Back to Journals » Clinical Ophthalmology » Volume 8
An open-label, one-year, noncomparative study to evaluate the safety and tolerability of intravitreal pegaptanib sodium in patients with diabetic macular edema
Authors Sivaprasad S , Browning R, Starita C
Received 28 May 2014
Accepted for publication 10 July 2014
Published 21 August 2014 Volume 2014:8 Pages 1565—1571
DOI https://doi.org/10.2147/OPTH.S68498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Sobha Sivaprasad,1 Richard C Browning,2 Carla Starita2
1Consultant Ophthalmologist, King’s College Hospital, Denmark Hill, London, 2Pfizer Ltd, Walton Oaks, Dorking Road, Tadworth, Surrey, UK
Background: The purpose of this study was to evaluate the safety and tolerability of pegaptanib in patients with diabetic macular edema.
Methods: An open-label, multicenter, noncomparative, one-year study of approximately 500 patients was planned. Recruitment was terminated after enrollment of 46 patients. Enrolled patients were fully informed and reconsented; 12 patients elected to complete the study. Patients received intravitreal injections of pegaptanib 0.3 mg once every 6 weeks or less frequently, as determined by the investigator. Clinical benefit was evaluated after the patient received two or more injections. Ocular and nonocular adverse events were closely monitored throughout the study.
Results: Compared with baseline, mean best-corrected visual acuity increased by week 6. Ten patients reported ocular-related adverse events, none of which were severe, and eight patients reported nonocular adverse events, two of which were severe but unrelated to study treatment. Three serious adverse events, unrelated to study treatment, were reported.
Conclusion: In this limited set of patients with diabetic macular edema, pegaptanib appeared to be well tolerated with evidence of efficacy.
Keywords: pegaptanib, diabetic macular edema, safety, tolerability
Introduction
Diabetic macular edema (DME) is a complication of diabetic retinopathy occurring in patients with type 1 or type 2 diabetes.1–3 It is characterized by diffuse or cystic macular thickening with or without lipid exudation caused by breakdown of the inner and outer blood retinal barriers. If untreated, DME can lead to vision loss, and the condition is responsible for 4.8% of cases of blindness worldwide.4 Patient quality of life is affected at all stages of the disease, but may improve with treatment.1–3,5–7 As the incidence of diabetes increases worldwide,8–10 so does the prevalence of DME.11,12 Approximately 10% of all adults with diabetes experience vision-threatening diabetic retinopathy, and half of these progress to developing DME.13 Until recently, the standard treatment for DME has been laser photocoagulation, with no approved therapeutic options available for those who fail to respond to laser therapy. Therefore, clinical research has been performed to identify safe and effective treatments that improve both visual function and quality of life for patients with DME.
In the past few years, several studies have demonstrated that treatment with vascular endothelial growth factor (VEGF) inhibitors can result in statistically significant improvement of visual acuity in patients with DME.14–19 Pegaptanib sodium (Macugen®; Pfizer Inc, New York, NY, USA) is an aptamer that binds with high specificity and affinity to VEGF165, a protein implicated in the pathogenesis of age-related macular degeneration20 and DME.21–23 Thus, pegaptanib acts as a VEGF antagonist and is currently approved for the treatment of age-related macular degeneration but not for DME. A Phase II/III trial demonstrated that compared with sham treatment, administration of intravitreal pegaptanib every 6 weeks for one year resulted in statistically significant improvement in visual acuity as measured by ≥10-letter gains (P=0.0047) and patient quality of life as measured by a greater than five-point difference in the National Eye Institute Visual Functioning Questionnaire 25.3
This Phase IIIb study was designed to extend and further evaluate the safety and tolerability of pegaptanib in patients with DME. However, soon after study initiation, the sponsor decided to withdraw the regulatory application for DME. Further recruitment of patients was immediately stopped. Patients already enrolled in the study were informed and given the opportunity to either withdraw or continue treatment until the end of the study upon providing written informed consent.
Patients and methods
Patients
This open-label, multicenter, noncomparative Phase IIIb trial (ClinicalTrials.gov identifier NCT01189461) was conducted in patients aged ≥18 years with type 1 or type 2 diabetes and a documented clinical diagnosis of DME with proliferative or nonproliferative diabetic retinopathy and who, according to the investigator, could have benefited from anti-VEGF therapy. Over 500 patients were to be enrolled in the study. For enrollment, patients were required to have a best-corrected visual acuity (BCVA) letter score between 78 and 24 inclusive (20/32 to 20/320 Snellen equivalents), intraocular pressure ≤21 mmHg, clear ocular media, and adequate pupillary dilatation. Furthermore, the treating investigator needed to certify that focal laser treatment could be deferred for ≥18 weeks in the study eye. Key exclusion criteria were: prior scatter photocoagulation treatment within 4 months of study initiation or anticipated within the following 6 months; other reasons for macular edema, atrophy, scarring, or fibrosis involving the center of the macula; significant media opacities, including cataracts; any intraocular surgery within 4 months of study entry; previous vitrectomy; and previously documented glycated hemoglobin >10% or recent evidence of uncontrolled diabetes. All patients provided written informed consent.
