Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
An Observational Registry to Assess Urinary Albumin Evolution in Saudi Hypertensive Patients with the Current Treatment Local algorithm: Results of the RATIONAL Study
Authors Al Shamiri MQ , Al-Ghamdi SMG , Farahat RM , El Desouki HN, ElNazer MS, Saleh HEDM , Abo El Naga AA, Salih AM, Mahmoud KAA, Ahmad NA
Received 26 September 2019
Accepted for publication 20 March 2020
Published 23 April 2020 Volume 2020:13 Pages 75—83
DOI https://doi.org/10.2147/IJNRD.S232633
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Mostafa Qaid Al Shamiri, 1 Saeed MG Al-Ghamdi, 2 Rafif M Farahat, 3 Hosam Nasr El Desouki, 4 Mohammed Saeed ElNazer, 5 Hossam El Deen Moustafa Saleh, 6 Ashraf Abdulghani Abo El Naga, 7 Adil Mohammed Salih, 3 Khedr Abdul Aal Mahmoud, 8 Nasim Ahmad Ahmad 9
1Department of Cardiac Science, Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia; 2Department of Internal Medicine, King Abdulaziz Hospital, Jeddah, Saudi Arabia; 3Department of Internal Medicine, Suliman Habib Hospital Saudi Arabia, Riyadh; 4Department of Internal Medicine, United Doctors Hospital, Jeddah, Saudi Arabia; 5Department of Internal Medicine, Riyadh National Hospital, Riyadh, Saudi Arabia; 6Department of Internal Medicine, Mecca Medical Center, Mekkah, Saudi Arabia; 7Department of Internal Medicine, Omar Ajaji PC, Riyadh, Saudi Arabia; 8Department of Internal Medicine, New Jedaani Hospital, Jeddah, Saudi Arabia; 9Department of Internal Medicine, Almana Hospital, Jebail, Saudi Arabia
Correspondence: Mostafa Qaid Al Shamiri
Department of Cardiac Science, Faculty of Medicine, King Saud University, PO Box 7805(38), Riyadh 11472, Saudi Arabia
Tel +966 50 413 5042
Email [email protected]
Introduction: Hypertension causes microalbuminuria, which if left uncontrolled could progress to kidney damage. Antihypertensive treatment primarily aims at controlling blood pressure (BP), but is also shown to control urine albumin excretion. This renoprotective role of antihypertensive medications consists of halting or reverting albuminuria progression.
Patients and Methods: A national Kingdom of Saudi Arabia (KSA), multicenter, observational, longitudinal study (RATIONAL), evaluated the correlation between BP control and microalbuminuria evolution over 1 year. Adult hypertensive patients with kidney damage were enrolled, after giving written consent.
Results: Of 409 patients, 60% had uncontrolled BP at baseline, down to 34% at 12 months. Over 80% of patients were on mono or double antihypertensive therapy, and angiotensin-receptor blockers (ARB) topped the list of medication classes. Albumin–creatinine ratio (ACR) significantly decreased throughout the study, indicating that BP control is paramount to prevent target organ damage. BP change most strongly correlated with ACR change upon triple therapy (ARB + calcium channel blocker + β-blocker). Importantly, 25% (at 6 months) and 38% (at 12 months) of patients reverted back to normoalbuminuria, mostly upon renin-angiotensin system blockers. Around 80% of study patients had also diabetes, a common condition in KSA, which significantly hindered achievement of normoalbuminuria at 12 months.
Conclusion: A modest but solid correlation between BP control and ACR reduction was identified. Results underline proper BP management in KSA and success of antihypertensive treatment in reverting microalbuminuria or delaying its progress. The study duration might be insufficient to reflect conclusively the beneficial effect of longer-term BP control on microalbuminuria evolution.
