because protein misfolding occurs frequently in tumor cells, their survival and proliferation are more dependent on HSP than normal cells.90,91 Previous studies have reported that HSP plays a key role in the occurrence and development of HCC.92–94 We describe here the changes in HSP after IRFA. After the tumor cells were heated in a 50-degree water bath for 10 min, Yoshida et al found that HSP 27, 70, and 90 increased significantly on the fifth day after heat treatment and returned to the baseline level after 12 days.21 Similarly, Zaimoku et al reported that the induction of HSP70 promoter increased with the increase of culture temperature or heating time in HepG2 and Huh7.47 Another study demonstrated elevated heat-induced expression of HSP.39 Two studies reported that heat induction can cause increased expression of stat3, which binds to HSP90 as a complex to promote survival. HSP90 inhibitors can reduce the complex formation and promote apoptosis in tumor cells.25,53 Two studies by Chen et al found that the HSP90/Akt/mTOR pathway is involved in the signaling between autophagy and HSPs, and subsequent studies found that the HSP90 inhibitor 17-AAG, in combination with the autophagy inhibitor 3-mA, promoted apoptosis more significantly than monotherapy, suggesting an association between heat-induced heat shock processes and autophagy.39,43 HSP is also involved in the heat-induced EMT process. STIP1, which is increased by IRFA, has been reported to bind to HSP to form a complex involved in the development of EMT.25
Immune Microenvironment
Immunotherapy is penetrating all aspects of the therapy of cancer, and the immune microenvironment (TIME) is an important factor determining the efficacy of immunotherapy.95–97 Local treatment can alter the immune microenvironment of HCC and thus affect its efficacy.3,98 Studies by Zerbini et al and Mizukoshi et al have shown that RFA monotherapy can activate tumor-specific T cells,99,100 but is not sufficient to control tumor progression. Two clinical studies by Duffy et al showed that subtotal RFA combined with CTLA4 inhibitors resulted in an increase of CD8+ T cells in a tumor or peripheral blood,101,102 and similar results have been observed in animal studies.103 Notably, IRFA combined with PD-1 inhibitors may not yield better results. Shi et al found that complete ablation combined with PD-1 blockade therapy significantly increased CD8+ T cells, and this combination was found to achieve a better survival benefit in the experiments in vitro.104 However, in a follow-up study, Shi et al found that IRFA triggered an increase in infiltrating myeloid cells in residual tumors and suppressed T-cell function, which ultimately blocked the effect of PD-1 inhibitors.52 This contradicts the study by Duffy et al and may be due to differences in immunosuppressant or differences in the means of ablation. Nevertheless, the changes in TIME caused by IRFA are uncertain, and more research is needed, from clinical trials to experiments in vivo.
Hypoxia-Inducible Factor
Although hypoxia has a negative impact on tumor proliferation in some cases, however, the main aspect is to adapt the tumor cells to the oxygen and nutrient deficit, thus inducing cancer cell survival by activating autophagy, suppressing apoptosis.105,106
Hypoxia-inducible factor (HIF) expression is significantly elevated in the hypoxic tumor microenvironment, which contributes to mechanisms such as metabolic reprogramming, autophagy, angiogenesis, and EMT.107–109 Tong et al simulated the survival environment of residual cells after IRFA through heating and hypoxia culture. They found that HIF-1α is dependent on TGF-β to activate downstream pathways that contribute to EMT and survival of CSCs in MHCC97H and SMMC7721.24 Another study found that the use of heat alone also resulted in elevated HIF-1α expression,48 confirming that IRFA can influence surviving tumor cells through both heat and hypoxia.110,111 In addition, to adapt to the absence of oxygen and nutrient, the tumor will increase the growth of blood vessels as much as possible. IRFA leads to more severe hypoxia,60 and therefore, angiogenesis is more common. Three studies have shown that,48–50 similar to other ways,112,113 IRFA can also activate the HIF-1α-VEGF axis to enhance angiogenesis. HIF-1α has also been reported to be involved in the process of metabolic reprogramming,114,115 while sublethal heat stress-induced O-GlcNAcylation regulates the Warburg effect in HCC cells by promoting the stability of HIF-1α,34 suggesting that sublethal heat stress may act as a “switch” that triggers a stronger Warburg effect to drive HCC progression.
