Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 9
An indirect comparison of HbA1c treatment effect with albiglutide and exenatide 2.0 mg QW using the Bucher method
Received 18 November 2015
Accepted for publication 18 February 2016
Published 19 May 2016 Volume 2016:9 Pages 163—168
DOI https://doi.org/10.2147/DMSO.S100775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ming-Hui Zou
Alan A Martin,1 Daniel Parks2
1Department of Value Evidence Analytics, GlaxoSmithKline, Uxbridge, Middlesex, UK; 2Department of Value Evidence Analytics, GlaxoSmithKline, Collegeville, PA, USA
Abstract: No head-to-head comparisons exist between once-weekly (QW) glucagon-like peptide-1 receptor agonists; accordingly, this indirect comparison was conducted to evaluate the comparative efficacy of QW albiglutide vs QW exenatide. Following a systematic literature search, it was determined that HARMONY 7 and DURATION 6, Phase III trials for albiglutide and exenatide, respectively, were similar in study design and baseline characteristics and included a common comparator arm, making them suitable for an indirect comparison using the Bucher method. The primary endpoint of change from baseline in glycated hemoglobin (HbA1c) with albiglutide 50 mg QW and exenatide 2.0 mg QW was compared and tested for noninferiority. The indirect comparison showed a treatment difference of 0.0% (95% confidence interval: -0.189% to 0.189%) in mean change in HbA1c from baseline, and albiglutide 50 mg was noninferior to exenatide 2.0 mg QW at the noninferiority margin of 0.3%. In the absence of a head-to-head trial, these results can be used in pharmacoeconomic analysis and to inform health technology assessment and clinical decision making.
Keywords: albiglutide, exenatide 2.0 mg QW GLP-1 RA, Bucher method
Introduction
With the expanding number of classes and agents for the treatment of type 2 diabetes mellitus (T2DM), as well as the focus on patient-centered care,1 it is becoming increasingly important to understand the parameters for optimal clinical use of these agents. Albiglutide is a once-weekly (QW) glucagon-like peptide-1 receptor agonist (GLP-1 RA) recently approved for the treatment of T2DM. The GLP-1 RA class includes both weekly and daily administered injectable products; thus, frequency of administration may be a factor in the choice of a GLP-1 RA. If a QW GLP-1 RA is preferred, then the relative efficacy of the weekly GLP-1s currently approved for clinical use (albiglutide, exenatide 2.0 mg QW and dulaglutide2–4) is of interest. Because albiglutide was the second QW GLP-1 RA to achieve marketing authorization, comparative efficacy against exenatide 2.0 mg QW (the first weekly approved GLP-1 RA) is important for health technology appraisal.5 The outcomes of interest, in a pharmacoeconomic analysis of T2DM treatments, are long-term diabetes complications. These must be modeled from effects observed in trials; the key treatment effect being modification of blood glucose measured as glycated hemoglobin (HbA1c). In the absence of head-to-head data, the purpose of this analysis was to provide an indirect comparison of the efficacy of these two agents on HbA1c lowering.
Methods
This analysis was a single-step, indirect comparison of change from baseline in HbA1c with albiglutide 50 mg QW (the highest approved dose) vs exenatide 2.0 mg QW. Liraglutide 1.8 mg once daily (QD) served as the common comparator. The indirect comparison was made using the Bucher method6 and included data from two studies: HARMONY 7 and DURATION 6 (Table 1).7,8 The primary endpoints (change from baseline in HbA1c at 32 weeks and 26 weeks, respectively, for albiglutide and exenatide 2.0 mg QW) of each study were compared.
Potential studies for inclusion in an indirect comparison were identified from a systematic literature search of EMBASE, MEDLINE, MEDLINE in Process, and Cochrane library electronic databases performed on January 8, 2013, and then updated on May 12, 2014. Phase III randomized controlled trials in adults with type 2 diabetes that lasted for 24 weeks or more, with treatment arms for albiglutide, exenatide 2.0 mg QW and other available GLP-1 RAs (exenatide BID [twice daily], liraglutide), sitagliptin, insulin lispro, insulin glargine and placebo were searched for, with no limitation on publication language or year of publication. The search was conducted by two independent analysts, and only full publications and clinical study reports for albiglutide Phase III trials were included. Following de-duplication, a total of 2,078 records were identified for abstract screening, and ultimately 51 full-text papers and eight albiglutide clinical study reports for unique studies were identified for assessment; this included eight albiglutide studies7,9–15 (references refer to publications subsequent to this analysis for some HARMONY studies) and eight exenatide 2.0 mg QW studies.8,16–22
Studies were assessed as suitable for inclusion in an indirect comparison if they fulfilled the condition of similarity of study design and populations, and other factors which could be modifiers of relative treatment effect and thus where differences could bias results. Of the eight albiglutide studies, five (HARMONY 1–5)9–13 were substantially different in design to exenatide 2.0 mg QW studies: much longer total study duration (3 years vs 24–30 weeks for exenatide 2.0 mg QW studies), primary endpoints evaluated at 52 weeks or 104 weeks (vs 24–30 weeks for exenatide 2.0 mg QW studies), the use of optional uptitration of albiglutide from 30 mg to 50 mg in four of the studies, and an approach to subject inclusion and hyperglycemic rescue designed to be more real-world. HARMONY 614 compared albiglutide to insulin lispro, which is not suitable as a common comparator due to variability in insulin lispro formulation and regimen. HARMONY 815 was conducted in patients with renal impairment, and no exenatide 2.0 mg QW study was conducted in a similar population. Consequently, these were excluded due to study heterogeneity. HARMONY 7, however, which compared QW albiglutide to liraglutide 1.8 mg QD, was similar in design and study population to several exenatide 2.0 mg QW studies. The inclusion of only the HARMONY 7 albiglutide study meant that constructing a meaningful network was not possible; thus, studies with other potential intermediate comparators were not included. DURATION 6 was the only exenatide 2.0 mg QW study with a common comparator, liraglutide 1.8 mg QD, so HARMONY 7 and DURATION 6 were ultimately included in the indirect comparison.
