Back to Journals » Journal of Blood Medicine » Volume 11
An Open-Label Extension Study to Assess the Long-Term Efficacy and Safety of a Plasma-Derived von Willebrand Factor (VWF)/Factor VIII (FVIII) Concentrate in Patients with von Willebrand Disease (SWIFT-VWDext Study)
Authors Lissitchkov T, Klukowska A , Buevich E, Maltceva I, Auerswald G, Stasyshyn O , Seifert W, Rogosch T
Received 24 June 2020
Accepted for publication 27 September 2020
Published 9 October 2020 Volume 2020:11 Pages 345—356
DOI https://doi.org/10.2147/JBM.S268907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Toshko Lissitchkov,1 Anna Klukowska,2 Evgeny Buevich,3 Irina Maltceva,3 Guenter Auerswald,4 Oleksandra Stasyshyn,5 Wilfried Seifert,6 Tobias Rogosch6
1Specialized Hospital for Active Treatment (SHAT), Sofia, Bulgaria; 2Department of Paediatrics, Haematology and Oncology of Warsaw Medical University, Warsaw, Poland; 3GOUVPO Altaysky State Medical University of Roszdrav, Bernaul, Russian Federation; 4Prof. Hess Kinderklinik, Klinikum Bremen-Mitte, Bremen, Germany; 5Institute of Pathology and Transfusion Medicine AMN, Lviv, Ukraine; 6Clinical Development, CSL Behring, Marburg, Germany
Correspondence: Toshko Lissitchkov
Specialized Hospital for Active Treatment (SHAT) “JOAN PAVEL”, 67A Stoletov Blvd, Sofia 1233, Bulgaria
Tel +35 989 436 9096
Email [email protected]
Objective: Plasma-derived von Willebrand factor/factor VIII (pdVWF/FVIII; VONCENTO®, CSL Behring) is a high-concentration, low-volume, high-purity concentrate, which contains a high level of high-molecular-weight multimers and a VWF/FVIII ratio of ∼ 2.4:1. The SWIFT (“Studies with von Willebrand factor/Factor VIII”) program is evaluating pdVWF/FVIII in patients with von Willebrand disease (VWD). The long-term efficacy and safety profile of pdVWF/FVIII was investigated in this multicenter, open-label, extension study.
Methods: Pediatric, adolescent, and adult patients with VWD who required treatment of non-surgical bleeds (NSBs), treatment during surgical events or who were receiving prophylaxis and who had completed one of two previous clinical trials of pdVWF/FVIII were included. Efficacy and safety analyses were performed for on-demand (n=10), prophylaxis (n=8), or on-demand and prophylaxis (n=2) treatment in patients pre-treated with pdVWF/FVIII for ≥ 12 months.
Results: Seven patients experienced a total of 402 NSBs in the on-demand arm, of which 77 required treatment and nine NSB events in three patients were considered major. Nine patients reported 118 NSBs in the prophylaxis arm, with 96 events requiring treatment and seven patients experiencing 12 major NSB events. Excellent or good hemostatic efficacy was reported by the investigator for 98.7% (on-demand) and 97.9% (prophylaxis) of NSB events treated with pdVWF/FVIII, without relevant differences between subgroups by age. pdVWF/FVIII was well tolerated, and the adverse events seen were mild-moderate and consistent with the safety profile for this product seen in other studies. There were no cases of anaphylactic reactions and angioedema, development of VWF/FVIII inhibitors, thromboembolic events, or viral infections.
Conclusion: This contemporary comprehensive development program evaluating pdVWF/FVIII across all ages demonstrates long-term safety and efficacy for treatment and prevention of bleeds in patients with severe VWD, supporting the benefit–risk profile of pdVWF/FVIII.
Keywords: von Willebrand disease, hemostatic efficacy, safety, prophylaxis, on-demand, surgery
Plain Language Summary
Von Willebrand disease (VWD) is an inherited bleeding disorder where the main symptoms are mucosal bleeding and heavy menstrual bleeding. Many patients with VWD are successfully managed with the synthetic peptide desmopressin acetate (1-deamino-8-D-arginine vasopressin, DDAVP). For those patients where DDAVP is not effective or is contraindicated, von Willebrand factor/factor VIII (VWF/FVIII) concentrates derived from human plasma are the treatment of choice. We describe here an extension study, where patients who had participated in previous clinical trials for a plasma-derived (pd)VWF/FVIII for ≥12 months could continue with their treatment regimen. This allowed for evaluation of the long-term hemostatic efficacy and safety of pdVWF/FVIII in adult, adolescent and pediatric patients who were receiving on-demand or prophylactic treatment. Excellent or good hemostatic efficacy was reported for 98.7% and 97.9% of bleeding events in the on-demand and prophylaxis groups, respectively. No significant difference in efficacy was seen between different age groups. No adverse events of specific interest to this type of treatment (eg, allergic reaction, product inhibitors, infection, or thromboembolic events), and the adverse events observed were consistent with other treatment products of this type. These results indicate that over long-term exposure the efficacy and safety of pdVWF/FVIII were maintained, with similar efficacy and safety to that observed in the previous studies, even in patients with most severe VWD.