Growth Factors
Angiogenesis is associated with the growth and metastasis of tumors.116 VEGF has been shown to play a crucial role in tumor angiogenesis. The binding of VEGF to its receptors leads to new vessel formation by inducing mitogenesis and chemotaxis of normal endothelial cells and increasing vascular permeability.117,118 VEGF has long been shown to be highly expressed in HCC tissue.119,120 VEGF or its receptors tends to be more abundantly expressed in liver cancer tissue after IRFA, and as a result, more tumor angiogenesis was observed.24,46,48,49,54,57,121–123 Notably, Tan et al indicated that VEGFR-2 was not affected by temperature, whereas VEGFR-1 was significantly elevated following incomplete ablation and was strongly associated with tumor metastasis and stemness.46
HGF is mainly secreted by mesenchymal cells, and serum HGF levels are significantly higher in HCC patients compared to normal controls.124,125 HGF activates downstream c-met, causing a series of downstream biological programs that ultimately inhibit apoptosis and promote cell proliferation and invasion.126,127 Previous studies reported that the expression of HGF increased significantly in tumor tissue after heat treatment.54 Zhang et al found that activated HSCs clustered around residual HCC cells, and HGF expression was up-regulated in the fibrous stroma, suggesting that HGF may come from activated HSCs.42 Two studies have shown that after IRFA, HGF acts mainly through the HGF/c-met/MAPK pathway.42,128
TGF-β has also been found to play an important role after IRFA. In hepatocarcinogenesis, TGF- β plays a dual role as an inhibitor in the early stage, but once the cell gets rid of its cell inhibition, it will promote tumor progression in the later stage.129–131 It was reported that TGF- β expression was significantly increased in tumor tissues of patients after RFA.132 Similar results were reported in a study by HE et al who found that the hypoxic environment following IRFA caused increased TGF-β secretion and caused EMT via the HIF-1a/TGF-b1/Snail pathway.24 Table 2 shows the potential relations or mechanisms of growth factors and IRFA.
 |
Table 2 Growth Factors and IRFA |
Hepatic Stellate Cells and Tumor-Associated Endothelial Cells
HSCs infiltrating HCC secrete many cytokines, extracellular matrix proteins chemokines, growth factors, and consequently remodel tumor microenvironment. The level of HGF in HSC-CM was found higher than that in control medium, and Zhang et al found HGF switches autophagic survival to proliferation in residual HCC cells through the regulation of HGF/c-Met/ERK1/2 signaling from downstream autophagic axis of ATG5/Beclin1 to proliferative axis of cyclinD1.42 In addition, the POSTN in HSC-CM promotes EMT and regulates the stemness of residual HCC cells enhancing the malignancy of HCC.27,45
Similar to HSCs, tumor-associated endothelial cells (TAECs) are also an important component of the microenvironment of tumor, which play a key role in angiogenesis. TAECs were activated by IRFA and the invasiveness of HCC cells was promoted when HCC cells were cultured in the conditioned medium from TAECs after IRFA.58 Additionally, the expression of ICAM-1 in TACEs was elevated consequently activating platelets and increasing endothelial permeability which is associated with the growth and metastasis of HCC after IRFA.10
Therapeutic Strategies
Sorafenib is a multi-kinase inhibitor and is one of the important drugs in the systemic treatment of advanced HCC.133,134 Sorafenib is a commonly used drug in preclinical trials involving IRFA. It has been reported to eliminate the differences in survival rates, migratory capacity, and invasive ability between heated or non-heated HCC cells, and it can inhibit heat-induced morphological changes23,24 as well as intra-tumor angiogenesis.30,49 Additionally, sorafenib also inhibits or reverses EMT,135 however, it has been shown that IRFA can cause a significant increase in ATPase inhibitory factor 1 (IF1) expression, which can blunt the effect of sorafenib.30
In addition to anti-malarial effects, chloroquine has been widely reported as potential anti-cancer agent due to its blocking of autophagy.136,137 We have said above that autophagy is a key mechanism for tumor cell survival after IRFA. Chloroquine significantly increased the apoptosis of HCC cells after IRFA and inhibited the enrichment of CSCs, and this effect was significantly enhanced by the combination of C-MET inhibitors.37,38,40,42
Some researchers found inflammation contributed to tumor metastasis and suppression of T-cell function.52,138,139 Jiang et al used different doses of aspirin to treat New Zealand white rabbits after IRFA. A significant decrease was observed in the levels of serum IL-6, hs-CRP, and TNF-α, the laboratory biomarkers of inflammation, after the treatment of aspirin. Additionally, aspirin brought more survival time and the decrease of the expression of PCNA, MMP-9, and VEGF.121
As mentioned earlier, the effect of IRFA on the immune microenvironment is very complex. Previous studies have shown that IRFA results in enhanced systemic antitumor T-cell immune responses and tumor expression related to the increasing of dental cell infection,140 however, the inflammation from IRFA may lead to the failure of PD-1 inhibitors.52 A clinical trial found the immune system could potentially also recognize and kill residual cancer, and tremelimumab (anti-CTLA4 treatment) could enhance this effect,101 which suggests that different immunosuppressants may have different effects on IRFA.
Discussion
Consistent with what has been observed in clinical practice, residual cells after IRFA are accelerating the progression of residual HCC cells through EMT, tumor stem cells, autophagy, heat shock response, immune microenvironment, and hypoxia-inducible factors. In addition to these ways, recent studies have shown that various growth factors are also extensively involved in angiogenesis, cell survival, proliferation, and cell migration after IRFA.46,48–51,55,123,128,132 Overall, IRFA activates residual HCC cells through multiple pathways.
