Back to Journals » Drug Design, Development and Therapy » Volume 15
An Overview of Bimekizumab for the Treatment of Psoriatic Arthritis: The Evidence so Far
Authors G Oliveira D , Faria R , Torres T
Received 21 January 2021
Accepted for publication 25 February 2021
Published 9 March 2021 Volume 2021:15 Pages 1045—1053
DOI https://doi.org/10.2147/DDDT.S267405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Georgios Panos
Daniel G Oliveira,1 Raquel Faria,2,3 Tiago Torres3,4
1Department of Internal Medicine, Centro Hospitalar e Universitário do Porto, Porto, Portugal; 2Clinical Immunology Unit (UIC), Centro Hospitalar e Universitário do Porto, Porto, Portugal; 3Unit for Multidisciplinary Research in Biomedicine, Instituto de Ciências Biomédicas Abel Salazar – University of Porto, Porto, Portugal; 4Department of Dermatology, Centro Hospitalar e Universitário do Porto, Porto, Portugal
Correspondence: Tiago Torres
Department of Dermatology, Centro Hospitalar Universitário do Porto, Rua D. Manuel II, s/n, ex-CICAP, Porto, 4099-001, Portugal
Email [email protected]
Abstract: Psoriatic arthritis is a complex and heterogeneous disease with potential significant disability and impaired quality of life. Although in the last decades new treatment options have led to a better management of this disease, there are still significant unmet therapeutic needs. Dual inhibitor antibodies target two different cytokines simultaneously, potentially offering a better disease control. In psoriatic arthritis, there is evidence for a pathogenic role not only of IL-17A but also the structurally homologous IL-17F. It is postulated that differential expression of both in several targets of PsA could account for disparities in clinical response to IL-17A inhibition alone (such as with secukinumab or ixekizumab). Here we review the evidence so far for the use in psoriatic arthritis of bimekizumab, the first humanized monoclonal IgG1 antibody that selectively neutralizes both IL-17A and IL-17F. A Phase 2b trial reports better outcomes over both placebo and IL-17A inhibition alone. Very recently encouraging results from open-label extensions with regards to both safety and maintenance of response were presented. Phase III trials are ongoing with the first results awaited in 2021.
Keywords: psoriatic arthritis, psoriasis, bimekizumab, interleukin-17A, interleukin-17F, biologic therapy
Introduction
Psoriatic arthritis (PsA) is a complex and heterogeneous inflammatory disease that affects 20% to 30% of patients with psoriasis and is associated with substantial disability, impaired quality of life (QoL), and several comorbidities.1–3 It involves diverse clinical domains that extend beyond musculoskeletal manifestations (peripheral and axial arthritis, enthesitis and dactylitis): eg, nails, gut, and eyes, in addition to latent or manifest psoriasis.
Although there is still a huge gap in knowledge on the pathophysiology of PsA, what is known has fortunately turned into new treatment approaches that have improved symptoms and outcomes for PsA patients over the last two decades. Pro-inflammatory cytokines have been recognized as potential treatment targets in inflammatory diseases and have led to the creation of a number of anti-cytokine monoclonal antibodies that have revolutionized its treatment, such as TNFα and IL-12/23 inhibitors.4 More recently, the IL-17 pathway has been shown to play an important role in the pathophysiology of psoriatic disease and its blockage has shown to be clinically beneficial, as demonstrated with IL-17A inhibitors secukinumab and ixekizumab.4 Some patients, however, still do not respond, stop responding over time or suffer from side effects, leading to drug discontinuation, and other times combination strategies are required to control all PsA’s disease domains. Thus, there is still a great need for novel therapeutic options.5
Dual inhibitor antibodies target two different cytokines simultaneously potentially offering a better disease control. Interleukin (IL)-17A and IL-17F share structural homology and have a similar biologic function. IL-17A is classically considered to be the most biologically active, but recent studies have shown that IL-17F is also increased in psoriatic skin and synovial cell in psoriatic arthritis, supporting the rationale for targeting both IL-17A and IL-17F in psoriatic disease. Bimekizumab is the first-in-class monoclonal antibody designed to simultaneously target IL-17A and IL-17F.
This article aims to review the current knowledge on bimekizumab, the first dual inhibitor of IL-17A and IL-17F being studied to treat psoriatic arthritis.
