Back to Journals » Infection and Drug Resistance » Volume 14
An Investigation of the Male Genitourinary Abscess Originated from Urinary Tract in a Tertiary Hospital, Shanghai, China, from 2004 to 2019
Authors Tan J, Tian M, Zhao F, Deng S, Jin P, Wang Y, Wen H, Qin X, Gong Y
Received 19 December 2020
Accepted for publication 31 March 2021
Published 14 May 2021 Volume 2021:14 Pages 1795—1803
DOI https://doi.org/10.2147/IDR.S298250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sahil Khanna
Jiaying Tan,1,* Mi Tian,1,* Feng Zhao,1 Shuixiang Deng,1 Peng Jin,1 Yao Wang,1 Huimei Wen,1 Xiaohua Qin,2,3 Ye Gong1,4
1Department of Critical Care Medicine, Huashan Hospital, Fudan University, Shanghai, 200040, People’s Republic of China; 2Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, 200040, People’s Republic of China; 3Key Laboratory of Clinical Pharmacology of Antibiotics, Ministry of Health, Shanghai, 200040, People’s Republic of China; 4Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, 200040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaohua Qin
Institute of Antibiotics, Huashan Hospital, Fudan University, No. 12 Middle Urumqi Road, Shanghai, 200040, People’s Republic of China
Email [email protected]
Ye Gong
Department of Critical Care Medicine, Huashan Hospital, Fudan University, No. 12 Middle Urumqi Road, Shanghai, 200040, People’s Republic of China
Email [email protected]
Background: Male genitourinary abscess is one of the serious complications of urinary tract infections (UTIs). There were few researches on the clinical and pathogenic characteristics of male genitourinary abscess.
Patients and Methods: A retrospective observational study was conducted between January 2004 and April 2019. Male patients with genitourinary abscess originated from urinary tract, including sites of scrotum, testis, epididymis, spermatic cord, and prostate, were enrolled. Clinical and microbial records were collected and analyzed, and antimicrobial susceptibility testings were performed according to CLSI standard. Whole-genome sequencing was applied to detect the β-lactamase genes and virulence genes, as well as to determine the multilocus-sequence typing (MLST) of the collected Klebsiella pneumoniae (K. pneumoniae) isolates.
Results: A total of 22 male patients were included. The main clinical symptoms were fever (86.4%), scrotal swelling (68.2%), local skin warmth (59.1%) and ulceration (45.5%). Urinary irritation was often presented in prostate involved abscess. Ultrasound features had a 94.7% positive rate. Surgical treatment, including abscess drainage, was helpful to the prognosis. No matter where the specimens obtained from, including blood, urine or pus, multidrug-resistant K. pneumoniae was the dominant (11 cases, 50.0%) microorganism in positive cultures. Nine of eleven K. pneumoniae isolates had been preserved and recovered. As for MLST typing, all the nine available isolates of K. pneumoniae belonged to the ST11 type and characterized with blaKPC-2 carbapenemase gene. Virulence genes rmpA2, ybtS, kfuC, wzi, aerobactin genes (iucABCD and iutA) and type 3 fimbriae genes (mrkAD) were identified in all the K. pneumoniae isolates.
Conclusion: It seemed that more patients under 35 years old were vulnerable to genitourinary abscess. There was an increasing trend that multidrug-resistant K. pneumoniae isolates with multiple virulence genes were involved in male genitourinary abscess. Prompt and proper antibiotic use, combined with adequate drainage of the abscess, was important to prognosis.
