Back to Journals » Journal of Pain Research » Volume 10
An evaluation of the analgesic effect of AnestaGel™ on mechanical allodynia in a rat model of postoperative incisional pain
Authors Hutchins J, Taylor W
Received 19 July 2017
Accepted for publication 15 November 2017
Published 13 December 2017 Volume 2017:10 Pages 2807—2813
DOI https://doi.org/10.2147/JPR.S146759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Katherine Hanlon
Jacob Hutchins,1 William Taylor2
1Department of Anesthesiology, University of Minnesota, Minneapolis, MN, USA; 2InSitu Biologics, LLC, St Paul, MN, USA
Purpose: Sustained release hydrogel with bupivacaine (AnestaGel™) is a novel formulation of extended release bupivacaine in a biohydrogel Matrix™. We sought to compare the analgesic effects via mechanical allodynia, the pharmacokinetic characteristics via serum blood levels, and the local tissue effects via pathology, following injection of either sustained release hydrogel with bupivacaine, liposome bupivacaine, or hydrogel only (negative control group).
Materials and methods: Ninety rats (30 in each group) were randomized to receive a sciatic nerve block injection of either sustained release hydrogel with bupivacaine, liposome bupivacaine (Exparel®), or a biohydrogel matrix. The total force generated was obtained at varying time points. Pathologic analysis was undertaken on days 5 and 42 of the study. Six additional rats (two in each group) were randomized to receive a sciatic nerve block injection of either sustained release hydrogel with bupivacaine, liposome bupivacaine, or bupivacaine and pharmacokinetic data were obtained for up to 120 hours.
Results: The sustained release hydrogel with bupivacaine group had significantly better response to mechanical allodynia compared to the other two groups. The pathology showed no significant adverse events at 42 days in any group. Finally, bupivacaine was present longer in the serum of sustained release hydrogel with bupivacaine group than the other two groups.
Conclusion: The sustained release hydrogel with bupivacaine achieved longer lasting analgesia with no significant findings on pathology at 42 days when compared to both positive and negative controls.
Keywords: mechanical allodynia, local anesthetics, extended release, nerve block
Introduction
Pain management continues to remain an important part of intraoperative and postoperative patient care. Several papers and societies have advocated for a multimodal analgesic approach to the management of postoperative pain, with a local anesthetic being a component of that approach.1–4 However, local anesthetics are limited in their effectiveness by their duration of action. Local anesthetics can be given via a catheter technique to extend their duration of action, but these techniques are sometimes more difficult to place and are associated with catheter dislodgement.5
Additionally, there have been several extended release formulations of local anesthetics developed, which prolong the duration of action of the local anesthetic.6,7 Liposome bupivacaine (Exparel®; Pacira Pharmaceuticals Inc., Parsippany-Troy Hills, NJ, USA) is a multivesicular liposomal formulation of 1.3% bupivacaine. It has shown prolonged release compared to placebo in wound infiltration and peripheral nerve blocks, but its clinical results have been mixed when compared to bupivacaine hydrochloride.8–12
Sustained release hydrogel with bupivacaine (AnestaGel™; InSitu Biologics, LLC, St Paul, MN, USA) is a novel formulation of extended release bupivacaine in a biohydrogel Matrix™ (InSitu Biologics, LLC). This formulation allows for a single injection of bupivacaine hydrogel into the tissue to prolong the release of local anesthetic. The matrix biohydrogel is tunable, biocompatible, and bioabsorbable. Prior formulations of sustained release hydrogel with bupivacaine have shown prolonged efficacy, but to date, no good laboratory practice (GLP) studies have been performed using sustained release hydrogel with bupivacaine. The objectives of this study were to evaluate under GLP 1) the analgesic effects of sustained release hydrogel with bupivacaine on mechanical allodynia; 2) the local tissue effects following injection of sustained release hydrogel with bupivacaine; and 3) the pharmacokinetic characteristics of sustained release hydrogel with bupivacaine analyzed by measuring serum blood levels.
