Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 13
An Investigator-Initiated, Prospective, Single-Center, Open-Label Clinical Study to Evaluate Safety and Performance of Intra-Articular Hyaluronic Acid (IA-HA) (Biovisc Ortho) in Patients with Osteoarthritis (OA) of the Knee
Authors Gupta A , Channaveera C, Anand V , Sethi S
Received 15 January 2021
Accepted for publication 2 April 2021
Published 11 May 2021 Volume 2021:13 Pages 73—82
DOI https://doi.org/10.2147/CPAA.S298589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Ajay Gupta,1 Chethan Channaveera,2 Vijender Anand,1 Satyaranjan Sethi1
1Department of Physical Medicine & Rehabilitation, VMM College and Safdarjang Hospital, New Delhi, 110029, India; 2Department of Physical Medicine & Rehabilitation,AIIMS, Mangalagiri, Andhra Pradesh, 522503, India
Correspondence: Satyaranjan Sethi
VMM College and Safdarjang Hospital, New Delhi, 110029, India
Email [email protected]
Objective: IA-HA is injected into the osteoarthritis knee as a viscosupplementation for therapeutic purposes. This clinical trial was carried out for evaluating the efficacy and safety of Biovisc Ortho IA-HA (20 mg/2 mL) in a 2 mL prefilled syringe.
Design: The study was conducted as an open-label, single-center, single-arm clinical trial in India. Patients of knee OA with moderate to severe symptoms for a minimum duration of 3 months were included in the study. Five visits were conducted at weekly intervals and the investigational product was administered at each visit. Two follow-up visits were conducted at 3 and 6 months after the completion of the last injection cycle. The primary outcome variable was change in KOOS pain score from baseline. The secondary outcome variables were analyzed for other KOOS scales and safety of the device.
Results: Change in KOOS pain score at 6 months from baseline was 29.71± 15.74 and the change in mean KOOS score for pain was statistically significant (p< 0.0001) for all post-baseline visits. Statistically significant improvement was observed for mean values of efficacy assessments (KOOS) during the study period (6 months) for all the domains evaluated, including pain, joint function and quality of life.
Conclusion: Despite being an open, noncomparative study, the safety and efficacy results of IA-HA establish the therapeutic effect of the treatment throughout the study period of 6 months and are safe.
Keywords: osteoarthritis, hyaluronic acid, intra-articular, IA, viscosupplementation, KOOS
Introduction
Osteoarthritis (OA) is a chronic and progressive joint disease that leads to articular cartilage damage, bone deformation, osteophyte formation and synovial inflammation. Recent studies have shown that changes in the infrapatellar fat pad and synovial membrane may enhance the inflammation and pain in OA.1,2 OA symptoms primarily hamper physical activity and lead to radiographic changes with acute or chronic inflammation.3 Inflammatory mediators elicit pain and degrade extracellular matrix and synovial fluid. Degradation of the synovial fluid leads to decreased concentration and reduced molecular weight of the endogenous hyaluronic acid (HA). HA is a glycosaminoglycan molecule which acts as a rheostatic agent. The elasticity and viscosity of synovial fluid are maintained by the HA in normal knee joints.3–6
Intra-articular therapeutic modality is preferred over analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) for knee OA management due to significant toxicity and poor tolerability by OA patients with comorbidities.5 Intra-articular HA is either naturally derived from avian or through bacterial fermentation.6 IA-HA injection is termed as viscosupplementation as it is believed to restore the viscoelastic properties of the synovial fluid when injected into the knee joints. Among other treatments, viscosupplementation is preferred by OA patients and physicians because of none to lesser known drug–drug interactions and side effects.6,7 Moreover, recent evidence suggests IA-HA is more effective in the long term in addition to efficacy and safety.7
Biovisc Ortho is a sterile, transparent, homogeneous viscoelastic gel of high molecular weight HA. Biovisc Ortho contains 10 mg/mL of HA buffered phosphate saline solution. Biovisc Ortho is formulated with injectable grade HA of bio-fermentation origin. This clinical study is aimed to evaluate the efficacy and safety of Biovisc Ortho IA-HA (20 mg/2 mL) in a 2 mL prefilled syringe.
Method
Study Design
An open-label, single-center, single-arm clinical study was conducted in India. The trial participants received five injections at weekly intervals followed by two follow-up visits at the third and sixth months after completion of the last injection cycle.