The Role of Interleukin (IL)‑17A and IL‑17F in Psoriatic Arthritis
The IL-17 cytokine family comprises six different members (from A to F), of which IL-17A is the most studied. Known to be produced by a wide range of immune cells, IL-17A is involved in the pathophysiology of several inflammatory diseases including spondyloarthritis.6–8
Most non-hematopoietic cells possess IL-17 receptors, including fibroblasts, epithelial cells and synoviocytes,8 but despite this ubiquitous presence, IL-17 seems to have only moderate inflammatory capability per se, rather recruiting and amplifying other pathways, such as IL-6, IL-8, TNF and inflammatory-cell attracting chemokines.6,7,9,10
Still, evidence supporting the centrality of the IL-17 pathway in both PsO and PsA is available from a wide range of data.11 Th17 cells, IL-17 protein and related genes are elevated in both skin, blood and synovial fluid of PsO and PsA patients.11,12 In PsA, increased levels of IL-17+ CD4 and CD813,14, as well as IL-17A+Tγδ cells, have been found in the synovial fluid compared with peripheral blood. Specifically, the levels of IL-17+CD8+ cells in the synovial fluid distinguish PsA from rheumatoid arthritis (RA) and correlate with increased DAS28 scores, C-reactive protein levels, power-doppler findings of activity and prevalence of erosions.13 Inhibition of this pathway is capable of normalizing almost four times more disease-related genes than anti-TNFα treatments.11,15
Within the entire IL-17 family, IL-17F is the most structurally homologous (~50%) to IL-17A8 (Figure 1). They can both be secreted as homodimers (ie IL-17A/A or IL-17F/F) or as heterodimers of IL-17A/IL-17F,9 sharing signaling pathways through the same heterodimeric complex of IL-17 receptors A and C (IL-RA/RC) and biologic function.7–9
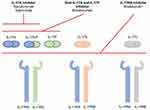 |
Figure 1 Summarized schematic of inhibition of the IL-17 cytokine family. *Not approved for psoriatic arthritis. Notes: Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, BioDrugs, Reis J, Vender R, Torres T. Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis, COPYRIGHT 2019.6 Abbreviations: IL, interleukin; IL-17RA, IL-17 receptor A; IL-17RB, IL-17 receptor B; IL-17RC, IL-17 receptor C; IL-17RE, IL-17 receptor E. |
The role of both IL-17A and F in psoriasis pathogenesis has been previously addressed.6,9,16
In enthesitis, a central pathologic process in PsA, Tγδ cells have recently been described that are capable of producing both IL-17A and IL-17F even independently of IL-23 stimulation.17 IL-17A and F had already been shown to promote osteogenic differentiation in in vitro models of human periosteum activated through the use of Th17 and Tγδ cells or through culture with serum from patients with ankylosing spondylitis,18 a mechanism potentially implied in the development of enthesitis. Importantly, both cytokines seem to be equipotent in this role, unlike in inflammatory processes where IL-17F seems to be less potent.18
Both IL-17A and IL-17F, when synergized with TNF, lead to increased production of pro-inflammatory cytokines, such as IL-8 and IL-6 in synoviocytes of PsA patients.9 IL-17A seems to be the most pro-inflammatory of the two cytokines.9,19 However, despite some inconsistencies in the literature regarding IL-17F detection levels which might be attributable to differences in methodology,19 IL-17F levels have been reported to be 30–50 times higher in some cytokine microenvironments, such as in psoriatic skin lesions of PsA patients20 or the synovium,21 which might dilute differences in relative potency. Additionally, IL-17F seems to be significantly increased in the synovium of PsA compared to osteoarthritis (OA) patients, unlike IL-17A.21 Dual neutralization of both IL-17A and IL-17F (using bimekizumab) resulted in greater downregulation of pro-inflammatory cytokine production than a single blockade in synovial fibroblasts.9,19 Critically, in in vitro models, anti-TNF blockade alone did not reduce the production of IL-8 as much as both IL-17A and F neutralization or even just anti-IL17A alone.9,19 In in vitro models of human periosteum dual blockade of IL-17A and F was also more effective in suppressing osteogenic differentiation than the blockade of either cytokine individually.18
Interestingly, in Tγδ cells, the predominant IL-17 production seems to be the F subtype.18 Also of note is the recent description that the IL-17receptorC (IL-17RC) competes with IL-17RA for IL-17F, IL-17A and IL-17A/F heterodimers,22 suggesting the possibility of IL-17RA-independent signaling pathways (and thus not targeted by brodalumab, an anti-IL17RA monoclonal antibody).