Keywords: male genitourinary abscess, carbapenem-resistant Klebsiella pneumoniae, virulence genes, MLST type
Introduction
Urinary tract infection (UTI) is one of the most common infections in both community and hospital.1–3 Approximately 50% of women have at least once UTI episode in their lifetime.4 It has been reported that Gram-negative bacteria such as Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae) were the most frequently implicated pathogens in both uncomplicated and complicated UTIs, accounted for 57.1% and 11.3%, in Europe during 2018.5 Concerns have been aroused about anaerobes, which might have been the significant pathogens of UTIs.6 Furthermore, the antimicrobial resistance rate of urinary Enterobacteriaceae was high, especially for extended spectrum β-lactamase (ESBL) phenotypes. It has been reported that the urinary K. pneumoniae isolates had a 7.7% resistance rate to meropenem according to the European SENTRY surveillance program in 2018.5 During the same year in China, the meropenem resistance rate of K. pneumoniae in urine isolates was 19.8%.7 Although previous study mentioned that plant essential oils (EOs) might be helpful to tackle the problem of antimicrobial resistance,8 it is still an enormous challenge at present.
UTIs are not extremely common in healthy male adults. As one of the serious complications, genitourinary abscess formation is rare to encounter. Once abscess emerges, it may lead to testicular infarction, which can bring disastrous result of infertility. It has been reported that Gram-negative enteric organism, Mycobacterium tuberculosis, Brucella and Candida were the main pathogens in non-sexually transmitted genitourinary infections,9 and obstructive urinary disease, urinary tract surgery or instrumentation were risk factors.10 The clinical and pathogenic characteristics, such as antimicrobial resistance and virulence factors, might have changed in recent years, but few researches on male genitourinary abscess have specified this issue.
Herein, we have attempted an investigation of the male genitourinary abscess originated from urinary tract in a single-centered retrospect of the last 15 years hospitalized male patients.
Patients and Methods
Study Design and Patients
A retrospective observational study was conducted between January 2004 and April 2019 in Huashan Hospital, Fudan University, a large academic tertiary hospital in Shanghai, China. The enrolled male patients had the diagnosis of genitourinary abscess, including sites of scrotum, testis, epididymis, spermatic cord, and prostate. Patients younger than 18 years old or with genitourinary abscess not spread from urinary tract were excluded.
Medical records were reviewed to extract the following clinical information: demographics, diagnosis, comorbidities, clinical symptoms, blood and urine detections, imaging or ultrasound features, length of hospital stay, medical instrumentation, surgery, microbial cultures, antimicrobial treatments, and clinical outcomes.
Microbiology and Antimicrobial Susceptibility Testing
The specimens were inoculated on the screening plates containing CHROMagar chromogenic media for urine culture (CHROMagarTM Microbiology Co., Ltd., Shanghai, China). The blue colonies were K. pneumoniae. Species were further identified with the VITEK 2 GN ID Panel according to the manufacturer’s instructions. Susceptibility testings were performed in the hospital microbiology laboratory using the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) criteria,11 or the VITEK 2 compact automated system (bioMérieux) following the instrument specifications, and the results were interpreted according to CLSI standard.
String Test
The mucoviscous phenotype of K. pneumoniae was identified by a positive string test, which is defined as the formation of a string >5 mm in length when a colony grown over night on a blood agar plate at 37°C was stretched by a bacteriology inoculation loop.12
Whole-Genome Sequencing
Bacterial DNA of the nine K. pneumoniae isolates were extracted from overnight cultures using the DNA mini Kit (Takara, Dalian, China) according to the manufacturer’s instructions. The assessment of DNA quality was performed by a NanoDrop Nd-1000 spectrophotometer (Thermo Fisher Scientific, United States). The genomic DNA was stored at −20°C. Sequencing libraries were generated using NEB Next Ultra DNA Library Prep Kit (Illumina, NEB) using 700 ng of DNA from each sample, following manufacturer’s instructions, and index codes were added to attribute sequences to each sample. The library polymerase chain reaction (PCR) products were purified (AMPure XP system), and the quality was evaluated on the Agilent Bioanalyzer 2100 system. The clustering of index-coded samples was processed on a cBot Cluster Generation System according to the manufacturer’s instructions. Then, the library preparations were sequenced using an Illumina HiSeq X Ten platform and 300 base pair (bp) (150 bp×2) paired-end reads were generated.