With only two studies, and therefore no networks with a closed loop, the Bucher method was selected for the analysis. Calculations were carried out using SAS (SAS Institute, Inc., Cary, NC, USA). The indirect treatment difference in mean change from baseline in HbA1c was calculated; noninferiority of albiglutide 50 mg vs exenatide 2.0 mg QW was tested. The noninferiority margin of 0.3% was chosen to be consistent with the HARMONY 7 study and was based on the US Food and Drug Administration guidance for industry.23
Results
Baseline characteristics
Baseline characteristics of patients in HARMONY 7 and DURATION 6 were similar, with no obvious differences between the two studies that could bias the results of the indirect comparison (Table 2).
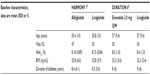  | Table 2 Baseline characteristics of patients enrolled in HARMONY 7 and DURATION 6 |
Indirect comparison of albiglutide vs exenatide 2.0 mg QW on HbA1c
Using the Bucher method, albiglutide 50 mg demonstrated noninferiority to exenatide 2.0 mg QW with regard to HbA1c lowering based on a difference of 0.0% with a 95% confidence interval of −0.189% to 0.189% at the noninferiority margin of 0.3% (P=0.002; Table 3, Figure 1).
  | Figure 1 Forest plot of albiglutide vs exenatide 2.0 mg QW. |
Discussion
Exenatide 2.0 mg QW was not yet approved when the HARMONY program was initiated. Thus, no head-to-head study comparing albiglutide and exenatide 2.0 mg QW exists. Based on the timing of approvals (exenatide 2.0 mg QW was the first QW GLP-1 RA to receive marketing authorization, and albiglutide was the second), comparative efficacy data vs the compound most likely to be displaced is an important component for reimbursement in countries where health technology appraisal is conducted. In the absence of head-to-head data, this indirect comparison was conducted using the Bucher method and demonstrated that albiglutide is noninferior to exenatide 2.0 mg QW with respect to HbA1c reduction, with a noninferiority margin of 0.3%. Even if a more stringent noninferiority margin of 0.25% had been used, noninferiority would still have been achieved.
The similarity assumption is central to indirect comparison24 and requires that the inclusion criteria and baseline characteristics be similar across the two trials, that the liraglutide titration schedule be similar, that missing data be unrelated to treatment efficacy, and that effect-modifying factors exhibit a similar distribution across studies. Based on the similarity of the inclusion criteria and baseline characteristics and the fact that the majority of patients who withdraw from GLP-1 RA studies do so for reasons unrelated to efficacy, this assumption has been met. Because only two studies were compared, the heterogeneity assumption and the consistency assumption could not be checked statistically.
Other factors deserve consideration as potential sources of bias: these are the increasing HbA1c trajectory observed with both exenatide and liraglutide at 26 weeks in DURATION 6, the difference in timing of endpoints (26 weeks in DURATION 6 vs 32 weeks in HARMONY 7), and the difference in HbA1c reduction observed with liraglutide 1.8 mg in DURATION 6 and HARMONY 7. Although increasing, HbA1c trajectories in DURATION 6 are increasing for both exenatide 2.0 mg QW and liraglutide, but not converging Buse et al,8 and thus treatment difference is not changing. The Bucher analysis is based on treatment difference, not on absolute effect, therefore the indirect comparison should not be affected. In HARMONY 7, the trajectory of HbA1c for albiglutide and liraglutide 26–32 weeks is stable Pratley et al,7 so comparisons at 26 weeks and 32 weeks would give similar results. The difference in HbA1c reduction observed with liraglutide in HARMONY 7 and DURATION 6 is not out of line with the range in magnitude, from 1.0% to 1.5%, seen with liraglutide 1.8 mg in other Phase III studies.25,26 Reductions in HbA1c can vary across studies for reasons other than differences in population, such as patient behavior with respect to diet and exercise and study design factors such as structure and duration of run-in periods, and also simply due to chance. Because the Bucher method adjusts for differences in absolute effect size and the two studies satisfied the similarity condition, this was not seen as a significant source of bias.