Materials and methods
This study was conducted in accordance with the US Food and Drug Administration Regulations on Good Laboratory Practice for Nonclinical Laboratory Studies CFR Title 21 Part 58, with the exception of blood sample processing. The pharmacokinetic portion of the study was not in accordance with GLP, but in accordance with the medical research organization NAMSA’s Institutional Animal Care and Use Committee protocol #17-12-8, which also approved this study. For the GLP portion of the study, 90 Sprague Dawley male rats weighing 150–250 g were selected for the analgesic and pathologic portions of this study. An additional six Sprague Dawley male rats weighing between 350 and 450 g were chosen for the pharmacokinetic portion of the study. Thus, the total number of rats used in the study was 96.
Figure 1 displays a summary of the GLP portion of the study. These animals were received, acclimated, and verified to be in good health prior to use. Within 2 days of the study, they underwent baseline nociceptive testing to assess the withdrawal threshold to mechanical stimulation using electronic von Frey (eVF) fibers. Animals were randomly divided into three groups. The test group (n=30) received sustained release hydrogel with bupivacaine (Ref No. P105C, Lot No. NB 100.101.1865, p.1) with a two-part hydrogel formulation consisting of drug reservoir particles suspended in a binding hydrogel matrix. Drug reservoir particles contained 200 mg/mL 5.5% tyrosine-substituted hyaluronan (TsHA). The binding matrix was formulated with 10 mg/mL 1.2% TsHA. The overall sustained release hydrogel with bupivacaine dose contained 105 mg/mL of bupivacaine. The second group (n=30) was a positive control and received liposome bupivacaine (Exparel; 1.33%, NDC 65250-266-20). The third group (n=30) received a negative control consisting of the sustained release hydrogel without any bupivacaine added.
  | Figure 1 The study design for the mechanical allodynia and pathology portion of the study. |
Animals were transferred to the procedure room, anesthetized with inhaled isoflurane, and the left hind paw was prepared for aseptic surgery. Surgical creation of a 1 cm longitudinal incision along the plantar aspect of the left foot was performed and the incision was closed in the standard fashion. Following the incisional procedure, each animal received an injection of 0.1 mL of the corresponding treatment targeting the sciatic nerve between the greater trochanter and the ischial tuberosity. All animals recovered from anesthesia and returned to their general housing area. Nociceptive testing was performed at 2, 6, 10, 24, 48, 72, 96, and 120 hours postinjection. Mechanical allodynia was tested using eVF Anesthesiometer (IITC Life Science, Woodland Hills, CA, USA). The testing was on the plantar surface of the ipsilateral and contralateral hind paw of each animal. This was repeated a total of three times at each time period to obtain an average force number for each time period. Pressure was applied with the probe tip with increasing force within 1 mm of the midline of the incision. Animal observations occurred at least once a day.
Twenty animals from each group (a total of 60 rats) survived a total of 5 days. Additionally, 10 rats from each group (a total of 30 rats) survived a duration of 42±3 days. All animals were humanely euthanized and submitted for gross necropsy. The injection sites of each animal, including the sciatic nerve and the local lymph nodes (popliteal, iliac, and/or prefemoral), were collected, processed for histology, and submitted to a board-certified veterinary pathologist for analysis. Tissue samples were prepared and hematoxylin and eosin slides were prepared. The pathologist tested for local effects after implantation, including inflammation, hemorrhage, foreign debris, neovascularization, and necrosis. Scoring criteria for pathology was on the scale of absent 0, minimal 1, mild 2, moderate 3, and marked 4 (Table 1). Irritant rank scores were also calculated. This was accomplished by totaling the implant scores (inflammatory cells + tissue response) for each implant site scored. The Group Average was equal to the sum of the total scores for that group divided by the number of implant sites, rounded to the nearest 10th. The Irritant Ranking Score was derived as follows: Test Article Group Average Score – Control Article Group Average Score = The Irritant Ranking Score. Nonirritant was a score of 0.0–2.9; slight irritant was a score of 3.0–8.9; moderate irritant was a score of 9.0–15.0; finally, severe irritant was a score larger than 15.0.