The mechanisms of sorafenib,23,24,28,30,49,141 bevacizumab,48 melatonin,110 chloroquine,37,38,40,42 aspirin,121 metformin45,123 in suppressing residual HCC after IRFA has been successively proposed, and these drugs all work after RFA. Notably, tumor invasion and metastasis seem to occur very rapidly after IRFA, as reflected by the fact that EMT or autophagy-related molecules are rapidly elevated after RFA,21,35,42 which prompts us to use drug treatment as soon as possible after RFA. Although we have many ways to cope with incomplete ablation after surgery, it is difficult to kill the tumor cells completely due to the combined effect of these multiple pathways. Therefore, we should emphasize the complete killing of tumor cells intraoperatively, and it has been reported that at least a sufficiently safety margin (3 mm) can reduce the chance of tumor progression,142,143 but due to the large size, irregular shape, and “heat sink effect” of the tumor,144–146 it is difficult to complete ablation and ensure sufficient safe margin. The evaluation of complete RFA is based on follow-up imaging or postoperative pathology, therefore, it is important to develop new techniques which can help us immediately detect residual tumors or ensure sufficient safe margin. Kan et al recently developed a “one-stop-shop” interventional oncologic technology “Intraprocedural real-time optical imaging guidance for complete tumor ablation”, they can identify residual tumors in real-time during operation, to conduct repeated ablation and completely kill tumor cells.147 In addition, the European Association for the Study recognized the value of fusion imaging (FI) which tackled the limitations of each single imaging modality (computed tomography, ultrasound and magnetic resonance imaging).1 The FI technique provides more accurate determination and tumor location improving the rate of complete ablation.148
In conclusion, we summarized relevant animal and cell models for IRFA that have been used and could help subsequent researchers construct more suitable models. A series of mechanisms from EMT to growth factors were described in this review, and corresponding therapeutic strategies were summed up. Even so, IRFA is still an important reason for the progression of liver cancer. We need earlier and more effective imaging methods to evaluate early progress to facilitate repeated ablation. Considering that multiple pathways are activated, the combination of multiple targeted drugs and immunosuppressants may be an effective means. Additionally, we would like to point out that hepatocarcinogenesis is a typical multistage process and most HCC patients have underlying liver diseases which play key roles in tumor microenvironment,149,150 therefore, diverse animal models with liver diseases (such as cirrhosis) reflecting the clinical setting more closely are needed. As far as we know, although many studies have been published in this field, there is still a lack of relevant review. It is certain that with the deepening understanding of IRFA, we will help patients after ablation achieves more survival benefits.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
2. Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15(1):126. doi:10.1186/s12957-017-1196-2
3. Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29(9):5045–5051. doi:10.1007/s00330-019-06189-6
4. Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–A 10-year experience at a single center. Radiology. 2016;278(2):601–611. doi:10.1148/radiol.2015142489
5. Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307. doi:10.1016/j.jhep.2013.04.009
6. Cheung TT, Ma KW, She WH. A review on radiofrequency, microwave and high-intensity focused ultrasound ablations for hepatocellular carcinoma with cirrhosis. Hepatobiliary Surg Nutr. 2021;10(2):193–209. doi:10.21037/hbsn.2020.03.11
7. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
8. Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2021;273(3):564–571. doi:10.1097/SLA.0000000000003268
9. Lee S, Kang TW, Cha DI, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69(1):70–78. doi:10.1016/j.jhep.2018.02.026
10. Kong J, Yao C, Dong S, et al. ICAM-1 activates platelets and promotes endothelial permeability through VE-cadherin after insufficient radiofrequency ablation. Adv Sci. 2021;8(4):2002228. doi:10.1002/advs.202002228
11. Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. doi:10.1097/SLA.0b013e3181efc656
12. Seki T, Tamai T, Ikeda K, et al. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13(3):291–294. doi:10.1097/00042737-200103000-00014
13. Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res. 2003;27(2):163–167. doi:10.1016/S1386-6346(03)00207-9
14. Portolani N, Tiberio GA, Ronconi M, et al. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepato-Gastroenterology. 2003;50(54):2179–2184.
15. Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8(5):332–335. doi:10.1007/s10147-003-0328-6
16. Ruzzenente A, Manzoni GD, Molfetta M, et al. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World j Gastroenterol. 2004;10(8):1137–1140. doi:10.3748/wjg.v10.i8.1137
17. Lam VW, Ng KK, Chok KS, et al. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol. 2008;15(3):782–790. doi:10.1245/s10434-007-9733-9
18. Ahmed M, Brace CL, Lee FT
19. Nijkamp MW, Hoogwater FJ, Steller EJ, et al. CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. J Hepatol. 2010;53(6):1069–1077. doi:10.1016/j.jhep.2010.04.040
20. Dong S, Kong J, Kong F, et al. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med. 2013;11(1):273. doi:10.1186/1479-5876-11-273
21. Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58(5):1667–1680. doi:10.1002/hep.26526
22. Zhang N, Wang L, Chai ZT, et al. Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-catenin signaling. PLoS One. 2014;9(12):e115949. doi:10.1371/journal.pone.0115949
23. Dong S, Kong J, Kong F, et al. Sorafenib suppresses the epithelial-mesenchymal transition of hepatocellular carcinoma cells after insufficient radiofrequency ablation. BMC Cancer. 2015;15:939. doi:10.1186/s12885-015-1949-7
24. Tong Y, Yang H, Xu X, et al. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci. 2017;108(4):753–762. doi:10.1111/cas.13191
25. Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–3527. doi:10.1038/s41388-018-0169-4
26. Zeng J, Cai X, Hao X, et al. LncRNA FUNDC2P4 down-regulation promotes epithelial-mesenchymal transition by reducing E-cadherin expression in residual hepatocellular carcinoma after insufficient radiofrequency ablation. Int j Hyperthermia. 2018;34(6):802–811. doi:10.1080/02656736.2017.1422030
27. Zhang R, Lin XH, Ma M, et al. Periostin involved in the activated hepatic stellate cells-induced progression of residual hepatocellular carcinoma after sublethal heat treatment: its role and potential for therapeutic inhibition. J Transl Med. 2018;16(1):302. doi:10.1186/s12967-018-1676-3
28. Zhang R, Ma M, Lin XH, et al. Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment. BMC Cancer. 2018;18(1):901. doi:10.1186/s12885-018-4820-9
29. Zhang N, Li H, Qin C, et al. Insufficient radiofrequency ablation promotes the metastasis of residual hepatocellular carcinoma cells via upregulating flotillin proteins. J Cancer Res Clin Oncol. 2019;145(4):895–907. doi:10.1007/s00432-019-02852-z
30. Kong J, Yao C, Ding X, et al. ATPase inhibitory factor 1 promotes hepatocellular carcinoma progression after insufficient radiofrequency ablation, and attenuates cell sensitivity to sorafenib therapy. Front Oncol. 2020;10:1080. doi:10.3389/fonc.2020.01080
31. Li Z, Jiang M, Zhang T, Liu S. GAS6-AS2 promotes hepatocellular carcinoma via miR-3619-5p/ARL2 axis under insufficient radiofrequency ablation condition. Cancer Biother Radiopharm. 2020;36:879–889.
32. Ma D, Gao X, Liu Z, Lu X, Ju H, Zhang N. Exosome-transferred long non-coding RNA ASMTL-AS1 contributes to malignant phenotypes in residual hepatocellular carcinoma after insufficient radiofrequency ablation. Cell Prolif. 2020;53(9):e12795. doi:10.1111/cpr.12795
33. Zhou T, Liu B, Wang Y, et al. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition mediated by interleukin-6/signal transducer and activator of transcription 3/Snail pathway in the H22 cells. J Cancer Res Ther. 2020;16(5):1112–1118. doi:10.4103/jcrt.JCRT_12_20
34. Chen Y, Bei J, Liu M, et al. Sublethal heat stress-induced O-GlcNAcylation coordinates the Warburg effect to promote hepatocellular carcinoma recurrence and metastasis after thermal ablation. Cancer Lett. 2021;518:23–34. doi:10.1016/j.canlet.2021.06.001
35. Fan Z, Gao Y, Zhang W, et al. METTL3/IGF2BP1/CD47 contributes to the sublethal heat treatment induced mesenchymal transition in HCC. Biochem Biophys Res Commun. 2021;546:169–177. doi:10.1016/j.bbrc.2021.01.085
36. Su T, Huang M, Liao J, et al. Insufficient radiofrequency ablation promotes hepatocellular carcinoma metastasis through N6-Methyladenosine mRNA methylation-dependent mechanism. Hepatology. 2021;74(3):1339–1356. doi:10.1002/hep.31766