Bioinformatic Analysis
Raw data from paired-end sequencing were quality checked with the Falco (v0.2.1) and adapter sequences were removed using Trimmomatic (v0.36).13,14 The cleaned paired-end sequencing data for each strain was error-corrected and assembled using SPAdes (v3.13.0), with the default parameters.15 Multilocus-sequence typing (MLST) was performed in silico by using the tool MLST (www.github.com/tseemann/mlst) and the Pasteur database (www.bigsdb.web.pasteur.fr/klebsiella/). Virulence genes and antimicrobial resistance genes, especially β-lactamases and efflux pump, were identified according to the BIGSdb Klebsiella genome database.16 The allelic information for virulence and antimicrobial resistance genes was shown by the heatmap produced by the Python (v3.7.5) with Matplotlib (v3.2.2).
Definitions
The following terms were defined prior to data analysis. The diagnosis of UTI is based on symptoms and the presence of pathogens and/or white blood cells in urine. Complicated UTIs (cUTIs) are those associated with neurogenic dysfunction or bladder outlet obstruction, obstructive uropathy from any cause, intermittent or long-term bladder catheterization, urologic instrumentation or indwelling stent, urinary tract post-surgical modifications, chemotherapy or radiation-induced damage, renal impairment, diabetes mellitus and immunocompromised conditions.17 Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection,18 with the Sequential Organ Failure Assessment (SOFA) score ≥2 points consequent to the infection. Immunocompromise was defined as the condition of patient received immunosuppressant, chemotherapy, radiation, long term or recent high-dose steroids (prednisone 1–2mg/kg/d or other equivalent dose glucocorticoid), or had a disease that was sufficiently advanced to suppress resistance to infection such as leukemia, lymphoma or acquired immune deficiency syndrome (AIDS).19 Antimicrobial treatment was extracted firstly after the diagnosis of genitourinary abscess. The carbapenem-resistant K. pneumoniae (CRKP) isolates were defined as resistant to ertapenem and/or imipenem and/or meropenem.
Statistical Analysis
The results are expressed as the mean±standard deviation (SD) or median (interquartile range[IQR] or range) (continuous variables) or as percentages of the group from which they were derived (categorical variables). Data were processed and analyzed using the STATA statistical software (StataCorp LP, version 16).
Results
Figure 1 shows the flowchart of the patient inclusion process. During the period of January 2004 and April 2019, 39 male patients were diagnosed with genitourinary abscess. All of these patients were adults. Moreover, only 56.4% (22/39) patients were genitourinary abscess consequent on UTIs. Abscess sites were further categorized into three sites: prostate only, scrotum only, or both. Scrotum included scrotum, testis, epididymis and spermatic cord. The vast majority (18 of 22, 81.8%) of cases occurred in the last five years, and there was a growing trend year by year (Figure 2).
 |
Figure 1 Flowchart of the study selection process. |
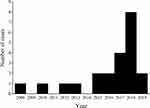 |
Figure 2 Case number of the male genitourinary abscess originated from urinary tract throughout the study period. |
Clinical Findings
The mean age of 22 patients was 54.5±16.5 years old (median, 57.5 years; range, 20 to 81 years). Regarding to the categories of abscess site, 14 (63.6%) cases were scrotal involved, 5 (22.7%) were prostatic, and the remaining 3 (13.6%) were both scrotal and prostatic involved. All these patients did not present the evidence of pyogenic liver abscess (PLA). Only two patients (9.1%) were uncomplicated UTIs. It was noteworthy that diabetes (22.7%) was more common than immunocompromised conditions (13.6%). Second to fever (86.4%), scrotal swelling was prevalent (68.2%) as clinical symptoms in those 22 patients. The local lesion of skin, including warmth (59.1%) and ulceration (45.5%), was also an apparent sign to genitourinary abscess. Urinary irritation was often presented in prostate involved abscess. Concerning the severity, 4 patients encountered sepsis and two of them progressed to septic shock (Table 1). Ultrasound features had a high positive rate up to 94.7% (Table 2). More than half patients suffered from hypoalbuminemia. Surgical treatment was helpful to suspend the process of complications. Abscess drainage was the most preferred method. If it was invalid and lesion was uncontrollable, resection was inevitable. There was one patient further underwent unilateral orchiectomy, and another underwent bilateral epididymectomy. These two patients had both scrotal and prostatic abscess. Male genitourinary abscess generally had a favorable prognosis. It did not directly lead to fatal outcome, and the 28-day mortality was zero.