Treatment
Patients were administered intravitreal pegaptanib 0.3 mg in the study eye under aseptic conditions by ophthalmologists experienced in the procedure. Patients were treated at baseline and at subsequent visits once every 6 weeks after BCVA evaluation, biomicroscopy, dilated fundus examinations in both eyes, and tonometry measurements. After the first two injections, additional injections could be administered less frequently than once every 6 weeks, as determined by the investigator. Clinical benefit was evaluated after two or more injections. Retreatment was left to the discretion of the investigator, and patients who demonstrated a clinical benefit could continue to receive intravitreal pegaptanib injections for up to 48 weeks.
Endpoints
The primary endpoint was the incidence of ocular and nonocular adverse events (AEs), defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment. One secondary endpoint was the incidence of ocular and nonocular serious AEs, defined as any AE resulting in, but not limited to, death, is life-threatening, hospitalization, persistent disability, or congenital anomaly. All observed and reported AEs were recorded using Medical Dictionary for Regulatory Activities (MedDRA) version 15.0 throughout the study. Other secondary endpoints included the mean number of injections per patient and efficacy of treatment as evaluated by change in BCVA from baseline to end of treatment. BCVA was measured using retroilluminated modified Ferris-Bailey Early Treatment Diabetic Retinopathy Study charts starting at 4 m. Complete ophthalmological examinations (including slit-lamp biomicroscopy, ophthalmoscopy, tonometry, BCVA measurements, and fundus examinations) were performed at screening, baseline, each treatment visit, and at follow-up. Applanation tonometry was performed for all patients at screening and to verify postinjection intraocular pressures ≥30 mmHg lasting for >30 minutes post injection or for a reading of ≥30 mmHg at any other time.
Statistical analyses
In total, 500 patients were to be enrolled based on a requirement of 459 patients, which would provide a >99% chance of detecting at least one occurrence of any AE with a true underlying rate of one or more in 100. Descriptive statistics were used for reporting efficacy (BCVA scores and change from baseline in BCVA scores to each visit and end of study) and safety endpoints. Statistics are presented using observed data with no imputation for missing values.
Results
Patient disposition
Fifty-five patients were screened prior to termination of recruitment. Of these, 46 patients were enrolled and 12 completed the study (Figure 1). The baseline characteristics of the 46 patients enrolled prior to termination of enrollment are given in Table 1. When enrollment was stopped, patients already entered into the study were informed and given the option of withdrawing or continuing in the study.
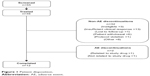  | Figure 1 Patient disposition. |
Of the 46 patients enrolled, 42 (91.3%) had received prior drug treatment for conditions other than diabetes or DME (Table 2) and an equal number were receiving concomitant treatments for conditions other than diabetes or DME during the study (Table 3). The median duration of study treatment for the 46 patients was 13.6 weeks.
Efficacy and safety
Compared with baseline, mean BCVA increased noticeably by week 6 and remained steady, with further small increases thereafter (Table 4). Owing to withdrawals, the results at later visits are based on a small number of patients. Overall, the mean total number of injections in all patients was 3.2, with a median of 3.0 and a range of 1.0–6.0. The mean interval between injections was 7.7 weeks, with a median of 6.5 weeks.
  | Table 4 Mean ± SD BCVA scores by study visit |
The individual data for the 12 patients who completed the study are given in Table 5. The BCVA change between baseline and the follow-up visit was ≥10 letters in four of 12 patients, and >5 letters in seven of 12 patients.
  | Table 5 Relevant data for individual patients completing study |
Sixteen (34.8%) of the 46 patients enrolled in the study reported AEs, of which four (8.7%) reported treatment-related AEs (Table 6). Seventeen ocular-related AEs (none severe) were reported by ten (21.7%) patients. Nineteen nonocular, all-causality AEs (two severe but not related to treatment) were reported by eight (17.4%) patients (Table 7). One moderate hypersensitivity skin reaction was observed, but was reported to be unrelated to study treatment. Three patients had serious AEs (cerebrovascular accident, myocardial infarction, and lung malignancy) that were reported as unrelated to study treatment by the investigator.