Keywords: hypertension, microalbuminuria, diabetes, Saudi Arabia, antihypertensive medication class
Introduction
Hypertension is a main cause of microalbuminuria; an increase in the intraglomerular pressure leads to albumin ultrafiltration.1 Microalbuminuria has been detected in up to 25% of hypertensive patients.2–4 Uncontrolled microalbuminuria could evolve to macroalbuminuria and organ damage.5 According to the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC), impaired kidney function promotes cardiovascular events and mortality.6
A 2002 Dutch study investigated the relationship between urinary albumin excretion and all-cause mortality in 80,000 subjects, and found that urine albumin excretion was a robust predictor of all-cause mortality and cardiovascular disease (CVD).7 A 2004 British study underlined this finding, proposing microalbuminuria as an indicator of fatal CVD8 and kidney failure.5 These studies endorsed the 1998 suggestion that microalbuminuria screening is a simple and accurate method to identify hypertensive patients at CVD risk.9 Given the interplay between microalbuminuria and hypertension, lowering urinary albumin excretion by lowering blood pressure (BP) would prevent/delay irreversible kidney damage. Although most antihypertensive classes achieve BP control, inhibitors of the renin-angiotensin-aldosterone system (RAS) seem most effective at also reducing albumin excretion,10,11 though antihypertensive medications might not achieve urine albumin normalization.1
Diabetes aggravates microalbuminuria and kidney damage. In diabetic patients, BP control is as important as glycaemia control to prevent/halt kidney damage.12 Furthermore, microalbuminuria is an early sign of diabetic nephropathy and a predictor for CVD in diabetic patients. The American Diabetes Association recommends testing for microalbuminuria upon diabetes diagnosis and at least yearly thereafter.13
In the Kingdom of Saudi Arabia (KSA), microalbuminuria is frequently detected in people with no prior diagnosis of kidney disease, and with known or newly discovered hypertension.14,15 Alarmingly, the 2010 study15 reported a macroalbuminuria prevalence of 5.3% in Riyadh residents with no known urine albumin anomaly, of which 30% were within the younger age group (18–30 years). This finding endorsed an earlier study reporting a 10.3% prevalence of microalbuminuria in a group of 2000 young Saudi military recruits,14 equivalent to albuminuria prevalence in American adults.16 A study in 2014 analyzed close to 55,000 diabetic people in KSA; it underlined a shortage of screening programs and prevalence of diabetic nephropathy in about 11% of the study population, closely linking it to diabetes duration and hypertension.17 Other studies had also addressed kidney damage in the context of diabetes; hence the relevance of properly managing these concomitant conditions to prevent/delay complications.18,19
Data is scarce in KSA on the prognostic value of microalbuminuria in hypertensive patients or on its evolution upon antihypertensive treatment. The purpose of the RATIONAL study was to evaluate urinary albumin evolution in hypertensive patients following the local treatment algorithm. An observational study design was chosen to reflect real-life conditions and draw on the efficacy of pharmacological management of hypertension in controlling albuminuria.
Methods and Participants
Study Design and Participants
A national, multicenter, observational, longitudinal study (RATIONAL) evaluated the correlation between antihypertensive treatment effect and microalbuminuria evolution over 12 months, between May-2016 and July-2018.
Eligible patients were over-20-year-old adults, with hypertension and microalbuminuria at inclusion, treated per routine practice in KSA; with or without diabetes and CVD and having given written consent. Patients with secondary hypertension, congenital kidney disease or those participating in any clinical trial were excluded.
Ethical approval was obtained from the Institutional Review Board at the College of Medicine at King Saud University. All participants signed written consent forms.. The study was carried out in compliance with the Declaration of Helsinki.
Investigators were randomly selected from an exhaustive list of potential investigators in KSA, complying with good clinical practices. To avoid bias and allow for result extrapolation to the broadest possible population, all consecutive patients who met the eligibility criteria were asked to participate in the registry, as per study protocol. For this study, 31 investigators enrolled patients in 25 centers throughout KSA.
Data Collection
Data were collected on individual Case Report Form (CRF) completed by the investigator/delegated staff at baseline, 6-month and 12-month visits. Collected data included BP measurements, urine albumin levels, antihypertensive medication classes, and demographic, biochemical and anthropometric profile of patients. The antihypertensive generic name was handwritten on the CRF. Generic names were given codes according to the World Health Organization Drug Dictionary (WHO-DD) and grouped into antihypertensive classes.
Microalbuminuria is assessed by measuring albumin levels in a random urine spot sample, 24-hour excretion rate or timed urine collection;20 which all seem consistent.21 To minimize missing data on urine albumin levels and reliably reflect routine practice, the CRF accommodated for multiple techniques of urine albumin measurement (dipstick measurement, turbidimetry and radioimmunoassay), in different urine samples (24-hour pooled urine, overnight urine collection, first-morning urine and spot-urine sample). Calculation of albumin–creatinine ratio (ACR) and conversions to one unified unit (mg/24h) were performed.