Bimekizumab
Bimekizumab is a humanized monoclonal IgG1 antibody that selectively neutralizes both IL-17A and IL-17F. In in vitro models, bimekizumab appears to be as potent as ixekizumab at inhibiting IL-17A (also more potent than secukinumab)8 but, unlike those drugs, also possesses the unique ability to inhibit IL-17F as well, functioning as a dual inhibitor. Unlike brodalumab, an IL-17 receptor A blocker – which targets not only IL-17A and F signaling but also IL-17 C, D and E – bimekizumab spares IL-17E (also known as IL-25), for example, which is believed to have anti-inflammatory properties.6
Bimekizumab demonstrates dose-proportional linear pharmacokinetics, with a half-life ranging from 17 to 26 days, and its distribution is restricted to the extravascular compartment.23 Currently, bimekizumab is in advanced clinical development for psoriasis, but also for psoriatic arthritis, and ankylosing spondylitis (both currently in phase III).
Bimekizumab in PsA – Efficacy
Phase I
The first bimekizumab clinical trial in PsA was a phase Ib randomized, double-blind, placebo-controlled clinical trial that included 53 patients (39 treated with bimekizumab, 14 with placebo) with active psoriatic arthritis who had failed conventional disease-modifying antirheumatic drugs (DMARDs) and/or one biologic DMARD. Patients in the active treatment arm were randomized to four different treatment regimens of varying loading doses (ranging from 80 to 560 mg) and maintenance doses (from 40 to 320 mg) at weeks 0, 3 and 6. Patients were followed for up to 20 weeks.9
Patients treated with bimekizumab had a faster response, compared to placebo. This was first detected at week two, with maximal or near-maximal responses maintained up to week 20, for both arthritis and skin psoriasis. ACR20, 50 and 70 responses were maximal at week 8 (80%), week 12 (57%) and week 16 (37%), respectively. For patients with skin involvement, PASI75 and PASI100 responses at week 8 were 100% and 87%, respectively (Table 1).
 |
Table 1 Results from Published Trials Involving Bimekizumab in Psoriatic Arthritis |
Phase II
BE ACTIVE10 was a 48-week multicentric, international, phase 2b dose-ranging, randomized, double-blind placebo-controlled trial to assess the efficacy and safety of bimekizumab. Two hundred and six adult patients (out of 308 screened) with active (tender and swollen count >3) PsA (diagnosed according to CASPAR criteria) were enrolled in 5 treatment arms (placebo, 16 mg, 160 mg with single 320 mg loading dose, 160 mg, 320 mg bimekizumab dose, with SC injections every 4 weeks). Concurrent use of TNF inhibitors was not permitted but conventional DMARDs (if on a stable dose and kept throughout the study), corticosteroids (equal or less 10mg/day) and NSAIDs were allowed. Sixteen-milligram bimekizumab (a much lower dose than other treatment arms) was tested with a programmed re-randomization at week 12 to either 160 or 320 mg dosing (meaning no placebo arm after 12 weeks). All patients received treatment up to week 48.
The primary outcome was ACR50 response at 12 weeks, a much more stringent outcome than used for other IL-17 inhibitors. The prespecified analysis was not possible due to the absence of a statistically significant difference versus placebo for the 320 mg group at week 12. All other outcomes were thus considered exploratory, rendering this a failed primary endpoint with no active comparator group.
At 12 weeks, significant ACR50 responses were present for every bimekizumab group, although lower in both the 16 mg and 320 mg dose group (Table 1 reports average values for all bimekizumab treatment groups). The 160 mg dosing had the greatest ACR and PASI response rates. These were confirmed to be increasing response rates up to week 24 and stability thereafter up to week 48, where the results of both 160 and 320 mg were similar. There were also responses in PASI scores, enthesitis, HAQ-DI and SF-36 across all bimekizumab doses. There was no loss of efficacy by week 48.