The study commenced only after written approval was obtained from the institute ethics committee of VMM College & Safdarjung Hospital, New Delhi. The study was conducted by the protocol pertinent requirements of the Indian Council of Medical Research (ICMR) Ethical guidelines, International Council for Harmonization (ICH) “Guidance on Good Clinical Practice” (E6), ISO 14,155, Medical Device Directives of Global Harmonization Task Force and European Union and “Declaration of Helsinki”. The study was registered at Clinical Trials Registry – India (CTRI registration number - CTRI/2018/01/011174).
After the informed consent form (ICF) was signed by the patient, the patient was assessed for eligibility criteria. For a patient who could not read such as sight compromised, blind or illiterate patients, the content of the ICF was read to the patient in the language the patient understands in the presence of a legally acceptable representative (LAR) or impartial witness. Investigators ensured that the patient has understood the contents of the ICF. For patients who were unable to sign such as those who were illiterate, lame or having a fractured hand, the left thumb impression (or left great toe in case of lame patients) was taken on the ICF. In both the above cases, the signature of the LAR or impartial witness was taken on the ICF. The informed consent form was signed in the same language as the consent form. This article is reported as per the CONSORT 2010 Statement. All data of the study will be maintained with the investigators for 5 years and de-identified data may be provided on request after approval from the institute ethics committee and Clinical Trials Registry of India.
Study Population
The investigational population was patients of any gender of age ≥40 years and ≤75 years with the following inclusion criteria: documented primary OA knee diagnosis with uni- or bilateral involvement as per the ACR (American College of Rheumatology) criteria;8 radiologically grade II and III OA of the knee as per Kellgren and Lawrence classification;9 with consistent symptoms (joint pain, swelling, crepitus, effusion alone or a combination of these symptoms) of knee OA for more than 3 months before screening; a minimum 3 months of unsuccessful non-surgical treatment. The non-surgical treatment includes (but is not limited to) acetaminophen, anti-inflammatory medication, cortisone injection, physical therapy and bracing; but not limited to those willing to discontinue all NSAIDs or other analgesic medication taken for any condition, including their knee pain. However, patients were allowed to use only acetaminophen or aspirin as a rescue pain medication during the study period. The patients must abstain from medication use 24 hours before any study visit; and patients are able to understand and follow the study procedures and provide written informed consent. Patients were excluded if they belonged to the following exclusion criteria: having previously undergone surgery on the target knee, including arthroscopy; having neurological deficit in the lower extremities; suffering from primary inflammatory joint disease, IA tumors; having comorbidities that impaired the ability to participate in functional daily activities; with any significant OA symptoms in other joints apart from knees which may require pharmacological treatment during the study; treated with IA-HA in last 6 months; treated with IA steroids or articular lavage of the target knee in last 3 months; oral intake of diacerein, chondroitin and glucosamine sulfate in last 3 months; with history of allergy or hypersensitivity to HA; and participated in any other clinical study in last 3 months and any surgery scheduled for next 8 months from the date of study that can affect directly the result of the present study.
Treatments
The eligible patients were treated with five injection cycles of IA-HA 20 mg/2 mL (Biovisc Ortho 1% prefilled syringe) with a molecular weight of 2.8–3.5 mio Da at weekly intervals. Patients were allowed to use only acetaminophen or aspirin as a rescue pain medication during the study period and to abstain from medications use 24 hours prior to any study visit.
Trial Procedures
Patients were screened as per the protocol inclusion/exclusion criteria. All of the patients were assessed for eligibility criteria, including medical history before the start of the study procedure. Patients who were eligible and ready to sign a consent form were included in the study. Compliance with investigations and laboratory assessment were assessed during the study. Laboratory tests of complete blood count, erythrocyte sedimentation rate, fasting blood sugar and X-ray were performed for all of the patients during the screening process.
Assessment of compliance with the therapy was critically evaluated until the sixth month from the last injection cycle. At each visit, the patients were enquired about the compliance of the treatment and recorded. If it was necessary to examine a patient other than at a scheduled visit date, the unscheduled visit procedures were followed.