Outcome Definitions
According to ESH/ESC 2013 Guidelines, grade 1 hypertension: systolic BP (SBP) of 140–159 mmHg or diastolic BP (DBP) of 90–99 mmHg; grade 2 hypertension: SBP of 160–179 mmHg or DBP of 100–109 mmHg; grade 3 hypertension: SBP ≥ 180 mmHg or DBP ≥ 110 mmHg. BP was measured in a sitting position by the standard methodology in the office by trained personnel with the conventional, automated or manual BP machines according to the practice of the investigators.
A healthy person loses daily below 30 mg of proteins, mostly albumin.22 Microalbuminuria develops from changes in kidney function leading to increased albumin excretion (30–299 mg/24h or 20–199 μg/min). Macroalbuminuria corresponds to urine albumin levels beyond 300 mg/24h. According to the World Health Organization, a body mass index (BMI) <18.5 kg/m2 indicates underweight, 18.5 ≤ BMI < 25 kg/m2 is healthy, 25 ≤ BMI < 30 kg/m2 indicates overweight and BMI ≥ 30 kg/m2 indicates obesity.
Statistical Considerations
Sample size was determined regarding primary endpoint: correlation between antihypertensive treatment and microalbuminuria evolution. The Spearman coefficient of correlation (r) was computed; a correlation is non-significant if |r|≤0.4 and significant if |r| >0.4. According to the formula developed by Bonnet and colleagues,23 a sample size of 304 patients is required to estimate the Spearman coefficient correlation with a precision of ±0.09 using a Fisher two-tailed 95% confidence interval (CI). Assuming that 30% of patients might not have paired BP and urinary albumin measures, this registry aimed at enrolling 435 patients.
Primary evaluation criteria included correlation coefficients between SBP and DBP change from one side, and urinary albumin change from the other side, with its two-tailed 95% CI at 6 and 12 months. Secondary evaluation criteria included changes from baseline in the urinary albumin values and in BP parameters, according to antihypertensive classes; in addition to the proportion of patients with normoalbuminuria, microalbuminuria and macroalbuminuria at each data point, and the proportion of patients with controlled and uncontrolled BP.
The statistical analysis was exploratory and descriptive. A paired Student’s t-test or a Wilcoxon/Mann–Whitney signed rank test was used to test the evolution of parameters over time depending on data normality. Missing data were not replaced and did not participate in percentage/probability calculation. Statistical significance was set at P < 0.05. Statistical analysis was performed using SAS (version 9.2; SAS Institute, Cary, NC, USA).
Results
Out of the 415 patients who gave informed consent, 409 patients were eligible for inclusion (reference population). In addition, 21 patients had their visits outside the accepted timeframe, 89 patients did not complete the study, and 158 patients had missing urine albumin values at study visits. In total, 326 patients attended all 3 study visits, but only 151 patients had urine albumin data at all visits (the completers). Table 1 displays the baseline characteristics of the reference population.
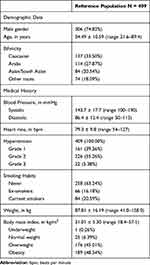 |
Table 1 Baseline Characteristics of Study Patients |
Clinical Profile at Inclusion
All registry patients had microalbuminuria (276 [99.28%]) or macroalbuminuria (2 [0.72%]) at inclusion; ACR varied from 30.0 to 552.2 mg/24h (278 patients). Over 50% had grade 2 hypertension (226 [55.26%]) and 22 (5.38%) patients had grade 3 hypertension. In addition, 340 (83.13%) patients also reported co-morbidities: diabetes only (241 [58.92%]), CVD only (15 [3.67%]), diabetes and CVD (84 [20.54%]). The proportion of study patients having concomitant kidney damage, hypertension and diabetes almost reached 80% and about 50% of patients presented vascular complications, which motivated analysis of the diabetic subset of patients.
Overall, age, smoking status, BMI, and microvascular complications were noticeably different at baseline among the diabetic and nondiabetic subsets of patients.
Overview of Hypertension Management Throughout the Study
The different therapeutic strategies to promote and maintain BP control were tightly followed in this study.
Out of the 409 patients in this study, only 133 (32.52%) had their BP controlled at baseline, almost doubling at 6 months (214 of 344 patients [62.21%]). At 12 months, 227 out of 344 patients (65.99%) had also achieved BP control.