For the pharmacokinetic portion of the study, six male Sprague Dawley rats weighing between 350 and 450 g were chosen. The six animals were divided into three groups. The first group (n=2) received sustained release hydrogel with bupivacaine (Ref No. P105C, Lot No. NB 100.101.1865, p.1) with a two-part hydrogel formulation consisting of drug reservoir particles suspended in a binding hydrogel matrix. Drug reservoir particles contained 200 mg/mL 5.5% TsHA. The binding matrix was formulated with 10 mg/mL 1.2% TsHA. The overall sustained release hydrogel with bupivacaine dose contained 105 mg/mL of bupivacaine. The second group (n=2) was a positive control and received liposome bupivacaine (1.33%, NDC 65250-266-20). The third group (n=2) was a positive control and received bupivacaine hydrochloride 0.75%.
For the non-GLP pharmacokinetic portion of the study, animals were anesthetized using inhaled isoflurane, and then each animal received 0.1 mL injection of their corresponding injectate between the greater trochanter and ischial tuberosity targeting the sciatic nerve. Blood sampling occurred via an implanted jugular catheter. The blood sampling was performed at 15 minutes, 45 minutes, 2 hours, 6 hours, and 24 hours for all three groups. Then, for the sustained release hydrogel with bupivacaine and liposome bupivacaine groups, blood sampling occurred at 48, 72, 96, and 120 hours. Blood samples were then sent to BASi laboratory for analysis of serum bupivacaine levels. No further pathologic testing occurred in these six rats.
Statistical analysis
Statistical analysis was performed by Technomics Research, LLC (Long Lake, MN, USA). The total force generated was analyzed using an unpaired t-test and calculated using the average force from each rat at each time point from 2 to 72 hours for 0–72 hours and from 2 to 120 hours for 0–120 hours. The difference between the right paw and left paw was evaluated using a repeated-measures analysis of variance. The area under the curve analysis was performed using the left paw average force value data and the difference was tested by an unpaired t-test.
Results
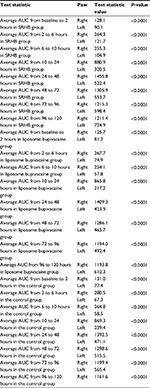
Ninety rats with 30 in each group were included in the GLP portion of the study testing both mechanical allodynia and pathology. Additionally, six rats were included in the final non-GLP pharmacokinetic analysis. We first analyzed the total force generated from 2 to 72 hours after injection in the left (injured) paw. We found that the sustained release hydrogel with bupivacaine group had significantly higher force generated than the control (P=0.0004; Table 2) and the liposome bupivacaine (P=0.0002; Table 3) groups. We then evaluated the total force generated from 2 to 120 hours after injection. The sustained release hydrogel with bupivacaine group had significantly higher force generated when compared to the control group (P=0.0024) and the liposome bupivacaine group (P=0.0005), as shown in Tables 2 and 3. Finally, we compared the right (uninjured) to left (injured) paw values for each group and found that the right paw generated significantly higher force than the left at all time points for all three groups (Table 4).
The results of the pathology tests illustrated that at day 5, five rats (out of 20) in the sustained release hydrogel with bupivacaine group had pathology consistent with either minimal or mild nerve damage. Minimal or mild nerve damage was characterized as axonophagia and/or myelinophagia. On day 42, five rats (out of 10) showed minimal nerve damage in the sustained release hydrogel with bupivacaine group. Neither the liposome bupivacaine group nor the biohydrogel matrix group showed any signs of nerve damage at both day 5 and day 42.
The irritant rank scores for all three groups at 5 and 42 days are listed in Table 5. At the 5-day time point, under the conditions of this study and based on the irritant rank score, sustained release hydrogel with bupivacaine was considered a moderate irritant when compared to liposome bupivacaine and a nonirritant when compared to the biohydrogel matrix group. At the 5-day time point, under the conditions of this study and based on the irritant rank score, liposome bupivacaine was considered a nonirritant when compared to the biohydrogel matrix group. At the 42-day time point, under the conditions of this study and based on the irritant rank score, sustained release hydrogel with bupivacaine was considered a slight irritant when compared to liposome bupivacaine and a nonirritant when compared to the biohydrogel matrix group. At the 42-day time point, under the conditions of this study and based on the irritant rank score, liposome bupivacaine was considered a nonirritant when compared to the biohydrogel matrix group. The material present and the corresponding tissue response and inflammation for the sustained release hydrogel with bupivacaine group led to the ranking of moderate irritant at 5 days and slight irritant at 42 days, compared to liposome bupivacaine.