At the recent American College of Rheumatology (ACR) congress, additional data on BE ACTIVE were reported. BASDAI scoring was improved on the 93 patients in the treatment arm (160–320 mg bimekizumab) who had a baseline score >4 (mean 6.2 ± 1.42). BASDAI50 response rates were 43% and 56% at week 12 and 48, respectively.24
Regarding patient-reported outcomes (PROs), the Health assessment questionnaire Disability Index (HAQ-DI) and the psoriatic arthritis impact of disease-9 (PsAID-9) questionnaire developed specifically to assess health-related quality of life (QoL) in PsA were used on 206 patients from the BE ACTIVE trial. Rapid improvement was registered by week 12 and this response was sustained up to 48 weeks. Better QoL was associated with the better clinical outcomes reported in that study.25,26
Open-Label Extension Study (OLE)
Results from the 108 weeks of follow-up in the open-label extension study of BE ACTIVE (BE ACTIVE2, NCT03347110) have been recently presented.27,28 All patients who completed all 48 weeks of the BE ACTIVE trial were enrolled and switched to the 160 mg dosing regardless of previous treatment dose regimen. Over 108 weeks (an additional 60 weeks of OLE study over the 48 of the original BE ACTIVE trial) there was a 66.7% and 75.4% ACR 50 and body surface area (BSA) 0% response, respectively. Dactylitis and enthesitis were also significantly improved completely resolving in 65.9% and 77.9% of patients, respectively.27 Regarding week 12 responders, ACR20/50/70 and BSA 0% responses were maintained until week 108 in 80/78/81% and 72%, respectively.27 MDA/VLDA responses and DAPSA remission were maintained by 81/72/76% of Week 12 responders, respectively, to Week 120 (MDA/VLDA), and Week 108 (DAPSA remission).
Bimekizumab in PsA – Safety
Phase I
Over 90% of reported adverse events, in both arms, were mild or moderate. In the treatment arm, two fungal infections (oropharyngeal and vulvovaginal candidiasis) were reported, both treated with oral medication. There was no increased incidence of other infections. There were no deaths or severe adverse events resulting from treatment, and no patient discontinued bimekizumab.9
Phase II
No difference was found in the frequency of adverse events between placebo and treatment arms by week 12 in the BE ACTIVE trial. After reallocation (after week 12) and up to the 48 weeks of the trial 151 (74%) of the total 204 patients who ever received bimekizumab reported some AE (exposure adjusted incidence rate 166.8/100 patient-years). Most AE were mild or moderate (the most frequently reported were nasopharyngitis and upper respiratory tract infections) and there was no direct association with bimekizumab dose.
Nine patients (8 of which received bimekizumab) had serious adverse effects. These included one patient with drug-induced liver injury. Another patient also had severe liver enzyme elevation. Both had been given the 320 mg dosing. From the hepatic point of view, the other 11 patients were noted to have increased liver enzymes (>3x ULN). There was no relation with bimekizumab dose, and most were on DMARDs and one was on TB prophylaxis. At least two serious adverse events were related to infections across the entire study period (28 weeks) – 1 hepatitis E infection, 1 cellulitis (both with the 160 mg dosing). Non-severe Candida infection was reported in 7% of the patients, none led to treatment discontinuation. Other serious AEs reported were melanoma in situ (160 mg), suicidal ideation (160 mg loading dose), and neutropenia (320 mg dosing) (only in one patient each).10 In summary, this safety profile overlaps with those of other anti-IL17 therapies.29
In the OLE study, at week 108, serious adverse events occurred in 9.3% of patients (no deaths or major adverse cardiac events) and a total of 8.8% of patients withdrew from the study due to side effects. Full publication is still pending but the authors share that the safety profile observed in the OLE study reflected previous observations.27
Discussion
Dual inhibitor antibodies represent a novel therapeutic strategy, and a logical extension of the success monoclonal antibodies has had over the last couple of decades.
Here we review the most recent information on IL-17A and F inhibition in psoriatic arthritis through the first-of-its-class bimekizumab, a dual inhibitor of both cytokines.
The importance of the IL-17 pathway in psoriatic arthritis, already suggested by preclinical data, was reinforced by the excellent results obtained by secukinumab30 or ixekizumab31 in the control of the disease in the last few years.