At baseline, the vast majority of patients were on single antihypertensive therapy (191 [46.70%] patients) or on double therapy (145 [35.45%] patients). Angiotensin receptor blockers (ARB, majorly irbesartan) topped the list of antihypertensive medication classes reported in this study. Other antihypertensive classes were also reported in this study; amlodipine was the major calcium channel blocker (CCB), bisoprolol the main β-blocker, hydrochlorothiazide the main diuretic and perindopril the main angiotensin-converting-enzyme inhibitor (ACEI). Table 2 reports proportions of patients on single, double, triple therapy and beyond, in addition to patients receiving each of the 7 antihypertensive medication classes (solo or in combination), and those who saw their antihypertensive treatment modified at 6 or 12 months.
 |
Table 2 Antihypertensive Medications Taken by Study Patients |
Achieving BP Control in Hypertensive Patients with Uncontrolled BP
All 409 patients who entered the study were hypertensive, but 276 patients had entered the study with uncontrolled BP and were prescribed a different antihypertensive treatment. ARBs prescribed solo at baseline achieved BP control in 32 out of the 55 patients (58.18%). CCB, β-blockers, diuretics and ACEI were also successful in controlling BP 6 months post-baseline in patients with initially uncontrolled BP. Importantly, ARB in combination with a CCB, diuretic or β-blocker achieved BP control in most patients.
Triple antihypertensive therapy prescribed at baseline seemed beneficial for patients with uncontrolled BP at study entry. In fact, around 50% of patients prescribed a combination of diuretics, ARB and CCB or quadruple therapy achieved BP control at 6 and at 12 months post-baseline.
Throughout the study, SBP and DBP declined by 6.39 ± 12.76 mmHg and 6.04 ± 12.41 mmHg, respectively, at the 6-month visit and by 7.21 ± 13.91 mmHg and 7.01 ± 13.52 mmHg at the 12-month visit. SBP and DBP decrease from baseline was highly significant (P < 0.001).
Monitoring of Albuminuria Throughout the Study
Albuminuria was measured by several methods in this study. Radioimmunoassay was the most frequently used method, followed by dipstick measurement; at each data point, a spot urine sample was collected and analyzed for over 50% of study patients. At baseline, patients had their urine albumin levels assessed on average 2.93 ± 6.56 weeks (range 0–52.3 weeks) prior to study entry; but consecutive urine albumin measurements became closer together at 6 months (1.66 ± 3.54 weeks) and then at 12 months (1.45 ± 3.25 weeks).
Antihypertensive Treatment Halted Albuminuria Progression
This study aimed primarily at determining the correlation between urine albumin change with BP change upon antihypertensive treatment, in an attempt to understand whether pharmacological management of hypertension is reversing, delaying or halting microalbuminuria evolution.
For patients who had ACR values reported at all 3 study visits, ACR significantly decreased at 6 months (−4.29 ± 92.05%, P < 0.001) and further at 12 months (−19.74 ± 71.34%, P < 0.001). Taken separately, BP parameters differentially correlated with ACR change. Among the reference population, only DBP change correlated with ACR evolution from baseline to 12-month visit (r = 0.41), while among completers, SBP change was found to correlate with ACR evolution at 12 months (r = 0.43).
The highest correlation between BP change upon 12-month monotherapy and ACR change was for patients treated with a β-blocker or a CCB (r = 0.55). BP change upon double therapy also strongly correlated with ACR change; β-blocker and ARB combination was associated with the highest correlation at 6 months (r = 0.60). However, the greatest correlation was observed upon 12-month triple therapy with a β-blocker, ARB and CCB (r = 0.84).
Relative changes of ACR from baseline showed a statistically significant difference among genders. The relative decrease in ACR at 6 months among females (a decrease of 23.56% ± 94.97) was more pronounced than in males (a decrease of 0.52% ± 98.23), with P = 0.019. At 12 months, ACR had increased among males (by 10.98% ± 172.35) and decreased among females (by 16.63% ± 156.65) with P = 0.014.