Efficacy Outcome Measures
The Knee Injury and OA Outcome Score (KOOS) scale was used to assess the change at baseline, third, fifth injection cycles and both follow-up visits. KOOS is a 42-item, patient-reported questionnaire to assess symptoms and problems of knee injury and OA of the following five dimensions: symptoms (seven items); pain (nine items); function, daily living (seventeen items); function, sports and recreational activities (five items); and quality of life (four items). A Likert scale was used to capture the scores except for pain. Pain scale ranges from 0 to 100 (best score, ie, no pain). Reported differences in pain scale score at 6 months from baseline were collected; so pain scale scores theoretically ranged from 0 (maximum pain) to 100 (no pain).
Safety Assessment
Any serious, non-serious adverse events and the systemic events were assessed during the study period. Additionally, procedural complications at the injection site were also assessed.
Sample Size
Sample size was calculated at 90% study power with a significance level (α) of 0.05 assuming an estimated standard deviation of differences of 15.0 to detect a mean of paired differences of 7.0; which came out to be 51. Considering a 20% dropout rate, 63 subjects were enrolled in the study.
Statistical Analysis
The primary efficacy end point was analyzed on a Per Protocol (PP) population and a modified Intent-To-Treat (mITT) population. The analysis based on the PP population was definitive while the analysis based on the mITT population was supportive. The PP population included the patients with the following criteria: i) the patients who received an enrollment number; ii) completed study in compliance with the protocol; and iii) not having any major protocol deviations. Patients with either serious noncompliance with regulatory or ICH Good Clinical Practice (GCP) guidelines, and violations of inclusion and exclusion criteria during the enrollment visit were excluded from the PP population and included in the mITT population.
The mITT population were the set of patients: i) who received an enrollment number; ii) administered with at least one dose of assigned study medication; and iii) appeared for at least one post-baseline visit. The patients, who have completed at least 3 months follow-up, were continued in the reporting. All patients lost to follow-up after 3 months were taken for efficacy evaluation based upon the last observation carried forward (LOCF) population.
The safety population included a set of all patients who signed informed consent, received an enrollment number and applied at least one dose of assigned study medication. The differences in paired mean were evaluated by paired t-test. Statistical analyses were performed using Statistical Analysis System (SAS®), version 9.4.
Results
Patient Disposition and Baseline Characteristics
Figure 1 illustrates the patient disposition in the study. Sixty-three patients were screened, enrolled and included for safety analysis. Of the 63 patients, 60 (95.24%) patients completed the study and were included in the mITT and PP population. In this study, the mITT and PP population remained the same. Hence, the PP population analysis results were used throughout this article. Three (4.76%) patients discontinued from the study after withdrawing consent by their own accord due to no improvement from baseline symptoms.
Demographic characteristics of the safety population are shown in Table 1. A total of 63 patients with mean (±SD) age of 54.22 (±9.66) years were enrolled into the study. Out of 63 patients, 19.00 (30.16%) patients were male and 44.00 (69.84%) patients were female. A mean body mass index (BMI) of 25.09 (±4.14) kg/m2 was observed among the 63 enrolled patients.
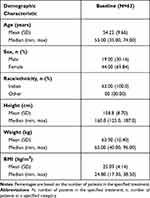 |
Table 1 Summary of Patient Demographic Characteristics – Safety Population |
Table 2 depicts the symptoms of knee OA. Out of the total 63 patients, 62 patients (98.41%) reported pain, 29 patients (46.03%) had swelling, 27 patients (42.85%) had bony tenderness, 1 patient (1.58%) had bony enlargement, 55 patients (87.30%) reported crepitus on active motion, 23 patients (36.50%) had the history of morning stiffness and 1 patient (1.59%) had the history of no warmth of touch during baseline. Moreover, 96.83% had bilateral knee pain, ie, 61 out of 63 patients.
 |
Table 2 Symptoms of Knee Arthritis Present for Last 3 Months in All Enrolled Patients |
Efficacy – Primary End point
For primary efficacy analyses of the PP population, the mean (±SD) of total KOOS score for pain at baseline was 42.93 (±12.51). The changes in total KOOS score for pain from baseline were 9.48 (±10.55), 16.62 (±12.25), 24.13 (±15.48) and 29.71 (±15.74) respectively for 3rd Biovisc Ortho Injection Cycle (Visit 3), 5th Biovisc Ortho Injection Cycle (Visit 5), 3 months±7 days after the last injection (Visit 6) and 6 months±7 days after the last injection (Visit 7). The change in mean KOOS score for pain is statistically significant (p<0.0001) for all post-baseline visits (Table 3). Figure 2 shows the overall summary of primary efficacy analyses. In other words, patients treated Biovisc Ortho injection showed consistent reduction in knee pain (during activities such as straightening, bending, walking going up or downstairs, sitting/lying, standing upright and at rest) and the reduction of pain observed up to 6 months after the last injection.