Introduction
Von Willebrand disease (VWD) is an autosomal dominant or recessive inherited bleeding disorder resulting from an abnormality, either quantitative or qualitative, of von Willebrand factor (VWF).1–4 The disease is usually classified into three types (types 1–3) on the basis of clinical and laboratory phenotypes. Type 1, accounting for the large majority of cases (70% to 80%), involves partial quantitative defects, while type 3 is characterized by a virtual absence of VWF in plasma.5,6 Type 2 variants consist of qualitative defects and can be sub-classified into 2A (lack of high- and intermediate-molecular-weight multimers/decreased binding to platelets), 2B (lack of high-molecular-weight multimers/increased affinity for platelets), 2M (normal multimer distribution/decreased affinity for platelets), and 2N (normal multimer distribution/decreased affinity for coagulation factor VIII [FVIII]).3
Patients with VWD experience excessive and frequent mucocutaneous bleeding episodes (epistaxis, menorrhagia and gastrointestinal bleeds); in severe cases, particularly in type 3 VWD, spontaneous bleeds into joints, soft tissues and other sites may occur.1
The aim of VWD therapy is to treat and prevent bleeding episodes, depending on the particular clinical situation. The treatment of choice for most patients with mild symptoms is administration of the synthetic peptide desmopressin acetate (1-deamino-8-D-arginine vasopressin, DDAVP), which induces the release of VWF from its endogenous stores.1 However, for those with more severe disease or in whom DDAVP is contraindicated, the treatment of choice is VWF/FVIII concentrates derived from human plasma.7 VWD patients who do not respond to DDAVP, and for many patients with type 2 VWD where DDAVP is not sufficiently effective or contraindicated, and for all type 3 VWD patients, the treatment of choice is VWF/FVIII concentrates derived from human plasma.7
Plasma-derived VWF/FVIII concentrate (pdVWF/FVIII; VONCENTO®, CSL Behring, Germany) is a high-concentration, low-volume, high-purity concentrate, which contains a large proportion of high-molecular-weight multimers and a VWF/FVIII ratio of ~2.4:1.8 pdVWF/FVIII is licensed by the European Medicines Agency (EMA) for the prevention and treatment of hemorrhage or surgical bleeding in adult, adolescent and pediatric patients with VWD.9
The SWIFT (“Studies with von Willebrand factor/Factor VIII”) program evaluated the hemostatic efficacy and safety of pdVWF/FVIII in pediatric (0 to <12 years of age; SWIFTLY-VWD study), or adolescent and adult (≥12 years of age; SWIFT-VWD) patients with VWD type 1, 2A, or 3 in whom treatment with a VWF product was required for prophylactic therapy, hemostatic control during surgery, or control of a non-surgical, spontaneous, or traumatic bleeding event.8,10 Following the completion of these studies, patients who had participated in either SWIFTLY-VWD or SWIFT-VWD were able to transition to an extension study (SWIFT-VWDext). The aim of this study was to build on the results of the previous studies to assess the long-term efficacy and safety of pdVWF/FVIII in pediatric, adolescent, and adult patients with VWD and to assist the clinical understanding regarding the use of on-demand treatment versus set prophylaxis treatment with a VWF product.
Materials and Methods
Study Design
The interventional, open-label extension study, SWIFT-VWDext (NCT01224808), was conducted in six centers in Bulgaria, Germany, Russia, Ukraine [one center each], and Poland [two centers] between October 2010 and March 2014. Pediatric (<12 years), adolescent (12 to <18 years), and adult (≥18 years) patients that participated in and completed either the SWIFTLY-VWD study (NCT01213446) or the SWIFT-VWD study (NCT00941616) were included.11 On entry to this extension study, patients continued with the treatment regimen (on-demand or prophylaxis) they were on when completing the SWIFTLY-VWD or SWIFT-VWD studies. pdVWF/FVIII (containing either 1200 or 2400 IU VWF:RCo and 500 or 1000 IU FVIII:C per vial, respectively) was used in this study. One patient in the on-demand arm did not experience any bleeding events during the study and was therefore excluded from all analyses. The patient disposition of the extension study is shown in Figure 1.
 |
Figure 1 Patient disposition. |
This study was carried out in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki and standard operating procedures for clinical research and development at CSL Behring and was compliant with the respective Committee for Medicinal Products for Human Use (CHMP) Guidelines.12 Ethics approval, individual informed consent (either from the patient, or from their legal guardian if the patient was under the relevant age of consent [12 or 18 years] as applicable in participating countries), and approval by independent ethics committees/investigational review boards of the participating centers (Ethics Committee for Multicenter Trials, Bulgaria; Ethics Committee of the State of Bremen, Germany; Bioethics Committee, Wroclaw, Poland; Ethics Committee at State Budget Educational Institution of Higher Professional Education, Barnaul, Russia; Ministry of Health of Ukraine, Ukraine) were obtained prior to enrollment.