Antihypertensive Treatment Induced Albuminuria Normalization
At study entry, all patients had microalbuminuria (276 [99.28%]) or macroalbuminuria (2 [0.72%]). Importantly, about 25% of patients (56 patients [24.67%]) for whom urine albumin levels were available at baseline and at the 6-month visit regressed from microalbuminuria to normoalbuminuria (ACR < 30 mg/24h) and up to 37.87% (89 patients) at the 12-month visit. Those patients were mostly on RAS blockers (10 out of 15 patients at the 6-month visit and 22 out of 29 patients at the 12-month visit were on monotherapy). Similar trends were observed for patients on double antihypertensive medications, where combinations involving ARB were the most widely prescribed medications. Very few patients progressed to macroalbuminuria: 10 patients (6 months) and 20 patients (12 months). Importantly, multiple antihypertensive therapies (beyond 3 classes) did not achieve better ACR outcomes. No difference was noticeable among genders.
Concomitant Diabetes Mellitus Hindered Normalization of Urine Albumin Levels
A large proportion of study patients also had diabetes mellitus (325 of 409 patients [79.46%]), and this subset more closely represented the overall population than did the nondiabetic subset (84 patients). In fact, correlation between BP parameters and ACR change within the diabetic subset was very comparable to that within the overall population.
Slightly more diabetic patients failed to achieve normoalbuminuria at 6 months (138 out of 180 diabetic patients [76.67%] versus 33 out of 47 nondiabetic patients [70.21%], P = 0.361); but this difference became more pronounced and reached statistical significance at 12 months (123 out of 186 diabetic patients [66.13%] versus 23 out of 49 nondiabetic patients [46.94%], P = 0.014, Figure 1 panel A).
These observations were not paralleled with changes in BP reported for the diabetic subset. In fact, though not statistically significant, a higher proportion of diabetic patients (63.94%) had achieved BP control at the 6-month visit than nondiabetic patients (57.53%), but these percentages were reversed at the 12-month visit with 64.81% of diabetic patients versus 72.22% of nondiabetic patients achieving BP control (Figure 1 panel A).
Figure 1 panel B also shows that, though not statistically significant, higher BMI was associated with lower proportions of patients achieving normoalbuminuria at 12 months and BP control at 6 and 12 months.
Antihypertensive Therapy Was Associated with Satisfactory Biochemical and Vital Sign Profile
Laboratory test results were reported for study patients. Total cholesterol levels decreased by around 5% at 6 months (P < 0.001) and by 2.5% at 12 months (P = 0.001); low-density lipoprotein (LDL)-cholesterol levels decreased by 2% at 6 months (P < 0.001) and by 1% at 12 months (P = 0.002); and high-density lipoprotein (HDL)-cholesterol levels increased by close to 4.5% at 6 months and by 8.5% at 12 months (P < 0.01). HbA1c levels, reflecting long-term blood glucose status, decreased by 4.5% and 7.5% at the 6- and 12-month visits (P < 0.001). Fasting blood glucose levels decreased by 8% at 6 months (P < 0.001) and by 9% at 12 months (P < 0.001). Average weight was mostly maintained throughout the study, and heart rate had decreased (P < 0.05).
Safety Reporting
Throughout the study, investigators were requested to properly report any safety events experienced by study patients. Only one event was reported: death of a 66-year-old patient, while participating in the study. His death was due to severe chest infection, with sepsis complicated by multiple organ dysfunction, severe acidosis and metabolic derangement; it was not attributed to any drug product.
Discussion
BP control in hypertensive patients is a milestone in preventing and delaying kidney damage. Albuminuria is associated with increased risk of CVD and target organ damage in diabetic patients.24 Little is known about the prevalence of these three conditions together in KSA adults, though diabetes is highly prevalent in the country.25 The RATIONAL study tackled hypertension management and diabetes in patients with kidney damage in KSA, given the tight reciprocal influence between these conditions.25,26 It aimed at identifying patterns of pharmacological management of hypertension and its modulation of microalbuminuria evolution over 1 year, by determining the correlation between the beneficial effect of BP control and ACR levels in hypertensive patients in general and with diabetes in particular.