This analysis assumes that HARMONY 7 and DURATION 6 reflect the real treatment difference between albiglutide or exenatide 2.0 mg QW and liraglutide 1.8 mg, and it is not known how the inclusion of other indirect evidence would have affected the results. Scott et al, in their network meta-analysis (NMA) comparing exenatide 2.0 mg QW and liraglutide,27 also included DURATION 6. However, although their models adjust for some sources of heterogeneity, significant inconsistencies were still present between the direct and indirect evidence for the comparison of exenatide 2.0 mg QW and liraglutide 1.8 mg, and liraglutide 1.8 mg and placebo. As described in the National Institute of Health and Care Excellence Decision Support Unit (NICE DSU) Technical Support Document 4,28 when inconsistency is present after adjustment for heterogeneity, it is advisable to reconsider the entire network, reviewing studies for effect modifiers that could be the source of the inconsistency and potentially excluding them. Scott et al considered that the inconsistency could be resolved by removing DURATION 6, but could not identify a reason, based on study and patient characteristics, for doing so. However, they do not report whether removing other studies with, for instance, different background therapies, different populations (eg, Asian), or different study duration also resolved the inconsistency. Judgment must be applied on what studies to include or exclude from the network, as there is no statistical method for doing so. In this situation, it is reasonable to assume that the direct evidence, ie, DURATION 6, reflects the real treatment difference between liraglutide 1.8 mg and exenatide 2.0 mg QW and indeed the study found that it was probable that liraglutide 1.8 mg reduces HbA1c more than exenatide 2.0 mg QW.
NMA and mixed treatment comparison (MTC) methods, with either frequentist or Bayesian approaches, are also available for conducting indirect comparisons, but were not used here. The key consideration was that only two studies (DURATION 6 and HARMONY 7) satisfied the similarity assumption (a fundamental requirement for NMA/MTC), placing constraints on methodology, as NMA and MTC are model-based approaches requiring a greater number of studies. For this reason, the Bucher method for adjusted indirect treatment comparison was chosen. Bucher is a frequentist method, and although a Bayesian approach would not normally be adopted with only two studies included, as an additional check a Bayesian analysis was also performed. In general, a frequentist (eg, Bucher) and a Bayesian approach should yield similar, although not necessarily identical, results – in this case, the results for the Bucher and Bayesian analyses were similar and conclusions were the same.
In addition to the limited number of studies suitable, based on study design, for inclusion in the indirect comparison, other limitations should be acknowledged. First, only the primary measure of efficacy (HbA1c) was compared. Other endpoints were considered for inclusion in the analysis, but because the primary goal was to provide a basis for quantitative health economic comparison, it was decided to focus on HbA1c, an approach also adopted in the Scott study. Second, the analysis did not include dulaglutide, another QW GLP-1 RA, because it was not available at the time of this analysis.
Conclusion
In conclusion, this indirect comparison demonstrates the noninferiority of albiglutide to exenatide 2.0 mg QW with respect to HbA1c lowering. This finding, in the absence of head-to-head data, can be used in pharmacoeconomic analysis and to inform health technology assessment and clinical decision making.
Acknowledgments
This study was funded by GlaxoSmithKline. Editorial support was provided by Joelle Suchy, PhD, and William Ho, PhD, MediTech Media, and funded by GlaxoSmithKline. Medical and clinical advice was provided by Dr Veronica Bainbridge, an employee of GlaxoSmithKline.
Disclosure
Daniel Parks and Alan Martin are employees of GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. | |
Tanzeum (albiglutide) [package insert]. Wilmington, DE: GlaxoSmithKline LLC; 2015. | |
Bydureon (exenatide) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. | |
Trulicity (dulaglutide) [package insert]. Indianapolis, IN: Eli Lilly and Company; 2015. | |
Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19–28. | |
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. | |
Pratley RE, Nauck MA, Barnett AH, et al. Once weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–297. | |
Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–124. | |
Reusch J, Stewart MW, Perkins CM, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab. 2014;16:1257–1264. | |
Nauck MA, Stewart MW, Perkins C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia. 2016;59(2):266–274. | |
Ahren B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37(8):2141–2148. | |
Weissman P, Carr M, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475–2484. | |
Home PD, Shamanna P, Stewart M, et al. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes Metab. 2015;17:179–187. | |
Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317–2325. | |
Leiter LA, Carr MC, Stewart M, et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care. 2014;37(10):2723–2730. | |
Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. | |
Diamant M, Van GL, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375(9733):2234–2243. | |
Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–439. | |
Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252–258. | |
Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–1310. | |
Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority study. Clin Ther. 2012;34(9):1892–1908. | |
Ji L, Onishi Y, Ahn CW, et al. Efficacy and safety of exenatide once-weekly vs exenatide twice-daily in Asian patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4(1):53–61. | |
Guidance for Industry. Non-Inferiority Clinical Trials. Silver Spring, MD: US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research; 2010. | |
Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. | |
Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224–1230. | |
Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. | |
Scott DA, Boye KS, Timlin L, Clark JF, Best JH. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab. 2013;15:213–223. | |
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE [homepage on the Internet]. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. 2011. [updated April 2012]; Available from: http://www.nicedsu.org.uk. Accessed January 18, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