37. Wang X, Deng Q, Feng K, et al. Insufficient radiofrequency ablation promotes hepatocellular carcinoma cell progression via autophagy and the CD133 feedback loop. Oncol Rep. 2018;40(1):241–251. doi:10.3892/or.2018.6403
38. Zhao Z, Wu J, Liu X, et al. Insufficient radiofrequency ablation promotes proliferation of residual hepatocellular carcinoma via autophagy. Cancer Lett. 2018;421:73–81. doi:10.1016/j.canlet.2018.02.024
39. Chen F, Bao H, Xie H, Tian G, Jiang T. Heat shock protein expression and autophagy after incomplete thermal ablation and their correlation. Int j Hyperthermia. 2019;36(1):95–103. doi:10.1080/02656736.2018.1536285
40. Jiang J, Chen S, Li K, et al. Targeting autophagy enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis for hepatocellular carcinoma. Int J Hyperthermia. 2019;36(1):499–510. doi:10.1080/02656736.2019.1600052
41. Xu WL, Wang SH, Sun WB, et al. Insufficient radiofrequency ablation-induced autophagy contributes to the rapid progression of residual hepatocellular carcinoma through the HIF-1α/BNIP3 signaling pathway. BMB Rep. 2019;52(4):277–282. doi:10.5483/BMBRep.2019.52.4.263
42. Zhang R, Lin XH, Liu HH, et al. Activated hepatic stellate cells promote progression of post-heat residual hepatocellular carcinoma from autophagic survival to proliferation. Int J Hyperthermia. 2019;36(1):253–263. doi:10.1080/02656736.2018.1558459
43. Chen F, Xie H, Bao H, Violetta L, Zheng S. Combination of HSP90 and autophagy inhibitors promotes hepatocellular carcinoma apoptosis following incomplete thermal ablation. Mol Med Rep. 2020;22(1):337–343. doi:10.3892/mmr.2020.11080
44. Li Q, Ni Y, Zhang L, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Targeted Ther. 2021;6(1):76. doi:10.1038/s41392-020-00453-8
45. Zhang R, Yao RR, Li JH, et al. Activated hepatic stellate cells secrete periostin to induce stem cell-like phenotype of residual hepatocellular carcinoma cells after heat treatment. Sci Rep. 2017;7(1):2164. doi:10.1038/s41598-017-01177-6
46. Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40. doi:10.1016/j.canlet.2019.05.041
47. Zaimoku R, Miyashita T, Tajima H, et al. Monitoring of heat shock response and phenotypic changes in hepatocellular carcinoma after heat treatment. Anticancer Res. 2019;39(10):5393–5401. doi:10.21873/anticanres.13733
48. Kong J, Kong J, Pan B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS One. 2012;7(5):e37266. doi:10.1371/journal.pone.0037266
49. Xu M, Xie XH, Xie XY, et al. Sorafenib suppresses the rapid progress of hepatocellular carcinoma after insufficient radiofrequency ablation therapy: an experiment in vivo. Acta Radiol. 2013;54(2):199–204. doi:10.1258/ar.2012.120249
50. Wu L, Fu Z, Zhou S, et al. HIF-1α and HIF-2α: siblings in promoting angiogenesis of residual hepatocellular carcinoma after high-intensity focused ultrasound ablation. PLoS One. 2014;9(2):e88913. doi:10.1371/journal.pone.0088913
51. Rozenblum N, Zeira E, Scaiewicz V, et al. Oncogenesis: an “Off-Target” effect of radiofrequency ablation. Radiology. 2015;276(2):426–432. doi:10.1148/radiol.2015141695
52. Shi L, Wang J, Ding N, et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun. 2019;10(1):5421. doi:10.1038/s41467-019-13204-3
53. Sun C, Bai M, Ke W, Wang X, Zhao X, Lu Z. The HSP90 inhibitor, XL888, enhanced cell apoptosis via downregulating STAT3 after insufficient radiofrequency ablation in hepatocellular carcinoma. Life Sci. 2021;282:119762. doi:10.1016/j.lfs.2021.119762
54. Ke S, Ding XM, Kong J, et al. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J Transl Med. 2010;8:73. doi:10.1186/1479-5876-8-73
55. Dai H, Jia G, Wang H, Yang J, Jiang H, Chu M. Epidermal growth factor receptor transactivation is involved in the induction of human hepatoma SMMC7721 cell proliferation by insufficient radiofrequency ablation. Oncol Lett. 2017;14(2):2463–2467. doi:10.3892/ol.2017.6463
56. Thompson SM, Jondal DE, Butters KA, et al. Heat stress induced, ligand-independent MET and EGFR signalling in hepatocellular carcinoma. Int J Hyperthermia. 2018;34(6):812–823. doi:10.1080/02656736.2017.1385859