About three-quarters of the study patients were males, matching worldwide hypertension prevalence among the male gender;27 80% of study patients had diabetes and best represented the overall study population. This observation alarmingly reflects diabetes prevalence among patients with microalbuminuria, and underlines the effect of diabetes on antihypertensive medication efficacy. Overall, across treatment classes, the percentage of patients with microalbuminuria at baseline (99.28%) went down to 70.93% at 6 months and further to 53.62% at 12 months, paralleled by increased proportion of patients reverting to normoalbuminuria. Successful BP management was thus accompanied by controlled urinary albumin excretion; emphasizing long-known facts that chronically elevated BP correlates with higher urine albumin levels and kidney damage,28 and that BP fluctuations could also predict kidney damage.29,30 Similarly, a study highlighted that better BP control associated with greater possibility of remission and reverting to normoalbuminuria.31
Around 50% of patients were on single antihypertensive treatment throughout the study, and 35–37% on dual therapy. A study published in 2007 reported that most patients with hypertension are prescribed two or more drugs for BP control.32 Some patients in the RATIONAL study had their antihypertensive treatment changed or escalated, and some were put on triple and quadruple therapy, highlighting the challenge of properly controlling BP.33 Nonetheless, over half of the patients who joined the study with uncontrolled hypertension saw their BP normalize over time. ARBs remained the most frequently prescribed medications in monotherapy or combined with CCB, diuretics or β-blockers, matching results from a 2018 study.34 Another recent study had also highlighted the preferred use of RAS blockers in hypertensive patients with kidney damage, irrespective of diabetes status,35 but warned against their use in combination in some patients.
Importantly, multiple antihypertensive therapy (beyond two classes) did not achieve better ACR control, suggesting that more aggressive antihypertensive treatment does not necessarily yield better urine albumin levels. Moreover, for the study population and timeframe, correlation between BP parameters and ACR change was rather modest, with only SBP correlating with ACR change at 12 months. Recent studies concluded that SBP variability can independently induce microalbuminuria in hypertensive patients with diabetes;36,37 which could explain correlation between SBP and ACR change in our study. A 2005 study also reported that even in patients with mild hypertension, onset of microalbuminuria was linked to poor BP control and gradual increase in glycaemia.38 In KSA, chronic kidney disease also prevailed in patients with higher glycaemia and waist/hip ratio, reflecting heavier weight and hypertension risk.15 Importantly, a study reported a fivefold increased risk of albuminuria in younger Saudi patients with diabetes,14 also aggravated by obesity and hypertension.
Given the prevalence of overweight/obesity and associated metabolic conditions like diabetes and hypertension in KSA, results from the current study could be extrapolated to the hypertensive population, but also to the otherwise healthy population who remains at risk of hypertension and diabetes, both of which strongly associate with target organ damage.
Study Limitations
ACR values were missing for a large proportion of patients, though we accepted several techniques of obtaining urine microalbuminuria, which reflect clinical practice.
This study did not investigate nocturnal/diurnal BP changes, which strongly influence urine albumin excretion rates; loss of nocturnal BP decline correlates with accelerated kidney damage.39 Future studies may integrate a 24-hr ambulatory BP monitoring to obtain dipping data and its relation to ACR. This study did not evaluate dietary habits, which impact clinical profile and kidney damage progression.40 Given the prevalence of overweight/obesity and associated metabolic conditions such as diabetes and hypertension in the Saudi population, results from this study could be extrapolated to the general hypertensive population in KSA, but also to the otherwise healthy population who remains at risk of developing hypertension and diabetes, both of which strongly associate with kidney damage.
Conclusion
This study gave an overview of pharmacological management of hypertension in kidney damage patients, and shed light on high diabetes prevalence in this patient population. RAS blockers, especially ARBs, have been used solo or in polypharmacy in most patients, achieving BP control in two-thirds of the study population. A noticeable proportion of patients also saw their ACR normalize after 6 and 12 months of antihypertensive treatment, and very few patients progressed to macroalbuminuria. This study revealed a modest but solid correlation between BP control and ACR decrease. The study duration might be insufficient to conclusively reflect the beneficial effect of longer-term BP control on microalbuminuria evolution. A larger cohort with longer follow-up, accounting for potential confounders (age, lifestyle, diabetes management and nocturnal/diurnal BP fluctuations), could provide better insights on these chronic conditions in terms of management and outcomes.
Clinical Summary
- BP control was achieved in two-thirds of the study population, under the current antihypertension management scheme in KSA.
- RAS blockers were the most frequently prescribed medications, in mono- or multiple therapies.
- A noticeable proportion of patients had their ACR normalized after 6 and 12 months of antihypertensive treatment, and only very few patients progressed to macroalbuminuria.
Acknowledgment
The authors thank the patients, investigators and staff members who took part in this study.
The authors also acknowledge the contract research organization KBP-Biomak (funded by SANOFI) for managing the study and providing writing support. This study was sponsored by SANOFI group. The authors declare no other conflict of interest.