 |
Table 3 Change from Baseline in KOOS Pain Score – PP Population |
Efficacy – Secondary End points
Secondary efficacy analyses showed an overall improvement in symptoms, pain, function and daily living, function sports and recreational activities and quality of life. Figure 3A–E summarizes the KOOS scores, change from baseline and previous visits. The mean (±SD) of the total KOOS score at baseline was 237.24 (±60.59). The change in total KOOS score for overall improvement from baseline to last follow-up visit was 160.80 (Figure 3A). Symptoms KOOS score at baseline was 41.65 (±12.54) and the change from baseline to last follow-up visit was 21.08 (±14.25). The changes from baseline to final follow-up visit were 30.05 (±14.53) for function KOOS score for daily living, 27.42 (±15.66) for function KOOS score for sports and recreational activities, and 27.29 (±15.18) for quality of life. The changes were statistically significant (p<0.005) for all the end points and at all the post-baseline visits.
Safety
Overall, five patients had procedural complications during the injecting of the device, ie, blood oozing. The treatment was tolerated by the participants. None of the patients required a secondary procedure. There were no AEs, ADRs and any other serious complications reported or observed with the treatment.
Discussion
While the American College of Rheumatology recommends against the use of IA-HA,10 Osteoarthritis Research Society International conditionally recommends the use of IA-HA injection for the treatment of OA knee as a non-pharmacologic therapy.11 IA-HA as a treatment option in managing pain associated with knee osteoarthritis is well documented in various cellular and clinical studies.12,13 Patients who do not respond or cannot take acetaminophen or NSAIDs due to comorbidities IA-HA remains an ideal choice for treatment.14 If we go through various studies, there is a variation in dosage and frequency in the IA-HA being used and usually one may not be compared with another. In this study we are reporting a five-injection course of IA-HA containing 20 mg/2 mL (Biovisc Ortho 1% in a 2 mL prefilled syringe) by M/S Biotech Vision Pvt Ltd as the preparation may make a difference in outcome. Review and meta-analysis of many studies has demonstrated IA-HA to be safe and efficacious for the treatment of OA.6,7,14 The efficacy and safety for up to 26 weeks of a five-injection course of sodium hyaluronate toward treatment of knee OA was demonstrated in a number of randomized controlled clinical trials.15–17 The dosage, the frequency and the molecular weight of IA-HA play a vital role in alleviating the pain.18 A five-injection course of IA-HA containing 20 mg/2 mL (Biovisc Ortho 1% in a 2 mL prefilled syringe) was studied in patients with knee OA and proved as safe.15,19
Sodium hyaluronate-treated patients demonstrated significantly greater beneficial effects compared against patients in the saline control group, and similar pain relief when compared with the patients who received naproxen 500 mg twice daily.20 Adverse events were generally minimal and limited to injection-site pain or bruising, as opposed to the significantly higher rate of gastrointestinal complaints in the group receiving naproxen. Similar results were obtained from placebo-controlled trials.12,16 In our study, there were no adverse events reported. However, injection-site complication such as blood oozing was observed.