  | Table 6 Summary of treatment-emergent AEs |
Discussion
Several clinical and preclinical studies have demonstrated the role of VEGF in the pathogenesis of DME, and ranibizumab is currently the only anti-VEGF agent approved in the European Union for the treatment of visual impairment due to DME.3,19,24–28
Two clinical studies were performed to study the efficacy and safety of pegaptanib in patients with DME.19,24 The primary objective of the study reported here was to further assess the safety and tolerability of pegaptanib in patients with documented DME which, in the opinion of the treating physician, would benefit from anti-VEGF therapy. As such, neither assessment of central macular thickness nor specific standardized retreatment criteria were included in the study design so as to better reflect real-world clinical practice. However, recruitment for this study was stopped following the sponsor’s decision to withdraw the marketing application for this indication. Thus, the primary limitation of this study is the very small number of patients enrolled (46 of the planned 500). The study is further limited by only 12 patients deciding to complete the study after being informed of the sponsor’s decision. Therefore, the sample size for this study was too small for any statistical analyses of the data. Consequently, the data reported here must be interpreted with great caution. Nevertheless, the results of this study are consistent with those reported for another small study of 20 patients in whom pegaptanib was demonstrated to be efficacious and safe over a 12-month period.29
Treatment-related AEs were mild or moderate in severity in 46 patients receiving at least one dose of pegaptanib. There were no severe drug-related AEs observed or deaths reported, suggesting that pegaptanib was well tolerated in this cohort of patients with DME. These data are consistent with those reported for the Phase II/III trials comparing intravitreal pegaptanib injections with sham treatment.3,19,24,25
Evidence of a modest initial clinical benefit, measured as improvement in BCVA, was observed in the patients by week 6. Although data are limited, this benefit appears to be sustained throughout the study; of the 12 patients completing the study, about 30% gained >10 letters of BCVA, while approximately 60% gained >5 letters of BCVA. These data appear to be consistent with those reported in the Phase II/III trials.3,19,24 However, the number of patients in this study is too small to derive any definitive conclusions. The magnitude and duration of the benefit suggested in this study for patients with DME will need to be confirmed in a larger study powered to address these questions.
Acknowledgments
Statistical evaluation of the data and support was provided by Rachel Moate, formerly of Quanticate Ltd, Hertfordshire, UK. We are very grateful to all of the patients and investigators and the study team who were involved in the study and provided data for analysis.
Author contributions
SS recruited patients for the study, and participated in interpretation of the analysis and data, development of the manuscript, and final approval of the manuscript. RCB was involved in the design of the study, monitoring of safety, analysis and interpretation of the data, development of the manuscript, and final approval of the manuscript. CS was involved in the design of the study, analysis and interpretation of the data, development of the manuscript, and final approval of the manuscript.
Disclosure
SS has received research grants from and has attended advisory board meetings for Novartis, Allergan, and Bayer. RCB and CS are employees of Pfizer Ltd, which funded the study. Editorial support for writing and styling the paper for journal submission was provided by Mukund Nori of Engage Scientific Solutions and was funded by Pfizer Inc, New York, NY, USA.
References
Davidov E, Breitscheidel L, Clouth J, Reips M, Happich M. Diabetic retinopathy and health-related quality of life. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):267–272. | |||
Hariprasad SM, Mieler WF, Grassi M, Green JL, Jager RD, Miller L. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92(1):89–92. | |||
Loftus JV, Sultan MB, Pleil AM. Changes in vision- and health-related quality of life in patients with diabetic macular edema treated with pegaptanib sodium or sham. Invest Ophthalmol Vis Sci. 2011;52(10): 7498–7505. | |||
Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. | |||
Scanlon PH, Martin ML, Bailey C, Johnson E, Hykin P, Keightley S. Reported symptoms and quality-of-life impacts in patients having laser treatment for sight-threatening diabetic retinopathy. Diabet Med. 2006;23(1):60–66. | |||
Sharma S, Oliver-Fernandez A, Liu W, Buchholz P, Walt J. The impact of diabetic retinopathy on health-related quality of life. Curr Opin Ophthalmol. 2005;16(3):155–159. | |||
Tranos PG, Topouzis F, Stangos NT, et al. Effect of laser photocoagulation treatment for diabetic macular oedema on patient’s vision-related quality of life. Curr Eye Res. 2004;29(1):41–49. | |||
International Diabetes Federation. IDF Diabetes Atlas. 5th ed. 2011. Available from: http://www.idf.org/diabetesatlas. Accessed September 4, 2012. | |||
Farag YMK, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011;26(1):28–35. | |||
Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia. 2010;53(1):10–20. | |||
Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115(11):1859–1868. | |||
Rubino A, Rousculp MD, Davis K, Wang J, Girach A. Diagnosed diabetic retinopathy in France, Italy, Spain, and the United Kingdom. Prim Care Diabetes. 2007;1(2):75–80. | |||
Wong TY, Cheung N, Tay WT, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869–1875. | |||
Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077.e35. | |||
Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. | |||
Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086.e2. | |||
Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4): 615–625. | |||
Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175–2181.e1. | |||
Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011; 118(6):1107–1118. | |||
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81(2):154–162. | |||
Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. | |||
Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. | |||
Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. | |||
Cunningham ET Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. | |||
Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):915–930. | |||
Querques G, Bux AV, Fusco AR, Iaculli C, Delle Noci N. Pegaptanib sodium versus pegaptanib sodium combined with macular laser photocoagulation or laser alone for diabetic macular edema. J Ophthalmol. 2009;2009:672178. | |||
Querques G, Bux AV, Martinelli D, Iaculli C, Noci ND. Intravitreal pegaptanib sodium (Macugen) for diabetic macular oedema. Acta Ophthalmol. 2009;87(6):623–630. | |||
Adamis AP, Altaweel M, Bressler NM, et al. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113(1):23–28. | |||
Pacella E, La Torre G, Impallara D, et al. Efficacy and safety of the intravitreal treatment of diabetic macular edema with pegaptanib: a 12-month follow-up. Clin Ter. 2013;164(2):e121–e126. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