Indeed, IL-17 seems to be involved in all of the clinical domains of psoriatic arthritis. In preclinical trials, it has been shown that both IL-17A and F are capable of inducing pro-inflammatory cytokines, like IL-8 or IL-6, in synoviocytes, periosteum and the skin,23 and that this activation was greatly suppressed by blocking both these cytokines simultaneously. Research is expanding on the differential role of IL-17F in different environments,18,21 compared with the more studied IL-17A, as well as possible alternative signaling pathways.22 Taken together these findings could potentially explain different clinical phenotypes in PsA and treatment responses to anti-IL17A (secukinumab, ixekizumab) and IL-17RA (brodalumab) inhibitors furthering support for the use of dual cytokine blockade such as with bimekizumab (Figure 1).
Phase II trials, specifically BE ACTIVE results, have been encouraging. Bimekizumab has shown to be relatively fast-acting, with initial improvements detected by week 8 and well established by week 12. Additionally, at a dose of 160 mg every 4 weeks, bimekizumab has shown to be capable of retaining this level of response in a high percentage of patients for at least 2 years. These results are independent of prior exposure to anti-TNF therapy.10
As with all new drugs, there are still pending questions regarding its optimal use. In BE ACTIVE,10 in which patients received four different dosages through the first 12 weeks, the 160 mg seemed most effective. The initial lower response in the 320 mg group might have been produced by a higher proportion of refractory patients in which bimekizumab took longer to work. This impression is reinforced, in the author’s opinion, by the fact that response rates were different as early as week 4 in both 160 mg (loading dose) and 320 mg dose groups although by that time period both groups had received the same dose. Co-medication was balanced between both groups.
Whichever dose proves best, these results were achieved with mostly mild side-effects that did not lead to treatment discontinuation – most commonly nasopharyngitis, upper respiratory infections and candidiasis. Overall the available data have not revealed any unexpected adverse events. Nonetheless, the number of patients included in the trials is still small. Thirteen out of the 204 patients (6,4%) receiving any dose of bimekizumab in the BE ACTIVE trial had some hepatic adverse effect, raising the need for attentive monitoring by treating physicians. Co-medication needs to be well pondered in this setting as well, but if real-world outcomes of bimekizumab prove as beneficial as in the trials there might be a reduced need for concomitant use of other DMARDs. Although IL-17F has been shown to be associated with increased susceptibility in many forms of human cancer, it has shown a protective role in colon tumorigenesis in mice,32,33 mainly by regulating tumor angiogenesis.6 Longer and bigger trials will be needed to fully ascertain the safety of bimekizumab.
Overall the available results for this new therapeutic option in psoriatic arthritis are encouraging, although it is still early to completely understand the added value offered by bimekizumab. As of yet, however, there are no head-to-head trials directly comparing it to other treatment options in PsA. Anti-IL17A monoclonal antibodies have been evaluated against other therapies, such as anti-TNF inhibitors in the treatment of PsA with mixed results (using different endpoints).34,35
Right now we can only look to early reports from the more advanced Phase 3 trials in psoriasis, where bimekizumab was first studied, which already encompass hundreds of patients and compare bimekizumab with other biologics. A head-to-head comparison with ustekinumab was recently published36 involving 567 patients (321 randomized to bimekizumab, 163 to ustekinumab and 83 to a placebo arm that was switched to bimekizumab at week 16). Using a 320 mg dose of bimekizumab every 4 weeks (and not the 160 mg shown in BE ACTIVE to be the most efficacious in PsA) bimekizumab was superior to ustekinumab (85% vs 49.7% PASI 90 responses at week 16, p<0.001). This response was also sustained throughout the 52-week duration of the study (81.6% vs 55.8%, p<0.001). Similar responses (86.2% vs 47.2% PASI 90 at week 16, p<0.001) in the BE SURE trial comparing bimekizumab (320 mg every 4 weeks or 320 mg until week 16 and then every 8 weeks) and adalimumab (80 mg week 0, 40 mg week 1 and every 2 weeks) were recently presented.37 Switching adalimumab patients to bimekizumab resulted in increased response rates, comparable to rates in bimekizumab-randomized patients at week 56. UCB, the company developing bimekizumab, have also reported the superiority of bimekizumab against secukinumab.38
If nothing else, bimekizumab is a proof-of-concept for a novel avenue in treating inflammatory diseases. Up until now the clinical practice in inflammatory diseases has been to steer clear of the combination of monoclonal antibodies. The results of the trials reported here using bimekizumab to simultaneously inhibit two cytokines, even if related ones, are an important reminder of the redundant and overlapping nature of the immune system and of the multiple pathways through which one arrives at inflammatory disease.
