Back to Journals » Drug Design, Development and Therapy » Volume 13
An Efficient, Lung-Targeted, Drug-Delivery System To Treat Asthma Via Microparticles
Authors SreeHarsha N , Venugopala KN , Nair AB , Roopashree TS, Attimarad M , Hiremath JG , Al-Dhubiab BE , Ramnarayanan C, Shinu P , Handral M , Haroun M , Tratrat C
Received 22 May 2019
Accepted for publication 17 October 2019
Published 27 December 2019 Volume 2019:13 Pages 4389—4403
DOI https://doi.org/10.2147/DDDT.S216660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Nagaraja SreeHarsha,1,2 Katharigatta N Venugopala,1,3 Anroop B Nair,1 Teeka S Roopashree,4 Mahesh Attimarad,1 Jagadeesh G Hiremath,2 Bandar E Al-Dhubiab,1 Chandramouli Ramnarayanan,5 Pottathil Shinu,6 Mukund Handral,7 Micheline Haroun,1 Christophe Tratrat1
1Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Saudi Arabia; 2Department of Pharmaceutics, Vidya Siri College of Pharmacy, Bengaluru, India; 3Department of Biotechnology and Food Technology, Durban University of Technology, Durban 4001, South Africa; 4Department of Pharmacognosy, Government College of Pharmacy, Bengaluru, India; 5Department of Quality Assurance, Krupanidhi College of Pharmacy, Bengaluru, India; 6Department of Biomedical Sciences, College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Saudi Arabia; 7Department of Pharmacology, Faculty of Pharmaceutical Sciences, PES University, Bengaluru, India
Correspondence: Nagaraja SreeHarsha
Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, PO Box 400, Al-Ahsa, Saudi Arabia
Tel +96-653-548-5322
Email [email protected]
Background: Chronic diseases such as diabetes, asthma, and heart disease are the leading causes of death in developing countries. Public health plays an important role in preventing such diseases to improve individuals’ quality of life. Conventional dosage schemes used in public health to cure various diseases generally lead to undesirable side effects and renders the overall treatment ineffective. For example, a required concentration of drug cannot reach the lungs using conventional methods to cure asthma. Microspheres have emerged as a confirmed drug-delivery system to cure asthma.
Method: In this paper, a salbutamol-loaded poly lactic acid-co-glycolic acid-polyethylene glycol (PLGA-PEG) microsphere (SPP)-based formulation was prepared using a Buchi B-90 nanospray drier. Face-centered central composite design (CCD) was applied to optimize the spray-drying process.
Results: The drug content and product yield were found to be 72%±0.8% and 86%±0.4%, respectively; drug release (91.1%) peaked for up to 12 hrs in vitro. Microspheres obtained from the spray dryer were found to be shriveled. The experiments were carried out and verified using various groups of rabbits. In our study, the particle size (8.24 μm) was observed to be an essential parameter for drug delivery. The in vivo results indicated that the targeting efficacy and drug concentration in the lung was higher with the salbutamol-loaded PLGA-PEG SPP formulation (1,410.1±10.11 μg/g, 15 mins), as compared to the conventional formulation (92±0.56 μg/g, 10 min). The final product was stable under 5°C±2°C, 25°C±2°C, and 40°C±2°C/75%±5% relative humidity. In addition, these co-polymers have a good safety profile, as determined by testing on human alveolar basal epithelium A549 cell lines.
Conclusion: Our results prove that microspheres are an alternative drug-delivery system for lung-targeted asthma treatments used in public health.
Keywords: polylactic acid-co-glycolic acid, public health, spray dryer, microspheres
Introduction
In recent years, chronic diseases were found to be among the leading causes of death in developing countries.1 Such diseases include heart disease, asthma, diabetes, and others. Asthma is a chronic disease that has a significant impact on the lungs. Asthma causes the lungs to swell and the airways to narrow, resulting in wheezing. Asthma patients experience shortness of breath, coughing, wheezing, and chest tightness, which result in a reduced quality of life (QoL).2 Also, such patients require frequent hospitalization, which imposes a huge burden on national economies.3 It has also been observed that the use of high dosages during asthma treatments can result in severe adverse effects that impact human health.4
Advancements in technology have recently gained increased attention in public health. The major objective of public health is to improve living standards by efficiently and effectively preventing chronic diseases. In public health, studies are carried out on affected groups of people, which can include a community, a village, a city, or a country in some cases. These types of studies can help identify solutions to efficiently prevent and cure diseases. Public health is a vast field that includes other subfields, such as environmental health, mental health, behavioral health, community health, and so forth.5 Currently, public health offers some promising novel biological treatments, but the majority of such treatments are not feasible for use as they are expensive and may be designed for selected cases. For example, patients with seemingly severe asthma may have other (modifiable) causes of poor asthma control.6
The worldwide prevalence of asthma ranges from 1% to 18%.7,8 However, nearly 10% of patients suffer from severe symptoms, which remain difficult to control despite the use of a therapeutic inhaler.7 According to the Saudi Initiative for Asthma (SINA) report, more than 2 million people in Saudi Arabia are suffering from asthma.9 This number is growing each day due to the lack of care and medical resources available.