57. Zhang Y, Zhang Y, Wang J, Gu H. Amarogentin inhibits liver cancer cell angiogenesis after insufficient radiofrequency ablation via affecting stemness and the p53-dependent VEGFA/Dll4/Notch1 pathway. Biomed Res Int. 2020;2020:5391058. doi:10.1155/2020/5391058
58. Kong J, Kong L, Kong J, et al. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J Transl Med. 2012;10:230. doi:10.1186/1479-5876-10-230
59. Obara K, Matsumoto N, Okamoto M, et al. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int. 2008;2(1):116–123. doi:10.1007/s12072-007-9040-3
60. Nijkamp MW, van der Bilt JD, de Bruijn MT, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249(5):814–823. doi:10.1097/SLA.0b013e3181a38ef5
61. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. doi:10.1016/j.cell.2016.06.028
62. Singh M, Yelle N, Venugopal C, Singh SK. EMT: mechanisms and therapeutic implications. Pharmacol Ther. 2018;182:80–94. doi:10.1016/j.pharmthera.2017.08.009
63. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi:10.1016/j.tcb.2018.12.001
64. Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49(3):361–374. doi:10.1016/j.devcel.2019.04.010
65. Tashiro E, Henmi S, Odake H, Ino S, Imoto M. Involvement of the MEK/ERK pathway in EGF-induced E-cadherin down-regulation. Biochem Biophys Res Commun. 2016;477(4):801–806. doi:10.1016/j.bbrc.2016.06.138
66. Tian YC, Chen YC, Chang CT, et al. Epidermal growth factor and transforming growth factor-beta1 enhance HK-2 cell migration through a synergistic increase of matrix metalloproteinase and sustained activation of ERK signaling pathway. Exp Cell Res. 2007;313(11):2367–2377. doi:10.1016/j.yexcr.2007.03.022
67. Uttamsingh S, Bao X, Nguyen KT, et al. Synergistic effect between EGF and TGF-beta1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27(18):2626–2634. doi:10.1038/sj.onc.1210915
68. Lu L, Zhang Q, Wu K, et al. Hepatitis C virus NS3 protein enhances cancer cell invasion by activating matrix metalloproteinase-9 and cyclooxygenase-2 through ERK/p38/NF-κB signal cascade. Cancer Lett. 2015;356(2Pt B):470–478. doi:10.1016/j.canlet.2014.09.027
69. Ren D, Yang Q, Dai Y, et al. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-κB signaling pathway. Mol Cancer. 2017;16(1):117. doi:10.1186/s12943-017-0688-6
70. Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A. EMT factors and metabolic pathways in cancer. Front Oncol. 2020;10:499. doi:10.3389/fonc.2020.00499
71. Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15(1):18. doi:10.1186/s12943-016-0502-x
72. Li T, Xie J, Shen C, et al. Amplification of long noncoding RNA ZFAS1 promotes metastasis in hepatocellular carcinoma. Cancer Res. 2015;75(15):3181–3191. doi:10.1158/0008-5472.CAN-14-3721
73. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi:10.1038/nm.4409
74. Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 2017;50(3):117–125. doi:10.5483/BMBRep.2017.50.3.222
75. Park DJ, Sung PS, Kim JH, et al. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J Immunother Cancer. 2020;8(1):e000301. doi:10.1136/jitc-2019-000301
76. Yin T, Wang G, He S, Liu Q, Sun J, Wang Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell Immunol. 2016;300:41–45. doi:10.1016/j.cellimm.2015.11.009
77. Wang Y, Zhu P, Luo J, et al. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. EMBO J. 2019;38(17):e101110. doi:10.15252/embj.2018101110
78. Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12. doi:10.1186/s12943-020-1138-4
79. Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9(9):1167–1181. doi:10.1158/2159-8290.CD-19-0292
80. Eskelinen EL. The dual role of autophagy in cancer. Curr Opin Pharmacol. 2011;11(4):294–300. doi:10.1016/j.coph.2011.03.009
81. Singh SS, Vats S, Chia AY, et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37(9):1142–1158. doi:10.1038/s41388-017-0046-6
82. Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32(7):955–963. doi:10.1093/carcin/bgr031
83. Rao S, Tortola L, Perlot T, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. doi:10.1038/ncomms4056
84. Yang S, Yang L, Li X, et al. New insights into autophagy in hepatocellular carcinoma: mechanisms and therapeutic strategies. Am J Cancer Res. 2019;9(7):1329–1353.