Dose and Dose Regimen
The general dosing recommendation for the treatment or prevention of spontaneous or trauma-induced hemorrhage is 25–50 IU VWF:RCo/kg b.w. and 60–80 IU VWF:RCo/kg b.w. for surgeries. The dosage and treatment rationale was based on the guidelines from the European Medicines Agency,13 as well as the data collected from previous clinical studies of efficacy, which were conducted with pdVWF/FVIII in VWD patients (Table 1). Each patient’s treatment regimen and individual dose were determined by the investigator, based on the reason for use (ie, as prophylaxis, to treat a major bleeding, provide prophylaxis for surgery, etc.) and severity of VWD.
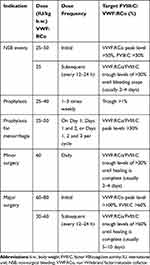 |
Table 1 Guidelines for Dosage During the Efficacy Component of the Study |
Efficacy Assessments
The clinical efficacy parameters assessed in the study were pdVWF/FVIII usage, hemostasis assessment (overall every 3 months by the investigator and for each non-surgical bleeding [NSB] event and surgical event by the investigator and patient), blood product transfusion requirements, and surgeon’s assessment of blood loss during a surgical procedure (“less”, “equivalent” or “more” compared to the expected blood loss from a patient without a bleeding disorder undergoing the same procedure). The severity of NSB events was assessed by the investigator as “major” or “minor”. “Major” NSB events included any bleeding into a joint or muscle, or a mucosal bleeding of the gastro-intestinal tract (excluding nasal or oral bleeding). All other NSB events were classified as “minor” unless the investigator assessment noted otherwise.
Clinical assessments of hemostatic efficacy were based on a four-point efficacy grading scale: “excellent” if hemostasis was achieved/cessation of bleeding occurred; “good” if slight oozing or partial but adequate control of bleeding occurred and no additional product was required for unplanned treatment; “moderate” if moderate bleeding or moderate control of bleeding occurred and additional product was required for unplanned treatment; “none” in cases of severe uncontrolled bleeding.
For prophylactic treatment, the patient was to perform a daily assessment of each NSB event as well as an overall monthly assessment. Additionally, an overall hemostatic efficacy assessment was performed by the investigator, based on the previous 3 months of pdVWF/FVIII usage, the number of NSB events that occurred during that 3-month period, and additional treatment requirements.
Safety
All patients who received at least one dose of pdVWF/FVIII were included in the safety analysis. Safety assessments included the reporting of serious adverse events (SAEs), the presence of VWF and/or FVIII inhibitors, laboratory parameters, such as biochemistry, hematology, and urinalysis, and a physical examination and vital signs assessment. All medications taken 30 days prior to screening and during the entire study duration were recorded.
VWD phenotype, presence of FVIII and VWF inhibitors, and the investigation of seroconversion for virus markers (indicative of hepatitis A virus [HAV], hepatitis B virus [HBV], hepatitis C virus [HCV] and human immunodeficiency virus [HIV] infection) were assessed/conducted in a central laboratory (Medilys Laborgesellschaft – Asklepios Institut, Hamburg, Germany). Presence of FVIII inhibitors was analyzed using the Bethesda method (Nijmegen modification). The presence of VWF inhibitors was analyzed using a Bethesda method-based assay with VWF:RCo activity; in addition, VWF:Ag and VWF:CB were determined for each inhibitor test. HAV- and HBV-negative patients were vaccinated prior to the first dose of pdVWF/FVIII. Virology reference samples were collected at Day 1 and at the final visit, but were not analyzed since analysis was not deemed necessary for this study.
Statistical Procedures
Continuous variables are summarized using descriptive statistics: mean and standard deviation (SD), and/or median and range. Numbers and percentages are presented in frequency tables for categorical variables. Missing data have not been replaced (ie, summaries are based on the observed data). No formal statistical tests were performed.