As of yet, however, there are no head-to-head trials directly comparing bimekizumab to conventional DMARDS or other bDMARDs in PsA although the results reported here seem encouraging. Upcoming trials (see Table 2) will hopefully fill this gap in knowledge.
 |
Table 2 Ongoing Trials of Bimekizumab in Psoriatic Arthritis |
Conclusion
Psoriatic arthritis can be a severe and disabling disease. Although improvements in its treatment have been achieved in the past decade, its pathogenesis is not completely known, and its treatment is still difficult particularly throughout all disease domains.
The IL-17 pathway has been implicated in disease pathogenesis and targeting IL-17A with secukinumab and ixekizumab has shown good results, although there is still a large proportion of patients that respond only partially. The simultaneous blockade of both IL-17A and IL-17F seems to have a synergistic benefit, with IL-17F inhibition contributing with a differentiated role in both osteogenesis and skin inflammation, important domains of PsA.
Bimekizumab uses a novel approach to biologic treatment in psoriatic arthritis through dual cytokine blockade. Mounting evidence from early trials has shown a good safety and efficacy profile, with rapid onset and sustained response, with results now extending to 108 weeks of follow-up. Moreover, clinical trials in skin psoriasis have also shown that bimekizumab is highly effective, confirming the importance of inhibiting these two cytokines in psoriatic disease.
In the near future, phase III trials will help to better understand the potential of bimekizumab in the treatment of psoriatic arthritis.
Disclosure
Tiago Torres has received honoraria for acting as a consultant and/or as a speaker from AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Fresenius Kabi Pharma, Janssen, Leo Pharma, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sandoz, Sanofi, UCB, Viatris. The authors report no other conflicts of interest in this work.
References
1. Raho G, Koleva DM, Garattini L, Naldi L. The burden of moderate to severe psoriasis: an overview. PharmacoEconomics. 2012;30(11):1005–1013. doi:10.2165/11591580-000000000-00000
2. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e19.
3. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. Longo DL, editor. N Engl J Med. 2017;376(10):957–970. doi:10.1056/NEJMra1505557
4. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–712. doi:10.1136/annrheumdis-2020-217159
5. Orr C, Veale DJ. Is there a need for new agents with novel mechanisms of action in psoriatic arthritis? Ann Rheum Dis. 2014;73(6):951–953. doi:10.1136/annrheumdis-2013-204934
6. Reis J, Vender R, Torres T. Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis. BioDrugs. 2019;33(4):391–399. doi:10.1007/s40259-019-00361-6
7. Taams LS, Steel KJA, Srenathan U, Burns LA, Kirkham BW. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol. 2018;14(8):453–466.
8. Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
9. Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–532. doi:10.1136/annrheumdis-2017-212127
10. Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395(10222):427–440. doi:10.1016/S0140-6736(19)33161-7
11. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clinic Rev Allerg Immunol. 2018;55(3):379–390. doi:10.1007/s12016-018-8702-3
12. Wade SM, Canavan M, McGarry T, et al. Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheum Dis. 2019;78(3):350–354. doi:10.1136/annrheumdis-2018-214138
13. Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014;66(5):1272–1281. doi:10.1002/art.38376
14. Chowdhury AC, Chaurasia S, Mishra SK, Aggarwal A, Misra R. Misra R. IL-17 and IFN-γ producing NK and γδ-T cells are preferentially expanded in synovial fluid of patients with reactive arthritis and undifferentiated spondyloarthritis. Clin Immunol. 2017;183:207–212. doi:10.1016/j.clim.2017.03.016
15. Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124(5):1022–1030.e395. doi:10.1016/j.jaci.2009.08.046
16. Watanabe H, Kawaguchi M, Fujishima S, et al. Functional characterization of IL-17F as a selective neutrophil attractant in psoriasis. J Clin Investig Dermatol. 2009;129(3):650–656. doi:10.1038/jid.2008.294
17. Cuthbert RJ, Watad A, Fragkakis EM, et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis. 2019;78(11):1559. doi:10.1136/annrheumdis-2019-215210
18. Shah M, Maroof A, Gikas P, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open. 2020;6(2):e001306. doi:10.1136/rmdopen-2020-001306
19. Burns LA, Maroof A, Marshall D, et al. Presence, function, and regulation of IL‐17F‐expressing human CD4 + T cells. Eur J Immunol. 2020;50(4):568–580. doi:10.1002/eji.201948138
20. Kolbinger F, Loesche C, Valentin M-A, et al. β-Defensin 2 is a responsive biomarker of IL-17A–driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139(3):923–932.e8. doi:10.1016/j.jaci.2016.06.038
21. van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16(4):426. doi:10.1186/s13075-014-0426-z
22. Goepfert A, Lehmann S, Blank J, Kolbinger F, Rondeau J-M. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL-17RA-independent signaling. Immunity. 2020;52(3):499–512.e5. doi:10.1016/j.immuni.2020.02.004
23. Glatt S, Helmer E, Haier B, et al. First‐in‐human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL‐17A and IL‐17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001. doi:10.1111/bcp.13185
24. Deodhar A, Gossec L, Mease PJ, et al. Bimekizumab treatment is associated with improvements in back pain and fatigue in patients with active psoriatic arthritis: 48-week results from a Phase 2b study.
25. Gossec L, Mease PJ, Gottlieb AB, et al. AB0778 Association between patient-reported outcomes and disease activity in bimekizumab-treated patients with psoriatic arthritis. Ann Rheum Dis. 2020;79(Suppl 1):1687. doi:10.1136/annrheumdis-2020-eular.4204
26. Gossec L, Mease P, Gottlieb A, et al. Bimekizumab improves patient-reported outcomes in psoriatic arthritis: 48-week results from a phase 2b study and association between patient-reported outcomes and disease activity.
27. Mcinnes I, Merola JF, Mease PJ, et al. SAT0403 efficacy and safety of 108 weeks’ bimekizumab treatment in patients with psoriatic arthritis: interim results from a phase 2 open-label extension studY. Ann Rheum Dis. 2020;79(Suppl 1):1153. doi:10.1136/annrheumdis-2020-eular.1850
28. Merola J, Behrens F, Kivitz A, et al. Bimekizumab maintenance of response in patients with psoriatic arthritis: 2-year results from a Phase 2b dose-ranging study and its open-label extension.
29. Benfaremo D, Paci V, Luchetti MM, Gabrielli A. Novel therapeutic approaches and treatment targets for psoriatic arthritis. CPB [Internet]; September 28, 2020 [cited January 3, 2021]:21. Available from: https://www.eurekaselect.com/186326/article.
30. COSENTYX (secukinumab) injection, Label changes, FDA [Internet]; [cited January 1, 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125504s001s002lbl.pdf.
31. TALTZ (ixekizumab) injection, SC, Label changes, FDA [Internet]; [cited January 1, 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125521s004lbl.pdf.
32. Tong Z, Yang XO, Yan H, et al. A protective role by interleukin-17f in colon tumorigenesis. Katoh M, editor. PLoS One. 2012;7(4):e34959. doi:10.1371/journal.pone.0034959
33. Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34–44. doi:10.1016/j.cyto.2015.09.011
34. McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496–1505. doi:10.1016/S0140-6736(20)30564-X
35. Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131. doi:10.1136/annrheumdis-2019-215386
36. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi:10.1016/S0140-6736(21)00125-2
37. Warren R, Blauvelt A, Bagel J, et al. Bimekizumab efficacy and safety versus adalimumab in patients with moderate to severe plaque psoriasis: results from a multicenter, randomized, double-blinded active comparator-controlled phase 3 trial (Be Sure). J Skin. 2021;5(1):s15. doi:10.25251/skin.5.supp.15
38. UCB Press Release – Bimekizumab superior to Cosentix in achieving complete psoriasis skin clearance [Internet]; [cited January 1, 2020]. Available from: https://www.ucb.com/_up/ucb_com_presscenter/documents/b611cc6eeb06894e.pdf.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