  | Table 5 Irritant rank scores for the three groups at 5 and 42 days postinjection |
The pharmacokinetic data are displayed in Table 6 which show serum bupivacaine levels from the three groups (sustained release hydrogel with bupivacaine, liposome bupivacaine, and bupivacaine) up through 120 hours. Six rats (who were not part of the mechanical allodynia and pathology portion of the study) were included in this analysis. The sustained release hydrogel with bupivacaine group showed serum bupivacaine cmax (peak serum concentration) levels at 579 and 1030 ng/mL for rats 1 and 2, respectively, both occurring 2 hours after injection. The cmax of rats 1 and 2 of the liposome bupivacaine rats was 27.0 and 42.1 ng/mL, respectively, both occurring at 6 hours postinjection. The cmax of rats 1 and 2 of the bupivacaine hydrochloride group was 129 and 138 ng/mL, respectively, both occurring at 45 minutes after injection.
  | Table 6 Serum bupivacaine levels (ng/mL) after injection near the sciatic nerve in rats Note: Values are the mean serum bupivacaine levels from two rats in each group. |
Discussion
This study illustrates that a single injection of sustained release hydrogel with bupivacaine administered near the sciatic nerve produced long-lasting analgesia in a rat model. When compared to both a negative control (sustained release hydrogel without bupivacaine) and a positive control (liposome bupivacaine), sustained release hydrogel with bupivacaine performed significantly better on assessing analgesia via mechanical allodynia produced from a sciatic nerve injection in rats from 0 to 72 hours and from 0 to 120 hours. This study is based on previous rat pain models which used similar incisions and force testing for assessment of analgesia.13,14 It must be noted, however, that while the volumes were the same between sustained release hydrogel with bupivacaine and the positive control, the dosages of bupivacaine were different. The concentration of bupivacaine in sustained release hydrogel with bupivacaine was 105 mg/mL and in liposome bupivacaine was 13.3 mg/mL.
This analgesic effect of sustained release hydrogel with bupivacaine on the injured paw was supported by the data regarding the right paw. There was no significant difference between the right paw data when comparing sustained release hydrogel with bupivacaine to control and sustained release hydrogel with bupivacaine to liposome bupivacaine. This suggests that all rats performed equally well with regards to force assessment via the eVF testing in their uninjured paw and, thus, further validates testing on the injured paw. Furthermore, as there were significant differences in force generation at all time points between the left and right paws for each group, we can conclude that again force assessment via the eVF was accurate as the injured paw performed significantly worse in force assessment when compared to the uninjured paw.
Previous studies have illustrated the neurotoxic effects of local anesthetics.15,16 Memari et al15 illustrated that when bupivacaine is injected near the sciatic nerve, neuronal injury can occur. The neuronal injury can be characterized as either perineural inflammation or decreased number of myelinated fibers. The exact mechanism of neuronal injury is unknown; however, recent data from Yu et al17 suggest different mechanisms depending on the type of local anesthetic used. Furthermore, they showed that as the concentration of bupivacaine increased, there was increased neurotoxicity. Consistent with these results, the sustained release hydrogel with bupivacaine group did show some nerve damage histologically, but this damage was minimal to mild at 5 days and minimal at the 42-day time point. This likely would resolve completely over time. The liposome bupivacaine (positive control) group did not show any measurable neurotoxicity, which was similar to previous pathologic findings obtained when injected perineurally in a porcine model.18 As described earlier, the concentration of sustained release hydrogel with bupivacaine was higher than that of liposome bupivacaine, which may account for the differences in neuronal damage on histopathology.