Results
Patients
A total of 20 patients were enrolled in the study. This included 18% of patients who completed the SWIFTLY-VWD study (three children aged <12 years), and 77% of patients who completed the SWIFT-VWD study (two adolescents [12 to <18 years] and 15 adults [≥18 years]). Of these patients, ten participated in the prophylaxis arm (including three children), eight in the on-demand arm (including one adolescent), and two in the prophylaxis and on-demand arm (including one adolescent). The two patients in the prophylaxis and on-demand arm, who had received on-demand treatment throughout the SWIFT-VWD study, started on prophylaxis treatment in this extension study, but were switched back to on-demand treatment at the discretion of the investigator following non-compliance with the prophylaxis regimen (one patient was switched at month 3 and the other patient at month 6). Patient characteristics are listed in Table 2. Overall, 18 patients (90%) completed the extension study. One patient in the on-demand arm died due to stomach ulcer in combination with pneumonia after participating for 754 days; data from this patient prior to death are included in the analysis. Additionally, one patient in the prophylaxis arm was withdrawn after 633 days because of a surgery at an external hospital that could not fulfil the sponsor’s requirements. The duration of study participation in these patients ranged from 7 to 34 months.
 |
Table 2 Baseline Characteristics |
Hemostatic Efficacy
On-Demand Arm
Seven patients (including one adolescent) who were treated on-demand experienced a total of 402 NSB events, resulting in a median (Q1; Q3) number of NSB events per patient of 35.0 (1–150). Of the total 402 NSB events, 325 did not require any pdVWF/FVIII administration. Three patients experienced a total of nine major NSB events, including one major mucosal and six joint events. The seven patients in the on-demand arm experienced a mean of 22.34 (SD 24.28) and a median 12.71 (range 0.96–57.25) NSB events per year. Overall, 70 out of the 77 medicated NSB events were treated at home (90.1%) while seven NSB (9.1%) were treated in the hospital. Hemostatic efficacy was assessed by the investigator as excellent or good for 98.7% NSB events treated with pdVWF/FVIII, and as moderate for the one remaining event (Table 3). Additionally, the hemostatic efficacy was assessed by the patient/legal guardian as excellent for 34.6% of the 107 assessed bleeding days, as good for 21.5%, and as moderate for 43.9% (those bleeding days with moderate efficacy were all associated with menstrual bleedings, predominantly in one patient).
 |
Table 3 Investigator’s Assessment of Hemostatic Efficacy per Treated Non-Surgical Bleeding Event |
The majority of bleeding events either required no pdVWF/FVIII treatment (80.8%) or only one (10.7%) or two (6.7%) infusions of pdVWF/FVIII. The remaining seven NSB events (1.7%) required a median of three infusions (range: 3–8).
Patients in the on-demand arm had a median pdVWF/FVIII dose of 54.6 IU VWF:RCo/kg b.w. per infusion (range: 40.0–86.0 IU VWF:RCo/kg b.w.).
The hemostatic efficacy for the one adolescent patient (excellent 64.0%; good 36.0%) in the on-demand arm was similar to that seen in the total on-demand arm (excellent 45.5%; good 53.2%). No children were included in the on-demand arm.
Prophylaxis Arm
Nine (90.0%) patients in the prophylaxis arm reported a total of 118 NSB events, 96 of which required treatment. The median (Q1; Q3) number of NSB events per patient was 8.0 (1–19). Seven patients experienced a total of 12 major NSB events, including five major mucosal and four joint events. The ten patients in the prophylaxis arm experienced a mean of 8.36 (SD 9.62) and a median of 4.37 (range 0–25.97) NSB events per year. One adult patient had no bleeding events in the prophylaxis regimen.
Over a 12-month on-demand treatment period in the SWIFT-VWD lead-in study, the seven adult patients in the prophylaxis arm reported 284 NSB events (median 31, range 18–82) including 116 (41%) major bleeds, 278 (98%) of which required treatment. Thus, these patients experienced a major decrease in bleeding frequency after switching to prophylactic treatment. Additionally, the number of joint bleeds was reduced from 99 in the on-demand phase to one while participating in the prophylaxis arm of this extension study.
Patients in the prophylaxis arm received a median number of 109 infusions (range: 19–406) at a median pdVWF/FVIII dose of 42.8 IU VWF:RCo/kg b.w. (range: 28.5–85.8 IU VWF:RCo/kg b.w.) for prophylaxis. Prophylaxis regimens were as follows: four patients were dosed once-weekly, three patients were dosed 2–3 times per week, two patients were dosed 3 times per week, and one patient was dosed 1–2 times per month (ie, every menstrual cycle). The majority of NSB events either required no additional pdVWF/FVIII treatment (18.6% of events) or only one (71.2%) or two (6.8%) infusions of pdVWF/FVIII with a median dose of 60.0 IU VWF:RCo/kg b.w. (range: 37.1–79.8 IU/kg b.w.) to treat the event. The remaining four NSB events (3.4%) required a median of 3.5 infusions (range: 3–5). Hemostatic efficacy was assessed by the investigator as excellent or good for 97.9% of NSB events treated with pdVWF/FVIII (Table 3). The patient’s/legal guardian’s assessment of hemostatic efficacy of the assessed 111 bleeding days (excellent [72.1%], good [23.4%], moderate [3.6%] and none [0.95%]) was generally in line with the investigator’s assessment (the one bleeding day with efficacy rated as “none” was associated with the first day of a nasal bleeding).