Controlled clinical trials are necessary for evaluating effectiveness. Therefore, restricting enrollment to patients with Kellgren grade II or III, and excluding patients with more advanced disease (Kellgren grade 4) were advised.16 Considering the above, we evaluated five injections of 20 mg/2 mL HA (Biovisc Ortho) at weekly intervals in patients suffering OA of the knee for its efficacy and its safety in single-center, open-label clinical study. A statistically significant score was observed, ie improvement from baseline was observed, after 6 months from the fifth injection in the evaluable population for all end points: overall KOOS score, pain, symptoms, daily living function, sports and recreational activity score and Quality of Life. Clinical improvement was observed in patients of the evaluable population receiving the five-injection Biovisc Ortho regimens compared to baseline score. The demographic and disease characteristics of the study population were typical of those of patients with knee OA; most of the patients (70%) were female. Biovisc Ortho was well tolerated and absolutely similar to available viscosupplements as presented in our study data. Furthermore, severe adverse reactions related to device or pseudo septic reactions were not reported during the period of study. Neither our study nor other studies of polyol containing intraarticular viscosupplements have shown any increased risk of device-related adverse events.21 The results presented in this study demonstrate that IA-HA of Biovisc Ortho given five times at weekly intervals is effective for reducing the pain and other KOOS score parameters. This benefit is maintained in the medium term in patients with knee OA over a period of 6 months. Improvement, which was statistically significant, after treatment was observed in all domains which are assessed by the KOOS score. This improvement is reflected in the domains which evaluate parameters related to pain, related to function and related to QoL. After the first treatment cycle, improvement in KOOS score was not only statistically significant but also maintained for the entire study period. The study period was a medium-term duration of 6 months. KOOS was selected for the study as it is a validated knee-specific instrument to assess both short- and long-term consequences of knee injury and osteoarthritis in five subscales of pain, other symptoms, functions (ADL, sports and recreation) and quality of life.22
In spite of being designed as an open label and non-comparative study, the efficacy results over the medium term of 6 months of IA-HA establish the therapeutic effect of the treatment throughout the study period of 6 months. Our study applies only to this particular injection and it may be difficult to make a larger interpretation of the IA-HA as a group of treatment. However, long duration studies with more follow-up(s) are advisable and recommended for decision regarding duration of efficacious effects and repetition of IA-HA.
Safety of treatment with IA-HA was the second objective in our study. The safety of IA-HA is reviewed in detail and the review assures that HA has an excellent safety profile for treatment of knee OA. Additionally, pseudogout, local pain, warmth and minimal swellings were reported for already available viscosupplements. A Hylan G-F 20 brand specific severe acute inflammatory or pseudo sepsis was reported which could be due to the formulation.6 Our study results never showed any similar serious adverse reactions throughout the entire study.
The efficacious effects and safety profile of IA-HA 20 mg/2 mL (Biovisc Ortho) manufactured by Biotech Vision Care Pvt Ltd was evaluated in patients with OA of the knee. Significant differences, or positive changes, in the KOOS score were observed at 3 and 6 months from baseline (p<0.05). The results confirm that repeated cycles of IA-HA of Biotech Vision are safe and improve knee OA and the effects lasts for at least 6 months after the last injection cycle.
Ethical Standard Statement
The study was performed in compliance with the principles of Good Clinical Practice (GCP), ISO 14,155 and the Declaration of Helsinki concerning medical research in humans and the country-specific regulations. Before enrollment, patients were asked to sign an informed consent form and were free to withdraw at any time for any reason. The patient informed consent form and the protocol, which complied with the requirements of the International Conference on Harmonisation (ICH), were reviewed and approved by V.M.M.C. and Safdarjung Hospital institute Ethics Committee (IEC/VMMC/SJH/Clinical Trial/November/2017). The study was registered prospectively in Clinical Trials Registry of India (CTRI/2018/01/011174). Patient’s identification information such as name, address, contact details etc. was masked to keep the patient’s personal identification confidential for the study-related process. Only some information that indirectly identified the subjects such as date of birth, gender and age was recorded. Investigators and the clinical research staff are committed to keep the patient’s identity confidential from any publication, presentation or reporting. The data may be shared with some agencies which need them for study related processes. The datasets used and/or analyzed during the current study will be de-identified and are available from the authors on reasonable request up to 2 years after publication. Patient’s data may be monitored and reviewed by the external or hospital monitors for scientific purposes. Regulatory authority of the country may also audit the data. In such cases, patient’s data with patient’s identification may be made available, but will not be publicly disclosed. All the data collected will be stored in paper and electronic format for processing, drawing conclusions and further scientific purposes. The outcomes will be published, presented, used for promotion of the product, but in all such cases patient’s identification will be kept confidential.
Acknowledgments
Dr Sushil Kumar, Dr Munim Tomar, Dr Gurpreet Singh, Dr Sakshi Jain, Dr Mohit Srivastava, Staff Nurse Vasundhara and Staff Nurse Sushila for assisting in the patient care and treatment program. Mr Tarun Jain for coordinating the supply chain. VMMC and Safdarjung Hospital for providing the infrastructure for the trial.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Role of the funding sources: M/s Biotech Vision provided the funding support and treatment devices.