Author Contributions
All authors contributed to study design, data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Saeed Al-Ghamdi reports grants from Sanofi during the conduct of the study; outside the submitted work. Dr Hossam El Deen Moustafa Saleh reports personal fees from Mecca medical center hospital during the conduct of the study. Dr Khedr Abdulaal Mahmoud reports personal fees from Sanofi pharmaceutical company during the conduct of the study. Dr Nasim Ahmad reports personal fees from Sanofi Aventis Group Co, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Crippa G. Microalbuminuria in essential hypertension. J Hum Hypertens. 2002;16(Suppl 1):S74–S77. doi:10.1038/sj.jhh.1001348published.
2. Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC study. microalbuminuria: a genoa investigation on complications. Hypertension. 1997;30(5):1135–1143. doi:10.1161/01.HYP.30.5.1135
3. Yudkin JS, Forrest RD, Jackson CA. Microalbuminuria as predictor of vascular disease in non-diabetic subjects. islington diabetes survey. Lancet. 1988;2(8610):530–533. doi:10.1016/S0140-6736(88)92657-8
4. Jensen JS, Feldt-Rasmussen B, Strandgaard S, Schroll M, Borch-Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35(4):898–903. doi:10.1161/01.HYP.35.4.898
5. de Alvaro F, Velasco O, Honorato J, Calvo C, Parrondo I. Microalbuminuria in hypertensive patients: evaluation of one-year treatment with irbesartan. Kidney Int Suppl. 2005;93:S29–S34. doi:10.1111/j.1523-1755.2005.09307.xpublished
6. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357. doi:10.1097/01.hjh.0000431740.32696
7. Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–1782. doi:10.1161/01.CIR.0000031732.78052.81
8. Yuyun MF, Khaw KT, Luben R, et al. A prospective study of microalbuminuria and incident coronary heart disease and its prognostic significance in a British population: the EPIC-Norfolk study. Am J Epidemiol. 2004;159(3):284–293. doi:10.1093/aje/kwh037
9. Rodicio JL, Campo C, Ruilope LM. Microalbuminuria in essential hypertension. Kidney Int Suppl. 1998;68:S51–S54. doi:10.1046/j.1523-1755.1998.06813.x
10. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi:10.1056/NEJMoa011161
11. Ibsen H, Olsen MH, Wachtell K, et al. Does albuminuria predict cardiovascular outcomes on treatment with losartan versus atenolol in patients with diabetes, hypertension, and left ventricular hypertrophy? The LIFE study. Diabetes Care. 2006;29(3):595–600. doi:10.2337/diacare.29.03.06.dc05-1724
12. TC E. P C. Diabetic nephropathy. Clin Diabetes. 2000;18:1.
13. Standards of Medical Care in. Diabetes-2018 abridged for primary care providers. Clin Diabetes, 2018; 36(1):14–37. doi:10.2337/cd17-0119
14. Abo-Zenah H, El-Benayan A, El Nahas AM. Prevalence of increased albumin excretion rate in young saudi adults. Nephron Clin Pract. 2008;108(2):c155–c162. doi:10.1159/000115328.
15. Alsuwaida AO, Farag YM, Al Sayyari AA, et al. Epidemiology of chronic kidney disease in the Kingdom of Saudi Arabia (SEEK-Saudi investigators) - a pilot study. Saudi J Kidney Dis Transpl. 2010;21(6):1066–1072.
16. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the. States U. JAMA. 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
17. Al-Rubeaan K, Youssef AM, Subhani SN, et al. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PLoS One. 2014;9(2):e88956. doi:10.1371/journal.pone.0088956
18. Alzaid AA, Sobki S, De Silva V. Prevalence of microalbuminuria in Saudi Arabians with non-insulin-dependent diabetes mellitus: a clinic-based study. Diabetes Res Clin Pract. 1994;26(2):115–120. doi:10.1016/0168-8227(94)90148-1
19. Qari FA. Profile of diabetic patients with end-stage renal failure requiring dialysis treatment at the king abdulaziz university hospital, Jeddah. Saudi J Kidney Dis Transpl. 2002;13(2):199–202.
20. Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83.