85. Ni HM, Woolbright BL, Williams J, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;61(3):617–625. doi:10.1016/j.jhep.2014.04.043
86. Zhao Y, Gong S, Shunmei E, Zou J. Induction of macroautophagy by heat. Mol Biol Rep. 2009;36(8):2323–2327. doi:10.1007/s11033-009-9451-4
87. Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat shock proteins and cancer. Trends Pharmacol Sci. 2017;38(3):226–256. doi:10.1016/j.tips.2016.11.009
88. Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40(2):253–266. doi:10.1016/j.molcel.2010.10.006
89. Chatterjee S, Burns TF. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci. 2017;18(9):1978. doi:10.3390/ijms18091978
90. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92(19):1564–1572. doi:10.1093/jnci/92.19.1564
91. Fuller KJ, Issels RD, Slosman DO, Guillet JG, Soussi T, Polla BS. Cancer and the heat shock response. Eur j Cancer. 1994;30a(12):1884–1891. doi:10.1016/0959-8049(94)00362-9
92. Leng AM, Liu T, Yang J, et al. The apoptotic effect and associated signalling of HSP90 inhibitor 17-DMAG in hepatocellular carcinoma cells. Cell Biol Int. 2012;36(10):893–899. doi:10.1042/CBI20110473
93. Wang B, Chen L, Ni Z, et al. Hsp90 inhibitor 17-AAG sensitizes Bcl-2 inhibitor (-)-gossypol by suppressing ERK-mediated protective autophagy and Mcl-1 accumulation in hepatocellular carcinoma cells. Exp Cell Res. 2014;328(2):379–387. doi:10.1016/j.yexcr.2014.08.039
94. Guo K, Kang NX, Li Y, et al. Regulation of HSP27 on NF-kappaB pathway activation may be involved in metastatic hepatocellular carcinoma cells apoptosis. BMC Cancer. 2009;9:100. doi:10.1186/1471-2407-9-100
95. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi:10.1038/s41591-018-0014-x
96. Taube JM, Galon J, Sholl LM, et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod pathol. 2018;31(2):214–234. doi:10.1038/modpathol.2017.156
97. El-Kenawi A, Hänggi K, Ruffell B. The immune microenvironment and cancer metastasis. Cold Spring Harb Perspect Med. 2020;10(4):a037424. doi:10.1101/cshperspect.a037424
98. Shen S, Peng H, Wang Y, et al. Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer. 2018;18(1):117. doi:10.1186/s12885-018-4011-8
99. Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66(2):1139–1146. doi:10.1158/0008-5472.CAN-05-2244
100. Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457. doi:10.1002/hep.26153
101. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–551. doi:10.1016/j.jhep.2016.10.029
102. Xie C, Duffy AG, Mabry-Hrones D, et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology. 2019;69(5):2048–2060. doi:10.1002/hep.30482
103. Zhang L, Wang J, Jiang J, Zhang M, Shen J. CTLA-4 blockade suppresses progression of residual tumors and improves survival after insufficient radiofrequency ablation in a subcutaneous murine hepatoma model. Cardiovasc Intervent Radiol. 2020;43(9):1353–1361. doi:10.1007/s00270-020-02505-6
104. Shi L, Chen L, Wu C, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res. 2016;22(5):1173–1184. doi:10.1158/1078-0432.CCR-15-1352
105. Bastani S, Akbarzadeh M, Rastgar Rezaei Y, et al. Melatonin as a therapeutic agent for the inhibition of hypoxia-induced tumor progression: a description of possible mechanisms involved. Int J Mol Sci. 2021;22(19):10874. doi:10.3390/ijms221910874
106. Mazure NM, Pouysségur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22(2):177–180. doi:10.1016/j.ceb.2009.11.015
107. Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758–770. doi:10.1016/j.trecan.2016.10.016
108. Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17(1):30–54. doi:10.1111/jcmm.12004
109. Schödel J, Grampp S, Maher ER, et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69(4):646–657. doi:10.1016/j.eururo.2015.08.007
110. Li M, Hao B, Zhang M, et al. Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct Targeted Ther. 2021;6(1):330. doi:10.1038/s41392-021-00745-7
111. Yamada S, Utsunomiya T, Morine Y, et al. Expressions of hypoxia-inducible factor-1 and epithelial cell adhesion molecule are linked with aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy. Ann Surg Oncol. 2014;21(Suppl 3):S436–42. doi:10.1245/s10434-014-3575-z
112. Zhu H, Zhang S. Hypoxia inducible factor-1α/vascular endothelial growth factor signaling activation correlates with response to radiotherapy and its inhibition reduces hypoxia-induced angiogenesis in lung cancer. J Cell Biochem. 2018;119(9):7707–7718. doi:10.1002/jcb.27120
113. Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor–HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97.
114. Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–S67. doi:10.1016/S1471-4914(02)02317-1
115. Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107(5):2037–2042. doi:10.1073/pnas.0914433107
116. Zhang L, Wang JN, Tang JM, et al. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol Biol Rep. 2012;39(5):5085–5093. doi:10.1007/s11033-011-1304-2
117. Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol. 2003;30(3 Suppl 6):39–50. doi:10.1016/S0093-7754(03)00124-6
118. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi:10.1038/35025215
119. Sharma BK, Srinivasan R, Chawla YK, Chakraborti A. Vascular endothelial growth factor: evidence for autocrine signaling in hepatocellular carcinoma cell lines affecting invasion. Indian J Cancer. 2016;53(4):542–547. doi:10.4103/0019-509X.204765
120. Moon WS, Rhyu KH, Kang MJ, et al. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod pathol. 2003;16(6):552–557. doi:10.1097/01.MP.0000071841.17900.69
121. Jiang T, Zhang X, Ding J, Duan B, Lu S. Inflammation and cancer: inhibiting the progression of residual hepatic VX2 carcinoma by anti-inflammatory drug after incomplete radiofrequency ablation. Int J Clin Exp Pathol. 2015;8(11):13945–13956.