The three children had a higher NSB frequency than the seven adults in the prophylaxis arm: 58 of the 96 treated NSB events occurred in these children. The hemostatic efficacy for the three children assessed by the investigator (excellent [91.4%] or good [8.6%]) was similar to that for the adults (excellent [72.9%] or good [25.0%]) participating in the prophylaxis arm. For each 3-month interval, the prophylactic hemostatic efficacy was assessed retrospectively by the investigator as either excellent or good (Table 4).
 |
Table 4 Investigator’s Assessment of Hemostatic Efficacy in the Prophylaxis Arm |
Prophylaxis and On-Demand Arm
Two patients (one adult and one adolescent) started with prophylaxis treatment but were switched to on-demand treatment after 3 and 6 months, respectively. These two patients reported 50 and 48 NSB events, respectively; all events occurred after the patients had been switched to on-demand treatment. Six major NSB events (three joint and three muscle events) were reported for the two patients.
Hemostatic efficacy was assessed by the investigator as excellent or good for all NSB events treated with pdVWF/FVIII (Table 3). Additionally, the hemostatic efficacy was assessed by the patients as excellent for 62.9% of the 97 assessed bleeding days and as good for the remaining 37.1% of bleeding days; all treated NSB events resolved within one day.
Both patients in the prophylaxis and on-demand arm received 15 infusions each as prophylaxis treatment, with average doses per prophylactic infusion of 22.0 and 30.1 IU VWF:RCo/kg b.w., respectively. The remaining number of infusions, 47 and 50, respectively, were administered to treat NSB events on-demand, using average doses of 24.5 and 32.5 IU VWF:RCo/kg b.w., respectively. None of the NSB events required more than one infusion of pdVWD/FVIII.
The hemostatic efficacy for the one adolescent patient in this arm was similar to that of the adult patients in the same arm and to the total on-demand arm.
Surgical Events
During the study, seven patients, including two children, underwent a total of 13 surgeries, four patients in the prophylaxis arm with one minor surgery each (two tooth extractions, circumcision, radiosynoviorthesis) and three patients in the on-demand arm with nine surgeries (two knee punctures, five tooth extractions, uterine biopsy, cervical conisation), of which three were major (two tooth extractions, cervical conisation).
The investigator’s assessment of hemostatic efficacy at discharge from the hospital was assessed as excellent for 11 surgical events (including the three major events) and as good for two events. Where available, the patient rated the hemostatic efficacy as either excellent or good for all days except for two major surgeries (teeth extractions) in the same patient which were rated as having moderate hemostatic efficacy on the first three days of the first event and on the first day of the second surgical event. Overall, the investigator assessed the hemostatic efficacy during surgical events per day of the hospital stay as excellent for 19 days (42.2%), as good for 22 days (48.9%), and as moderate for four days (8.9%). The post-surgery hemostatic efficacy assessment by the investigator was available for 12 surgical events and was excellent for eight of the 12 surgeries (66.7%; two major and six minor) and good for four surgeries (33.3%; one major and three minor).
Blood loss during surgery was assessed as equivalent compared to the expected blood loss from a patient without a bleeding disorder undergoing the same procedure in the four surgical events that occurred in four patients in the prophylaxis arm and seven of the nine surgeries in the on-demand arm, and less than expected in the remaining two surgeries in the on-demand arm. No patient required blood product transfusions during the entire study.
Bleeding Events and Disease Severity
As expected, patients with VWD type 3 experienced more NSB events (138) than those patients with VWD type 1 and type 2A (20 and 112, respectively) (Table 5). Overall, 0%, 6.25% and 14.5% of NSB events were classed as major in patients with VWD type 1, 2A and 3, respectively. The majority of NSB events reported by pediatric patients were nasal bleeds; 100% reported as minor in both patients with VWD type 3 and 89% reported as minor in the patient with VWD type 2A (data not shown).
 |
Table 5 Type, Location and Severity of Treated Bleeding Event by VWD Type |
Safety
The median total pdVWF/FVIII dose received by the seven patients in the on-demand arm was 703 IU VWF:RCo/kg b.w. (range: 40–4974 IU VWF:RCo/kg b.w.) at a median of 14 exposure days (range: 1–66 days), and the two patients in the prophylaxis and on-demand arm received total pdVWF/FVIII doses of 1482 and 1780 IU VWF:RCo/kg b.w. at 62 and 65 exposure days, respectively. The mean (range) treatment duration in the prophylaxis arm was 639 (211–848) days; only one patient remained in the study for less than 365 days. The ten patients in the prophylaxis arm received 5147 IU VWF:RCo/kg b.w. (range: 736–34,832 IU VWF:RCo/kg b.w.) at a median of 107 exposure days (range: 19–397 days). Taking the predecessor studies (SWIFT-VWD and SWIFTLY-VWD) into account the patients from the prophylaxis arm received a median of more than 10,000 IU VWF:RCo/kg b.w. (range: 1867–48,313 IU VWF:RCo/kg b.w.) at a median of 224 exposure days (range: 53–592).