Generally, smooth-muscle relaxants that target the beta2 (β2)-adrenergic receptor are used in the primary treatment of asthma,10 with salbutamol being the most commonly used. Salbutamol is available in oral and inhaled dosage forms.11 It has been observed that oral salbutamol demonstrates blood distribution and absorption power, with a bioavailability of about 44%.11 However, this drug suffers from first-pass hepatic biotransformation challenges in this form.12 The phenyl sulfotransferase enzyme is responsible for the biotransformation of salbutamol in the liver;11 renal excretion is also responsible for the elimination of salbutamol in the parent and conjugated forms.11 The biotransformation of salbutamol in the human body is highly enantioselective.7 There are two major types of salbutamol: (S)-salbutamol and (R)-salbutamol; both types differ in their elimination rate from the blood. S-salbutamol offers a very slow elimination rate of salbutamol from the blood as compared to R-salbutamol [4]. In this way, R-salbutamol is removed rapidly from the systemic circulation, resulting in the low bioavailability of salbutamol.9 Therefore, formulating and targeting the drug to the lungs is essential. Salbutamol is generally administered in the form of inhaled therapy; however, intravenous (IV) administration is suggested for patients in the event that the aforementioned treatment fails.13
PLGA is the U.S. Food and Drug Administration (FDA) approved copolymer which exhibits excellent biodegradable, biocompatible properties for sustained release, targeted drug delivery and protects preparations against degradation. Moreover, PLGA hydrolysis in the body leads to two monomers (lactic acid and glycolic acid) both familiar cell metabolites.14–16
We have proposed a new approach whereby approved drugs would be prepared into polymeric drug carriers, owing to the biodegradable, nontoxic, and biocompatible properties of (polylactic acid-co-glycolic acid-polyethylene glycol [PLGA-PEG]). Our solution is nontoxic, biodegradable, and composed of biocompatible microspheres that have the ability to control for particle size and passively target the lungs following IV injection. By developing a formulation that targets the lungs using this approach, there is a greater possibility that a new and efficient drug-delivery system can be developed featuring fewer side effects. Furthermore, this approach is also responsible for reducing the amount of drug distributed to highly perfused tissues.7,9,17
Microspheres are tiny, spherical particles that vary in diameter and range from 1 to 1,000 microns in size. Particles between 5 and 15 μm are widely distributed throughout the lung tissue after iv bolus injection, these particles get entrapped by a capillary network of the lungs thereby achieving passive lung-specific drug delivery. Smaller particles that are less than 5 μm migrates towards liver, spleen and blood. However, even smaller particles such as nanoparticles, aggregated proteins and colloids have been shown to localize in the brain, bone marrow or to target enhanced permeation and retention of tumors.18,19 It offers many significant advantages, such as size reduction, decreased dose toxicity, drug coating, site-specific drug delivery, and so forth.20
Several methods and techniques have been reported in the formulation of microspheres, such as the use of a reduced pressure–solvent evaporation method, coprecipitation, coacervation, solvent dispersion, solid-in-oil-in-water (S/O/W) emulsion, spray-freeze–drying and water-in-oil (w/o).21 The general drawback associated with these methods is the use of reactive organic solvents. These reactive organic solvents form toxic solutions and explosives.22 Furthermore, these techniques have a poor yield and cannot be scaled up commercially.
In this research, the spray-drying method is used by our team to formulate microspheres by following a one-step process to fabricate the microspheres, thus making it an efficient scheme for this purpose. This approach provides various advantages, including its low cost, swiftness, and the ability to adjust the physiochemical properties of the particles. Some common properties include particle size, size distribution, morphology, moisture within the particle, polymorphism, and particle density. On the basis of these properties, spray-drying can be used as a vital technique to prepare the microspheres required for various forms of drugs.23–27 To overcome all the aforementioned problems, we have developed and optimized salbutamol microspheres using PLGA-PEG as a polymer (SPP) to overcome the common side effects associated with this drug, including upset stomach, runny nose, throat irritation, and shakiness.