122. Liu Z, Dai H, Jia G, Li Y, Liu X, Ren W. Insufficient radiofrequency ablation promotes human hepatoma SMMC7721 cell proliferation by stimulating vascular endothelial growth factor overexpression. Oncol Lett. 2015;9(4):1893–1896. doi:10.3892/ol.2015.2966
123. Zhang Q, Kong J, Dong S, Xu W, Sun W. Metformin exhibits the anti-proliferation and anti-invasion effects in hepatocellular carcinoma cells after insufficient radiofrequency ablation. Cancer Cell Int. 2017;17(1):48. doi:10.1186/s12935-017-0418-6
124. Shiota G, Okano J, Kawasaki H, Kawamoto T, Nakamura T. Serum hepatocyte growth factor levels in liver diseases: clinical implications. Hepatology. 1995;21(1):106–112. doi:10.1002/hep.1840210119
125. Yu J, Chen GG, Lai PBS. Targeting hepatocyte growth factor/c-mesenchymal-epithelial transition factor axis in hepatocellular carcinoma: rationale and therapeutic strategies. Med Res Rev. 2021;41(1):507–524. doi:10.1002/med.21738
126. Nakayama N, Kashiwazaki H, Kobayashi N, et al. Hepatocyte growth factor and c-met expression in Long-Evans Cinnamon rats with spontaneous hepatitis and hepatoma. Hepatology. 1996;24(3):596–602. doi:10.1002/hep.510240323
127. Giordano S, Columbano A. Met as a therapeutic target in HCC: facts and hopes. J Hepatol. 2014;60(2):442–452. doi:10.1016/j.jhep.2013.09.009
128. Jia G, Li F, Tong R, et al. c-Met/MAPK pathway promotes the malignant progression of residual hepatocellular carcinoma cells after insufficient radiofrequency ablation. Med Oncol. 2020;37(12):117. doi:10.1007/s12032-020-01444-z
129. Fabregat I, Moreno-Càceres J, Sánchez A, et al. TGF-β signalling and liver disease. FEBS J. 2016;283(12):2219–2232. doi:10.1111/febs.13665
130. Russell WE, Coffey RJ
131. Gonzalez-Sanchez E, Vaquero J, Férnandez-Barrena MG, et al. The TGF-β pathway: a pharmacological target in hepatocellular carcinoma? Cancers. 2021;13(13):3248. doi:10.3390/cancers13133248
132. Iwahashi S, Shimada M, Utsunomiya T, et al. Epithelial-mesenchymal transition-related genes are linked to aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation. Cancer Lett. 2016;375(1):47–50. doi:10.1016/j.canlet.2016.02.041
133. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
134. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
135. Nagai T, Arao T, Furuta K, et al. Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2011;10(1):169–177. doi:10.1158/1535-7163.MCT-10-0544
136. Silva VR, Neves SP, Santos LS, Dias RB, Bezerra DP. Challenges and therapeutic opportunities of autophagy in cancer therapy. Cancers. 2020;12(11):3461. doi:10.3390/cancers12113461
137. Ferreira PMP, Sousa RWR, Ferreira JRO, Bezerra DP. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol Res. 2021;168:105582. doi:10.1016/j.phrs.2021.105582
138. Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8(20):3267–3273. doi:10.4161/cc.8.20.9699
139. Rajput S, Wilber A. Roles of inflammation in cancer initiation, progression, and metastasis. Front Biosci. 2010;2:176–183. doi:10.2741/s55
140. Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251(1):58–66. doi:10.1148/radiol.2511072175
141. Zhang R, Ma M, Dong G, et al. Increased matrix stiffness promotes tumor progression of residual hepatocellular carcinoma after insufficient heat treatment. Cancer Sci. 2017;108(9):1778–1786. doi:10.1111/cas.13322
142. Yoon JH, Lee JM, Klotz E, et al. Prediction of local tumor progression after Radiofrequency Ablation (RFA) of hepatocellular carcinoma by assessment of ablative margin using Pre-RFA MRI and Post-RFA CT registration. Korean j Radiol. 2018;19(6):1053–1065. doi:10.3348/kjr.2018.19.6.1053
143. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195(3):758–765. doi:10.2214/AJR.09.2954
144. Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175. doi:10.1007/s00270-012-0377-1
145. Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95(9):1931–1937. doi:10.1002/cncr.10892
146. Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28(5):897–908. doi:10.1016/j.bpg.2014.08.011
147. Kan X, Zhang F, Zhou G, et al. Interventional real-time optical imaging guidance for complete tumor ablation. Proc Natl Acad Sci U S A. 2021;118(41). doi:10.1073/pnas.2113028118
148. Lee DH, Lee JM. Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean j Radiol. 2018;19(4):545–559. doi:10.3348/kjr.2018.19.4.545
149. Baglieri J, Brenner DA, Kisseleva T. The role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma. Int J Mol Sci. 2019;20(7):1723. doi:10.3390/ijms20071723
150. Khan HA, Ahmad MZ, Khan JA, Arshad MI. Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance. HBPD Int. 2017;16(3):245–256. doi:10.1016/S1499-3872(17)60014-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