21. Aziz KM. Correlation of urine biomarkers: microalbuminuria and spot urine protein among diabetic patients. application of spot urine protein in diabetic kidney disease, nephropathy, proteinuria estimation, diagnosing and monitoring. Recent Pat Endocr Metab Immune Drug Discov. 2015;9(2):121–133. doi:10.2174/1872214809666150708111022
22. Stephen R, Jolly SE, Nally JV
23. DG B, TA W. Sample size requirements for estimating pearson, spearman and kendall correlations. Psychometrika. 2000;65(1):23–28. doi:10.1007/BF02294183
24. Alharf AA, Cleland S, Webster J, McInnes GT, Padmanabhan S. Microalbuminuria in subjects with hypertension attending specialist blood pressure clinics. J Hum Hypertens. 2016;30(9):527–533. doi:10.1038/jhh.2015.116.
25. Lary SA. Urinary enzymes and microalbuminuria as indicators of renal involvement in patients with diabetes mellitus in saudi arabia. Saudi J Kidney Dis Transpl. 2004;15(1):18–26.
26. Munakata M, Konno S, Ohshima M, Ikeda T, Miura Y, Ito S. High-normal blood pressure is associated with microalbuminuria in the general population: the Watari study. Hypertens Res. 2011;34(10):1135–1140. doi:10.1038/hr.2011.98.
27. Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol. 2015;61(1):1–17. doi:10.1080/19485565.2014.929488.
28. Bianchi S, Bigazzi R, Baldari G, Sgherri G, Campese VM. Diurnal variations of blood pressure and microalbuminuria in essential hypertension. Am J Hypertens. 1994;7(1):23–29. doi:10.1093/ajh/7.1.23
29. Li CL, Liu R, Wang JR, Yang J. Relationship between blood pressure variability and target organ damage in elderly patients. Eur Rev Med Pharmacol Sci. 2017;21(23):5451–5455. doi:10.26355/eurrev_201712_13934.
30. Yin LH, Yan WJ, Guo ZX, Zhou FZ, Zhang HY. Relation between blood pressure variability and early renal damage in hypertensive patients. Eur Rev Med Pharmacol Sci. 2017;21(9):2226–2231.
31. Nishimura M, Kato Y, Tanaka T, et al. Effect of home blood pressure on inducing remission/regression of microalbuminuria in patients with type 2 diabetes mellitus. Am J Hypertens. 2017;30(8):830–839. doi:10.1093/ajh/hpx050
32. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591–603. doi:10.1016/s0140-6736(07)61299-9.
33. Jones DW, Hall JE. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure and evidence from new hypertension trials. Hypertension. 2004;43(1):1–3. doi:10.1161/01.HYP.0000110061.06674.
34. Hosaka M, Inoue R, Satoh M, et al. Effect of amlodipine, efonidipine, and trichlormethiazide on home blood pressure and upper-normal microalbuminuria assessed by casual spot urine test in essential hypertensive patients. Clin Exp Hypertens. 2018;40(5):468–475. doi:10.1080/10641963.2017.1403617
35. Del Vecchio L, Teatini U, Locatelli F. Use of ACE inhibition and blood pressure management in deferring dialysis initiation. Panminerva Med. 2017;59(2):166–172. doi:10.23736/s0031-0808.17.03293-1.
36. Noshad S, Mousavizadeh M, Mozafari M, Nakhjavani M, Esteghamati A. Visit-to-visit blood pressure variability is related to albuminuria variability and progression in patients with type 2 diabetes. J Hum Hypertens. 2014;28(1):37–43. doi:10.1038/jhh.2013.36.
37. Takao T, Suka M, Yanagisawa H, Matsuyama Y, Iwamoto Y. Predictive ability of visit-to-visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract. 2017;128:15–23. doi:10.1016/j.diabres.2017.03.027
38. Pascual JM, Rodilla E, Gonzalez C, Perez-Hoyos S, Redon J. Long-term impact of systolic blood pressure and glycemia on the development of microalbuminuria in essential hypertension. Hypertension. 2005;45(6):1125–1130. doi:10.1161/01.hyp.0000167151.52825.11.
39. Mentari E, Rahman M. Blood pressure and progression of chronic kidney disease: importance of systolic, diastolic, or diurnal variation. Curr Hypertens Rep. 2004;6(5):400–404. doi:10.1007/s11906-004-0060-2
40. Weir MR. Dietary salt, blood pressure, and microalbuminuria. J Clin Hypertens. 2004;6(11 Suppl 3):23–26. doi:10.1111/j.1524-6175.2004.04066.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