Disclosure
The study was investigator initiated and supported by M/s Biotech Vision Care which is the manufacturer of Biovisc Ortho 1%. Prof. Dr Ajay Gupta reports non-financial support from Biotech Vision Care Pvt. Ltd., during the conduct of the study. Dr Chethan Channaveera reports non-financial support from Biotech Vision Care Private Limited, during the conduct of the study. Dr Vijender Anand report non-financial support from Biotech Vision Care Pvt Ltd, during the conduct of the study. Dr Satyaranjan Sethi reports non-financial support from Biotech Vision Care Pvt Ltd, during the conduct of the study. The authors reported no conflicts of interest for this work.
References
1. Zapata-Linares N, Eymard F, Berenbaum F, Houard X. Role of adipose tissues in osteoarthritis. Curr Opin Rheumatol. 2021;33(1):84–93. doi:10.1097/BOR.0000000000000763
2. Belluzzi E, Macchi V, Fontanella CG, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21(17):6016. doi:10.3390/ijms21176016
3. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi:10.1016/S0140-6736(19)30417-9
4. De Lucia O, Murgo A, Pregnolato F, et al. Hyaluronic acid injections in the treatment of osteoarthritis secondary to primary inflammatory rheumatic diseases: a systematic review and qualitative synthesis. Adv Ther. 2020;37(4):1347–1359.
5. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(3):351. doi:10.5312/wjo.v5.i3.351
6. Benke M, Shaffer B. Viscosupplementation treatment of arthritis pain. Curr Pain Headache Rep. 2009;13(6):440. doi:10.1007/s11916-009-0072-3
7. Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48(4):563-572. doi:10.1016/j.semarthrit.2018.06.002
8. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi:10.1002/art.1780290816
9. Kellegren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16(4):494–501. doi:10.1136/ard.16.4.494
10. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72:220–233. doi:10.1002/art.41142
11. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi:10.1016/j.joca.2019.06.011
12. O’Hanlon CE, Newberry SJ, Booth M, et al. Hyaluronic acid injection therapy for osteoarthritis of the knee: concordant efficacy and conflicting serious adverse events in two systematic reviews. Syst Rev. 2016;5(1):186. doi:10.1186/s13643-016-0363-9
13. Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217–228. doi:10.2147/JPR.S83076
14. Reid MC. Viscosupplementation for osteoarthritis: a primer for primary care physicians. Adv Ther. 2013;30(11):967–986. doi:10.1007/s12325-013-0068-6
15. Altman MD, Roy D. Status of hyaluronan supplementation therapy in osteoarthritis. Curr Rheumatol Rep. 2003;5:7–14. doi:10.1007/s11926-003-0077-6
16. Bowman S, Awad ME, Hamrick MW, et al. Recent advances in hyaluronic acid-based therapy for osteoarthritis. Clin Trans Med. 2018;7:6. doi:10.1186/s40169-017-0180-3
17. Altman RD, Moskowitz R. Intraarticular sodium hyaluronate in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol. 1998;25:2203–2212.
18. Dernek B, Duymus TM, Koseoglu PK, et al. Efficacy of single-dose hyaluronic acid products with two different structures in patients with early-stage knee osteoarthritis. J Phys Ther Sci. 2016;28(11):3036–3040. doi:10.1589/jpts.28.3036
19. Huang T-L, Chang -C-C, Lee C-H, Chen S-C, Lai C-H, Tsai C-L. Intra-articular injections of sodium hyaluronate (Hyalgan®) in osteoarthritis of the knee. A randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet Disord. 2011;12(1):221. doi:10.1186/1471-2474-12-221
20. Miller LE, Block JE. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. London, England: SAGE Publications Sage UK; 2013.
21. Conrozier T, Eymard F, Afif N, Balblanc J-C, Legré-Boyer V, Chevalier X. Safety and efficacy of intra-articular injections of a combination of hyaluronic acid and mannitol (HAnOX-M) in patients with symptomatic knee osteoarthritis: results of a double-blind, controlled, multicenter, randomized trial. Knee. 2016;23(5):842–848. doi:10.1016/j.knee.2016.05.015
22. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1):64. doi:10.1186/1477-7525-1-64
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