 |
Table 1 Characteristics of Patients Diagnosed with Male Genitourinary Abscess Originated from Urinary Tract |
 |
Table 2 Results of Auxiliary Examination in Patients Diagnosed with Male Genitourinary Abscess Originated from Urinary Tract |
Microbial Findings
Microbial cultures obtained from specimens in most related three locations revealed the pathogenic characteristics. Positive blood culture occurred in 6 patients, and two of them suffered from sepsis. No matter where the specimens obtained from, including blood, urine or pus, CRKP was the dominant microorganism in positive cultures (Table 3). K. pneumoniae was found in specimens from two of those three locations in 6 patients. Overall, 11 (50.0%) patients could be considered suffering from K. pneumoniae genitourinary abscess according to culture results.
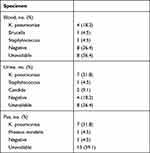 |
Table 3 Results of Microbial Cultures in Patients Diagnosed with Male Genitourinary Abscess Originated from Urinary Tract |
Focusing on the three patients suffered from both scrotal and prostatic abscess, all the pathogens were CRKPs which were simultaneously resistant to cefotaxime, ceftazidime, cefepime, cefoxitin, meropenem, imipenem, piperacillin-tazobactam, levofloxacin, amikacin and fosfomycin, only susceptible to tigecycline and colistin. Among them, such positive result was cultured from blood, urine and pus in a 67-year-old man, from blood and urine in a 20-year-old young man, and from urine in a 50-year-old man.
According to the results of MLST typing, all the nine isolates of K. pneumoniae which were available in clinical microbiology laboratory belonged to the ST11 type and carried KPC carbapenemase. The seven out of nine CRKP isolates detected both rmpA and rmpA2 genes were positive in the string test. Moreover, whole-genome sequencing showed that blaKPC-2, blaSHV, blaCTX, blaTEM and ramA genes were identified within all these isolates, but none of blaNDM, blaVIM, blaIMP or blaOXA-48 genes were detected. Virulence genes rmpA2, ybtS, kfuC, wzi, aerobactin genes (iucABCD and iutA) and type 3 fimbriae genes (mrkAD) were identified in all the K. pneumoniae strains. The iroN gene was identified in 77.8% (7/9) of the K. pneumoniae strains (Figure 3). Salmochelin (iroBCD), magA, wcaG or entB was detected negative in all the K. pneumoniae isolates.
 |
Figure 3 Heatmap of whole-genome sequencing. |
Discussion
Over the past decades, few systematic researches on the clinical and pathogenic characteristics of male genitourinary abscess have been reported. To our knowledge, this is the first retrospective cohort study concerning this type of infection originated from urinary tract in Chinese population.
The pathogens of UTI in male adult were difficult to forecast. Therefore, urine culture was necessary and further diagnostic evaluation should be started early in order to determine whether the patients had cUTIs. UTIs in male adults might cause genitourinary abscess which could further result in dramatic complications, although the ascending route was extremely rare.