Finally, the pharmacokinetic pilot study results suggest that bupivacaine remained longer in the blood of rats that received a sciatic nerve injection of sustained release hydrogel with bupivacaine than after injection of bupivacaine hydrochloride and liposome bupivacaine, indicating prolonged release. In rats weighing between 350 and 450 g, the concentrations of bupivacaine injected were between 23 and 30 mg/kg for sustained release hydrogel with bupivacaine and 3 and 3.7 mg/kg for liposome bupivacaine. Thus, the differences could be related to the differences in the concentration of bupivacaine injected. However, even at a lower concentration, liposome bupivacaine failed to produce measurable blood levels beyond 24 hours, whereas the sustained release hydrogel with bupivacaine produced measurable serum bupivacaine levels at 72 hours in one rat and 96 hours in another. Serum bupivacaine cmax levels of the sustained release hydrogel with bupivacaine are similar to previous studies involving larger dosages of liposome bupivacaine in animals.19
There are two limitations of this study. The first, as discussed earlier, is that the concentrations of bupivacaine between sustained release hydrogel with bupivacaine and liposome bupivacaine were not equivalent. This may have affected the analgesic, pathologic, and pharmacokinetic differences between the two groups. However, while this may be viewed as a limitation for comparison, it is an inherent advantage of the sustained release mechanism of action of the hydrogel with bupivacaine. Additionally, as the pharmacokinetic portion of the study consisted of only two rats in each group, it is difficult to draw definite conclusions regarding the differences observed in serum bupivacaine levels.
Conclusion
Sustained release hydrogel with bupivacaine provides long-lasting analgesia via release of bupivacaine from a biohydrogel matrix with no severe negative pathological findings in a rat model performed under GLP when compared to both positive and negative controls.
Acknowledgments
The authors would like to acknowledge Technomics Research, LLC, for their assistance with statistical analysis for this manuscript and NAMSA for their assistance with the GLP and pharmacokinetic portion of the study. This study was funded by InSitu Biologics, LLC.
Disclosure
Dr Hutchins is a consultant and owns stock with InSitu Biologics, LLC. William Taylor is an employee and owns stock with InSitu Biologics, LLC. The authors report no other conflicts of interest in this work.
References
Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. | ||
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. | ||
American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. | ||
Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–593. | ||
Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–925. | ||
Weiniger CF, Golovanevski L, Domb AJ, Ickowicz D. Extended release formulations for local anaesthetic agents. Anaesthesia. 2012;67:906–916. | ||
Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107–116. | ||
Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;2:CD011419. | ||
Hadzic A, Minkowitz HS, Melson TI, et al. Liposome bupivacaine femoral nerve block for postsurgical analgesia after total knee arthroplasty. Anesthesiology. 2016;124:1372–1383. | ||
Hutchins J, Delaney D, Isaksson Vogel R, et al. Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: a prospective randomized controlled study. Gynecol Oncol. 2015;138:609–613. | ||
Abildgaard JT, Lonergan KT, Tolan SJ, et al. Liposomal bupivacaine versus indwelling interscalene nerve block for postoperative pain control in shoulder arthroplasty: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2017;7:1175–1181. | ||
Vandepitte C, Kuroda M, Witvrouw R, et al. Addition of liposome bupivacaine to bupivacaine HCl versus bupivacaine HCl alone for interscalene brachial plexus block in patients having major shoulder surgery. Reg Anesth Pain Med. 2017;42:334–341. | ||
Brennan, T. Postoperative models of Nociception ILAR J. 1999;40:129–136. | ||
Chaplan SR, Bach FW, Progrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. | ||
Memari E, Hosseinian MA, Mirkheshti A, et al. Comparison of histopathological effects of perinerual administration of bupivacaine and bupivacaine-dexmedetomidine in rat sciatic nerve. Exp Toxicol Pathol. 2016;68:559–564. | ||
Drasner, K. Local anesthetic neurotoxicity: clinical injury and strategies that may minimize risk. Reg Anesth Pain Med. 2002;27:576–580. | ||
Yu X, Zhao W, Li Y, et al. Neurotoxicity comparison of two types of local anaesthetics: amide-bupivacaine versus ester-procaine. Sci Rep. 2017;7:45316. | ||
Damjanovska M, Cvetko E, Hadzic A et al. Neurotoxicity of perinerual vs intraneural—extrafascicular injection of liposomal bupivacaine in the porcine model of sciatic nerve block. Anaesthesia. 2015;70:1418–1426. | ||
Richard BM, Newton P, Ott LR, et al. The safety of EXPAREL® (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogs. J Drug Deliv. 2012;2012:962101. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.