Materials And Methods
PLGA, PEG, salbutamol sulphate, and terbutaline sulphate were purchased from Sigma-Aldrich Corporation (St Louis, MO, USA). Further, dialysis tubing cellulose membrane (2,000 molecular weight cutoff) was used. Human Alveolar basal epithelium A549 cells were purchased from the National center for Cell Science, Pune, India. The remaining chemicals used were of analytical grade.
Preparation Of Spray-Dried Microspheres
Initially, the salbutamol along with PLGA-PEG (weighted at 500 mg each) was dissolved in 250 mL of de-ionized distilled water:ethyl acetate (1:1). For experimentation, a Büchi B-90 Spray Dryer (a laboratory-scale spray dryer) was used to prepare the spray-dried particles. The conditions used during processing were as follows: inlet temperature, 100°C; aspiration rate, 100%; drying air flow rate, 160 L/h; pump setting, 100% (2.5 mL/minute).
The outlet temperature of 36°C was achieved using the aforementioned conditions. Following that, the spray-dried powders were collected from the collection vessel. Finally, the resultant spray-dried yield was reported. We repeated this process in triplicate.
Design Of Experiments (DoE)
The design of experiments (DoE) technique is used to provide a better and faster way to optimize the spray-drying procedure. It helps to investigate the eventual causes and relationships for three-process variables. The desired particle size for this experiment was between 5 and 15 μm. The DoE is carried out using the Design-Expert® software package, Version 7.0 trail (Stat-Ease Inc., Minneapolis, MN, USA). The central composite design was devised and created using 15 runs, as assigned by the software.
Particle Size Analysis
After DoE, the particle size was determined using a Malvern Zetasizer Nano Series (Nano-ZS; Malvern Instruments, Malvern, UK). Three milliliters of a suspension containing microspheres in 0.5% w/w Tween 20 solution were sonicated and transferred into a glass cuvette. Using this method, the mean size diameter and distribution were determined.28
Morphological Characteristics
Finally, the surface patterns of the spray-dried microspheres were determined using an ultra-thin coating of electrically conducting metal (platinum). To scan the microspheres, a Quanta 200 scanning electron microscopy system (Philips-FEO, Eindhoven, the Netherlands) was used. This helped to determine the external surface of the microspheres to examine any changes in their structure, such as their shape, hallow, cracks, and so forth. All samples were analyzed in triplicate.29
Percentage Yield
The spray-dried powder was collected using a rubber scapula by scraping it off the collecting vessel and weighed. The production yields of the microspheres were determined using the following equation:30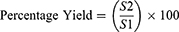
In this equation, S1 represents the total amount of solids sprayed, and S2 is the weight of the recovered microspheres.
Drug Content Analysis
In this process, a known quantity of 25 mg powder was weighed and transferred to a mortar and pestle. After that, it was crushed to determine the drug content of salbutamol in the microspheres. Deionized water was added to this powder to form a suspension. The suspension was transferred to a dialysis membrane and placed in a dissolution basket in phosphate-buffered saline (PBS). The basket was rotated for 2 hrs to release the drug from the microspheres. In the end, the drug content was measured at 276 nm with an ultraviolet (UV)-visible (VIS) spectrophotometer (Shimadzu UV 1601; Shimadzu Corporation, Tokyo, Japan), using the following equation:31,32
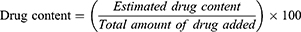
In Vitro Release Mechanism
The in vitro release profile was carried out according to the methods described in a recently published report with slight modification.33 For the in vitro release mechanism, the studies were carried out by adding 100 mg of the microsphere in 5 mL of PBS (pH 7.4). The suspension was transferred to a 3 X 1 dialysis bag (molecular weight cutoff: 12,000–14,000 Da; Sigma-Aldrich Co). After that, it was dialyzed against 100 mL of PBS (pH 7.4) and stirred at 50 rpm. Sink conditions were also maintained during the studies. The temperature of the dissolution medium was maintained at 37°C±2°C. Aliquots (2 mL) were withdrawn to determine the drug content at predetermined time intervals. Finally, the drug content was determined at 276 nm using a UV-VIS spectrophotometer (1601; Shimadzu Corporation). A similar procedure was carried out in triplicate using all SPP samples (mean ± standard deviation [SD], n=3). The obtained data were fitted to different kinetic models to understand the release mechanism using Sigmaplot software version 9.01 (Systat Software, Inc., San Jose, CA, USA).