It has been shown that the causative organism of male genitourinary infection like epididymo-orchitis usually correlated to the age of patients. The pathogens of Chlamydia trachomatis and Neisseria gonorrhoeae were more dominant in young men (<35y), while the culprits were inclined to E. coli or other enteric organisms in patients aged over 35 years.20,21 Other rare pathogenic microorganisms, such as anaerobes, Actinomyces and Actinomyces-like organisms (ALOs), had distributed in various ages of male genitourinary infection.6,22 In our study, the proportion of patients under 35 years old was 18.2% (4 cases all had cUTIs), which was higher than expectation. This might suggest a trend to the demographic change in severe male genitourinary infection. On the other hand, subtle change of microorganisms has also been in process. The multidrug-resistant K. pneumoniae or even CRKP instead of E. coli became the predominant pathogen isolated from blood, urine or pus in our patients. The multidrug-resistant pathogens could easily escape from the attack of antimicrobials. This led to the long-term persistence of K. pneumoniae in genitourinary tract. We speculated that the possible invasive characteristics of K. pneumoniae attributed to the genitourinary abscess formation.
It is notorious that K. pneumoniae strains were associated with a wide spectrum of infections, such as UTI, pneumonia, intra-abdominal infection, bloodstream infection (BSI), meningitis, PLA, necrotizing fasciitis and endogenous endophthalmitis.23–26 They carried a variety of virulence genes and had the capacity to acquire a versatile armament of antimicrobial resistance genes. This was the bacteriological basis of invasive or even disseminated infections caused by K. pneumoniae. Type 3 fimbriae genes mrkA and mrkD were detected positive in all the CRKP isolates in this study, while the type 3 fimbriae were an important uropathogenic virulence factor for K. pneumoniae to adhere to the urinary tract epithelium and to perform the biofilm.27
The hypervirulent K. pneumoniae (hvKP) was first reported in Taiwan starting in the mid-1980s, where patients presented with primary community-acquired hepatic abscess with a propensity for disseminated spread to extrahepatic sites.28 Through the worldwide reports and investigations in recent thirty years, it was generally considered that hvKP syndrome was associated with bacteremia, primary liver abscess, and metastatic infections.29 However, we have not observed this typical syndrome. None of the participants presented PLA, even in those four cases positive for K. pneumoniae in blood cultures.
Although hypervirulent and antimicrobial-resistant populations of K. pneumoniae were once considered largely nonoverlapping,16 isolates with combined virulence and resistance had been frequently reported in recent years, particularly in China.30–32 The multidrug-resistant and hypervirulent K. pneumoniae has become a great threat to public health till now. According to MLST typing, ST11 with blaKPC-2 gene has been reported as the most common type of CRKP in China,33,34 which was in coincidence with our study that all the available K. pneumoniae isolates were proved as ST11 CRKPs with blaKPC-2 gene. Furthermore, virulence genes rmpA2, ybtS, kfuC, wzi, aerobactin genes (iucABCD and iutA) and type 3 fimbriae genes (mrkAD) were also identified in all the K. pneumoniae isolates in our study. The K. pneumoniae isolates with both multidrug-resistant and virulent characteristics seemed more likely to cause male genitourinary abscess. This was probably due to their prolonged stay in urinary tract and invasive feature of multidrug-resistant K. pneumoniae isolates with multiple virulence genes.
In view of the pathogenic change in male UTIs, more information of microorganism and early diagnosis of genitourinary abscess was particularly important. With modern ultrasound equipment, which was non-invasive, inexpensive and repeatable, genitourinary abscess could be early detected, and it was usually repeated in order to rule out testicular malignancy. Compared to the imaging examination such as computed tomography (CT) or magnetic resonance imaging (MRI), sonography was the mainstay first-line investigation in genitourinary abscess. Once the diagnosis confirmed, acquisition of pathogens, antibiotic use, and adequate drainage of the abscess should be undertaken as soon as possible. Early intervention might prevent progression of genitourinary abscess, avoid genital excision, and reduce the risk of death. Considering the serious problem of antimicrobial resistance, bioactive components such as EOs from the leaves, rhizomes and other parts of plant might be the potential therapy as a supplement to antibiotics.35,36
Our results had limited validity since this was a retrospective, small sample size and single-center cohort study. The rarity of the male genitourinary abscess originated from urinary tract limited the sample size. The pathogenicity of multidrug-resistant K. pneumoniae isolates with multiple virulence genes in this study had not been confirmed, as lack of the in vivo experiment. More large-scale trials with controlled group of male patients suffered from K. pneumoniae UTI without genitourinary abscess are needed for further study.