During this extension study, 16 patients (84.2%) reported a total of 79 treatment-emergent adverse events (TEAEs): seven patients (70.0%) in the prophylaxis arm had 55 TEAEs, seven patients (100.0%) in the on-demand arm had 21 TEAEs, and the two patients in the prophylaxis and on-demand arm had three TEAEs. The most frequently reported TEAEs, each reported by three patients (15.8%), were anemia, arthralgia, nasopharyngitis, and upper respiratory tract infection. No TEAEs leading to discontinuation of pdVWF/FVIII and no TEAEs of special interest (development of VWF/FVIII inhibitors, virus transmissions, hypersensitivity reactions, and embolic and thrombotic events) occurred during this study.
Three patients reported a total of five serious TEAEs: one patient with diabetes insipidus, gastric ulcer, and pneumonia, and one patient each with dysfunctional uterine bleeding and mild carcinoma of the cervix (stage 0; resolved with sequelae after 15 days following surgery), respectively. The gastric ulcer and pneumonia events led to death of the patient; the gastric ulcer was present prior to the first pdVWF/FVIII administration, and this was considered causality for the pneumonia TEAE by the investigator. All TEAEs were considered to be not related or unlikely related to pdVWF/FVIII.
Other than TEAEs that are typical childhood diseases, there were no major differences in the TEAE reporting profile between the three children, two adolescents, and 14 adults included in the safety population.
Abnormal low laboratory values that were considered clinically significant were reported for hemoglobin, hematocrit, mean corpuscular volume, erythrocytes, and monocytes, but for no more than three patients. None of these values were considered related to pdVWF/FVIII. All patients had a negative inhibitor titer during the study period.
Discussion
This extension study was designed to generate data on the long-term efficacy and safety of the high concentration/low volume, high-purity VWF/FVIII complex concentrate pdVWF/FVIII in pediatrics, adolescents, and adults with severe VWD. The study aimed to further strengthen the clinical safety and efficacy data already established for pdVWF/FVIII for long-term use and to assist the clinical understanding regarding the use of on-demand versus prophylaxis treatment in patients with VWD.
In addition to pdVWF/FVIII (VONCENTO®), WILATE® (Octapharma AG), WILFACTIN®/WILLFACT® (LFB Biopharmaceuticals), and HAEMATE® P (CSL Behring) are plasma-derived VWF products approved in the EU for the treatment of VWD. These products have varying ratios of VWF:RCo to FVIII:C.14–20 The VWF:RCo/FVIII:C ratio for pdVWF/FVIII is ~2.4:1.21 A high ratio of VWF:FVIII may help to achieve therapeutic VWF plasma levels without causing long-term accumulation of FVIII levels, which have been associated with an increased risk of thromboembolic events.22–26
In all three treatment arms, the hemostatic efficacy was assessed by the investigator as either excellent or good for at least 97.9% of events, including all major NSB events. The hemostatic efficacy assessments by the patient/legal guardian during prophylaxis generally matched those by the investigator, and were similar between age groups. This is in line with results observed for WILATE, where efficacy was rated as excellent or good in 96% of all treated bleeding episodes and in 96% of surgical procedures performed in VWD patients.14,18 A further single-center study of WILATE use in pediatric patients reported excellent/good efficacy in the on-demand treatment of 97.7% of NSBs and 95.1% of surgeries.19 Similarly, a post-marketing study of WILFACTIN reported excellent or good efficacy for 94.0% of treated major bleeds and 98.2% of major surgical procedures.20
The median number of NSB events per year in the prophylaxis arm (children: 22.2 events; adults: 0.6 events) was similar to the median reported in the prophylaxis arms of the SWIFTLY-VWD pediatric and SWIFT-VWD adult/adolescent studies (23.5 and 1.0, respectively).8,10 In patients treated on-demand, the median number of bleeding events per year in this study (12.71 events) was lower than in the on-demand arm of SWIFT-VWD (19.5 events).8 This would be expected, as patients who experienced a higher rate of bleeding during the on-demand portion of the SWIFT-VWD study would have transitioned to prophylactic treatment in the second stage of that study and would have remained on prophylaxis during this extension study. Two patients started with prophylaxis treatment for 3 months and 6 months, respectively, but were switched to on-demand therapy. As a consequence, the number of annual bleeds came close to the number observed in the on-demand arm.
During this study, the calculated number of minor bleeds per year were approximately seven times higher during on-demand treatment than in the prophylaxis arm for patients ≥12 years. In addition, the bleeding rate for NSB events in the seven adult patients in the prophylaxis arm in the current study were markedly lower than during their on-demand treatment period in the SWIFT-VWD study, especially for joint and major bleeds. This indicates that a set prophylaxis treatment regimen with regular administrations every 2–3 days may improve bleeding outcomes in these patients.
The efficacy of pdVWF/FVIII observed in predecessor studies was maintained in this long-term extension study. pdVWF/FVIII was well tolerated and the safety profile was consistent with observations in previous studies27–30 and the safety profiles of other plasma-derived VWF/FVIII concentrates.14,17–20,31 Overall, the safety results observed in this study support a positive benefit–risk profile of pdVWF/FVIII.
Limitations of this study include the low number of patients included in this study, particularly pediatric patients and patients with type 1 VWD, which may limit the applicability of the results to the wider VWD population. It would be of interest to conduct further studies in a wider range of patients; however, this study was constrained by the nature of an extension study.
Conclusion
Long-term pdVWF/FVIII exposure in VWD patients who require prophylaxis or on-demand treatment with a VWF product was efficacious and well tolerated without affecting the benefit–risk profile, even in patients with most severe VWD.
Abbreviations
DDAVP, desmopressin acetate (1-deamino-8-D-arginine vasopressin); FVIII, factor VIII; FVIII:C, factor VIII:coagulant activity; IU, international units; NSB, non-surgical bleeding; pdVWF/FVIII, plasma-derived von Willebrand factor/factor VIII; SAE, serious adverse event; TEAE, treatment-emergent adverse event; VWD, von Willebrand disease; VWF, von Willebrand factor; VWF:Ag, von Willebrand factor:antigen; VWF:CB, von Willebrand factor:collagen binding; VWF:RCo, von Willebrand factor:ristocetin cofactor.
Data Sharing Statement
CSL will only consider requests to share Individual Patient Data (IPD) that are received from systematic review groups or bonafide researchers. CSL will not process or act on IPD requests until 12 months after article publication on a public website. An IPD request will not be considered by CSL unless the proposed research question seeks to answer a significant and unknown medical science or patient care question. Applicable country-specific privacy and other laws and regulations will be considered and may prevent sharing of IPD.
Requests for use of the IPD will be reviewed by an internal CSL review committee. If the request is approved, and the researcher agrees to the applicable terms and conditions in a data sharing agreement, IPD that has been appropriately anonymized will be made available. Supporting documents including study protocol and Statistical Analysis Plan will also be provided.
For information on the process and requirements for submitting a voluntary data sharing request for IPD, please contact CSL at [email protected].
Acknowledgments
The authors would like to thank Kazimierz Kuliczkowski for his input in the clinical study. Editorial support was provided by Meridian HealthComms and was funded by CSL Behring, Germany.
Funding
This study was supported by a grant from CSL Behring, Australia.
Disclosure
AK received fee for lectures and advisory board from CSL Behring, Sobi, Novo Nordisk, Takeda, Bioverativ, and Roche; GA received honoraria from CSL Behring for speeches at congresses and travel expenses; OS received fee for lectures from Novo Nordisk, Takeda and Roche; WS and TR are employees of CSL Behring. The authors report no other conflicts of interest in this work.
References
1. Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171–232.
2. Friedman KD, Rodgers GM. Inherited coagulation disorders. In: Greer JP, Foerster J, Rodgers GM, editors. Wintrobe’s Clinical Haematology.
3. Castaman G, Federici AB, Rodeghiero F, Mannucci PM. Von Willebrand’s disease in the year 2003: towards the complete identification of gene defects for correct diagnosis and treatment. Haematologica. 2003;88(1):94–108.
4. Sadler JE. A revised classification of von Willebrand disease. For the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1994;71(4):520–525. doi:10.1055/s-0038-1642471
5. Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand factor. J Thromb Haemost. 2006;4(10):2103–2114. doi:10.1111/j.1538-7836.2006.02146.x
6. Leebeek FW, Eikenboom JC. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067–2080. doi:10.1056/NEJMra1601561
7. Mannucci PM. How I treat patients with von Willebrand disease. Blood. 2001;97(7):1915–1919. doi:10.1182/blood.V97.7.1915
8. Lissitchkov TJ, Buevich E, Kuliczkowski K, et al. Pharmacokinetics, efficacy, and safety of a plasma-derived VWF/FVIII concentrate (VONCENTO) for on-demand and prophylactic treatment in patients with von Willebrand disease (SWIFT-VWD study). Blood Coagul Fibrinolysis. 2017;28(2):152–162.
9. European Medicines Agency. European Public Assessment Report (EPAR). Voncento: human coagulation factor VIII/von Willebrand factor; 2013. Available from: https://www.ema.europa.eu/en/documents/assessment-report/voncento-epar-public-assessment-report_en.pdf.
10. Auerswald G, Djambas Khayat C, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of a plasma-derived VWF/FVIII concentrate (Formulation V) in pediatric patients with von Willebrand disease (SWIFTLY-VWD study). J Blood Med. 2020;11:213–225. doi:10.2147/JBM.S236789
11. ClinicalTrials.gov. Extension study of Biostate in subjects with Von Willebrand Disease (NCT01224808); 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT01224808.