Conclusions
The present study suggested the change of demography and microorganisms in male genitourinary abscess originated from urinary tract in Chinese population. It was manifested that more patients under 35 years old and more multidrug-resistant K. pneumoniae isolates with multiple virulence genes have been involved in this disease. Once UTI occurred in male patients, the virulence and drug resistance information of microorganism should be gained as early as possible. Imaging or ultrasound in genitourinary system was significant to rule out abscess formation. Early interventions, such as antibiotic use and adequate drainage of the abscess, were helpful to a decent prognosis.
Abbreviations
UTI, urinary tract infection; K. pneumoniae, Klebsiella pneumoniae; E. coli, Escherichia coli; ESBL, extended spectrum β-lactamase; EO, essential oil; CLSI, Clinical and Laboratory Standards Institute; PCR, polymerase chain reaction; bp, base pair; MLST, multilocus-sequence typing; cUTI, complicated UTI; SOFA, Sequential Organ Failure Assessment; AIDS, acquired immune deficiency syndrome; CRKP, carbapenem-resistant Klebsiella pneumoniae; SD, standard deviation; IQR, interquartile range; PLA, pyogenic liver abscess; ALO, Actinomyces-like organism; BSI, bloodstream infection; hvKP, hypervirulent Klebsiella pneumoniae; CT, computed tomography; MRI, magnetic resonance imaging.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board (IRB) of Huashan Hospital, Fudan University. Specific patient consent to review their medical records, which contained in their consent form for admission, was not required by the IRB. The raw data were accessed from the photocopy medical record browser, which was granted by the director of Medical Record Administration through routine application procedure. The IRB approval included the statement covering patient data confidentiality and compliance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by National Natural Science Foundation of China (Grant No. 81401699), National Key R&D Program of China (Grant No. 2018YFC1312600 and 2018YFC1312604 to YG), Scientific Research Project from Shanghai Municipal Health Commission (Grant No. 202040422) and The Scientific Research Project supported by Huashan Hospital, Fudan University (Grant No. Y845).
Disclosure
The authors declare that they have no competing interests.
References
1. Zowawi HM, Harris PN, Roberts MJ, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12(10):570–584. doi:10.1038/nrurol.2015.199
2. Ahmed NH, Hussain T, Biswal I. Comparison of etiological agents and resistance patterns of the pathogens causing community acquired and hospital acquired urinary tract infections. J Glob Infect Dis. 2014;6(3):135–136. doi:10.4103/0974-777X.138515
3. Gajdács M, Ábrók M, Lázár A, Burián K. Comparative epidemiology and resistance trends of common urinary pathogens in a tertiary-care hospital: a 10-Year Surveillance Study. Medicina (Kaunas). 2019;55(7):356. doi:10.3390/medicina55070356
4. Schaeffer AJ, Schaeffer EM. Infections of the urinary tract. In: Wein AJ, Kavoussi LR, Campbell MF, editors. Campbell-Walsh Urology.