12. European Medicines Agency (EMA). Guideline on the clinical investigation of human plasma derived von Willebrand factor products (CPMP/BPWG/220/02); 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500067126.pdf.
13. European Medicines Agency (EMA). Guideline on the core SPC for human plasma-derived and recombinant coagulation factor VIII products (CPMP/BPWG/1619/1999); 2007. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-core-spc-human-plasma-derived-recombinant-coagulation-factor-viii-products-revision_en.pdf.
14. Windyga J, von Depka-Prondzinski M; European Wilate Study Group. Efficacy and safety of a new generation von Willebrand factor/factor VIII concentrate (Wilate®) in the management of perioperative haemostasis in von Willebrand disease patients undergoing surgery. Thromb Haemost. 2011;105(6):1072–1079. doi:10.1160/TH10-10-0631
15. Octapharma. WILATE® Summary of Product Characteristics; 2020. Available from: https://www.medicines.org.uk/emc/product/2873/smpc.
16. LFB Biopharmaceuticals. WILLFACT® Summary of Product Characteristics; 2016. Available from: https://www.medicines.org.uk/emc/medicine/29947.
17. Miesbach W, Krekeler S, Wolf Z, Seifried E. Clinical use of Haemate® P in von Willebrand disease: a 25-year retrospective observational study. Thromb Res. 2015;135(3):479–484. doi:10.1016/j.thromres.2014.12.017
18. Berntorp E, Windyga J. Treatment and prevention of acute bleedings in von Willebrand disease–efficacy and safety of Wilate, a new generation von Willebrand factor/factor VIII concentrate. Haemophilia. 2009;15(1):122–130. doi:10.1111/j.1365-2516.2008.01901.x
19. Khair K, Batty P, Riat R, et al. Wilate use in 47 children with von Willebrand disease: the North London paediatric haemophilia network experience. Haemophilia. 2015;21(1):e44–e50. doi:10.1111/hae.12497
20. Goudemand J, Bridey F, Claeyssens S, et al. Management of von Willebrand disease with a factor VIII-poor von Willebrand factor concentrate: results from a prospective observational post-marketing study. J Thromb Haemost. 2020;18(8):1922–1933. doi:10.1111/jth.14928
21. CSL Behring. VONCENTO® Summary of Product Characteristics; 2019. Available from: https://www.medicines.org.uk/emc/medicine/28757.
22. Coppola A, Franchini M, Makris M, Santagostino E, Di Minno G, Mannucci PM. Thrombotic adverse events to coagulation factor concentrates for treatment of patients with haemophilia and von Willebrand disease: a systematic review of prospective studies. Haemophilia. 2012;18(3):e173–e187. doi:10.1111/j.1365-2516.2012.02758.x
23. Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345(8943):152–155. doi:10.1016/S0140-6736(95)90166-3
24. Makris M, Colvin B, Gupta V, Shields ML, Smith MP. Venous thrombosis following the use of intermediate purity FVIII concentrate to treat patients with von Willebrand’s disease. Thromb Haemost. 2002;88(3):387–388. doi:10.1055/s-0037-1613227
25. Mannucci PM. Venous thromboembolism in von Willebrand disease. Thromb Haemost. 2002;88(3):378–379. doi:10.1055/s-0037-1613225
26. Mannucci PM, Chediak J, Hanna W, et al. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood. 2002;99(2):450–456. doi:10.1182/blood.V99.2.450
27. Dunkley S, Baker RI, Pidcock M, et al. Clinical efficacy and safety of the factor VIII/von Willebrand factor concentrate BIOSTATE in patients with von Willebrand’s disease: a prospective multi-centre study. Haemophilia. 2010;16(4):615–624.
28. Favaloro EJ, Lloyd J, Rowell J, et al. Comparison of the pharmacokinetics of two von Willebrand factor concentrates [Biostate and AHF (High Purity)] in people with von Willebrand disorder. A randomised cross-over, multi-centre study. Thromb Haemost. 2007;97(6):922–930. doi:10.1160/TH06-09-0495
29. Howman R, Barnes C, Curtin J, et al. The clinical efficacy and safety of the FVIII/VWF concentrate, BIOSTATE®, in children with von Willebrand disorder: a multi-centre retrospective review. Haemophilia. 2011;17(3):463–469. doi:10.1111/j.1365-2516.2010.02445.x
30. Shortt J, Dunkley S, Rickard K, Baker R, Street A. Efficacy and safety of a high purity, double virus inactivated factor VIII/von Willebrand factor concentrate (Biostate) in patients with von Willebrand disorder requiring invasive or surgical procedures. Haemophilia. 2007;13(2):144–148. doi:10.1111/j.1365-2516.2006.01430.x
31. Borel-Derlon A, Federici AB, Roussel-Robert V, et al. Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin): a prospective study of 50 patients. J Thromb Haemost. 2007;5(6):1115–1124. doi:10.1111/j.1538-7836.2007.02562.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