5. Critchley IA, Cotroneo N, Pucci MJ, Jain A, Mendes RE. Resistance among urinary tract pathogens collected in Europe during 2018. J Glob Antimicrob Resist. 2020;23:439–444. doi:10.1016/j.jgar.2020.10.020
6. Gajdács M, Ábrók M, Lázár A, Burián K. Microbiology of urine samples obtained through suprapubic bladder aspiration: a 10-year epidemiological snapshot. Dev Health Sci. 2019;2(3):76–78. doi:10.1556/2066.2.2019.012
7. Hu F, Guo Y, Yang Y, et al.; China Antimicrobial Surveillance Network (CHINET) Study Group. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
8. Yu Z, Tang J, Khare T, Kumar V. The alarming antimicrobial resistance in ESKAPEE pathogens: can essential oils come to the rescue? Fitoterapia. 2020;140:104433. doi:10.1016/j.fitote.2019.104433
9. Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744–749. doi:10.1177/0956462417699356
10. Mittemeyer BT, Lennox KW, Borski AA. Epididymitis: a review of 610 cases. J Urol. 1966;95(3):390–392. doi:10.1016/S0022-5347(17)63468-2
11. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
12. Ye M, Tu J, Jiang J, et al. Clinical and genomic analysis of liver abscess-causing klebsiella pneumoniae identifies new liver abscess-associated virulence genes. Front Cell Infect Microbiol. 2016;6:165. doi:10.3389/fcimb.2016.00165
13. de Sena Brandine G, Smith AD. Falco: high-speed FastQC emulation for quality control of sequencing data. F1000Res. 2019;8:1874. doi:10.12688/f1000research.21142.1
14. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi:10.1093/bioinformatics/btu170
15. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
16. Bialek-Davenet S, Criscuolo A, Ailloud F, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–1820. doi:10.3201/eid2011.140206
17. Grabe M, Bartoletti R, Bjerklund-Johansen TE, et al. European Association of Urology. Guidelines on urological infections; 2014. Available from: http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
18. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
19. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
20. Holmes KK, Berger RE, Alexander ER. Acute epididymitis: etiology and therapy. Arch Androl. 1979;3(4):309–316. doi:10.3109/01485017908988421
21. Dale AW, Wilson JD, Forster GE, Daniels D, Brook MG. Management of epididymo-orchitis in genitourinary medicine clinics in the United Kingdom’s North Thames region 2000. Int J STD AIDS. 2001;12(5):342–345. doi:10.1258/0956462011923066
22. Gajdács M, Urbán E. The pathogenic role of Actinomyces spp. and related organisms in genitourinary infections: discoveries in the New, Modern Diagnostic Era. Antibiotics (Basel). 2020;9(8):524. doi:10.3390/antibiotics9080524
23. Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151(8):1557–1559. doi:10.1001/archinte.1991.00400080059010
24. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi:10.1128/CMR.11.4.589
25. Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26(6):1434–1438. doi:10.1086/516369
26. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi:10.4161/viru.22718
27. Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect Immun. 2013;81(8):3009–3017. doi:10.1128/IAI.00348-13
28. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913–1916. doi:10.1001/archinte.1986.00360220057011
29. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi:10.1016/S1473-3099(12)70205-0
30. Liu Y, Li XY, Wan LG, Jiang WY, Yang JH, Li FQ. Virulence and transferability of resistance determinants in a novel Klebsiella pneumoniae sequence type 1137 in China. Microb Drug Resist. 2014;20(2):150–155. doi:10.1089/mdr.2013.0107
31. Zhang Y, Zeng J, Liu W, et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71(5):553–560. doi:10.1016/j.jinf.2015.07.010
32. Zhang R, Lin D, Chan EW, Gu D, Chen GX, Chen S. Emergence of carbapenem-resistant Serotype K1 hypervirulent klebsiella pneumoniae strains in China. Antimicrob Agents Chemother. 2015;60(1):709–711. doi:10.1128/AAC.02173-15
33. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi:10.1016/S1473-3099(13)70190-7
34. Liu L, Feng Y, Tang G, et al. Carbapenem-resistant isolates of the Klebsiella pneumoniae Complex in Western China: the Common ST11 and the Surprising Hospital-specific Types. Clin Infect Dis. 2018;67(suppl_2):S263–S265. doi:10.1093/cid/ciy662
35. Trong Le N, Viet Ho D, Quoc Doan T, et al. Biological activities of essential oils from leaves of paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (Miq.) Swingle. Antibiotics (Basel). 2020;9(4):207. doi:10.3390/antibiotics9040207
36. Donadu MG, Trong Le N, Viet Ho D, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of hornstedtia bella Škorničk. Antibiotics (Basel). 2020;9(6):334. doi:10.3390/antibiotics9060334
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