Tissue Distribution, Targeting Efficiency, And Pharmacokinetic Studies In Vivo
Healthy white New Zealand rabbits were used for the experiment, according to previously published articles.33,34 Six-month-old healthy male/female white rabbits weighing from 2.5 to 3 kg were selected for this experiment. The rabbits were kept in a specific environment under a controlled room temperature (25°C). During the experiment, the rabbits had access to ad libitum and rodent diet. These animals were divided into six groups containing six animals each to determine the drug release at predetermined time intervals (0.17 hrs, 0.5 hrs, 1 hr, 3 hrs, 6 hrs, and 12 hrs). Group 1 was the “control group” that received an intravenous dose of 60 mg/kg of salbutamol (pure drug). The other groups (2–6) received the formulation equivalent to 60 mg/kg of the drug.35 The drug release from different organs (the lungs, liver, spleen, and blood) was tested after being extracted from the rabbits. The drug content from the different organs was estimated using high-performance liquid chromatography.36 The known quality of salbutamol within the plasma was withdrawn into microtubes and an internal standard (terbutaline sulphate) was added. Then, this was centrifuged, and the supernatant was separated and injected into a C18 column (50 μm, 70 A). The animal studies were approved by the ethical committee at the College of Clinical Pharmacy, King Faisal University, Saudi Arabia (COCP/KFU-2145-65-19). It is confirmed that the experiments were performed in accordance with King Faisal University, research ethics committee, which is also in accordance with the National Committee of BioEthics (NCBE-Saudi Arabia) guidelines and regulations.
To analyze the targeted delivery mechanism of the clinical application of salbutamol in the formulation, three targeting parameters (re, te, and Te) were used in this study. These pharmacokinetic parameters were calculated according to the following formulas:37,38
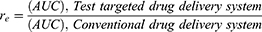
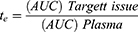
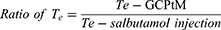
where Te is the targeting efficacy, Re is the relative targeting efficiency, and Te is the lung-targeting efficacy.
In Vitro Cytotoxicity Studies
An in vitro cytotoxicity test was performed in accordance with the method described earlier39 Briefly, human alveolar basal epithelium A549 cells were seeded at a density of 10,000 cells/well onto a 96-well microtiter plate and incubated for 24 hrs. After the incubation period, each well was exposed to different concentrations of SPP microspheres. The medium was aspirated and 100 µL of MTT was added and further incubated for 4 hrs. The response was stopped by adding an identical volume of solution containing 20% sodium dodecyl sulfate (SDS) in 50% N,N-dimethylformamide (DMSO) to solubilize the formed formazan crystal. The amount of formazan was measured at 545 nm, which is directly proportional to the number of living cells in culture.39
DNA Fragmentation Assay
A DNA fragmentation assay was performed according to the protocol developed earlier by Zhivotosky and Orrenius (2001) with a slight modification to investigate the fragmentation of genomic DNA.40,41 Briefly, HepG2 cells (1×105 cells/well) were cultured in 6-well plates and treated with a 100 µg/mL concentration of SPP/control and were subsequently incubated. Following incubation for 24 hrs, the cells were trypsinized and washed thrice with PBS and centrifuged at 2,500 rpm for 5 mins. The DNA was extracted by incubating the cells with 500 μL of lysis buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris–Cl [pH 8.0], 0.5% SDS, and 1 mg/mL of proteinase K) at 54°C with gentle agitation. DNA was pelleted by mixing with 65 μL of 10M ammonium acetate and 500 μL of ice-cold ethanol. The pellets were then dissolved in 50 μL of TE buffer and sample loading dye, and the fragmented DNA was resolved in 2% agarose gel.
Stability Studies
Stability testing was carried out to prove the stability of the formulation under different environmental factors, such as humidity and temperature. The drug-loaded PLGA-PEG microspheres (SPP) were filled and packed tightly in amber-color vials and exposed to stability testing according to the International Conference on Harmonisation (ICH) guidelines.42 The packed vials were kept at three different conditions: 5°C±2°C, 25°C±2°C, and 40°C±2°C/75%±5% relative humidity for 1 year. The formulations were further analyzed to examine their physical appearance, drug content, and particle size. The results obtained during this study are presented in Section 5.
Statistical Analysis
Statistical analysis of the in vitro release studies was carried out using the SigmaPlot Software (Version 9.01) (Systat Software, Inc., San Jose, CA, USA). Optimization studies were carried out using Design-Expert Version 7.0 (Trial) Stat-Ease, Inc. Minneapolis, MN, USA.
Results And Discussion
SPP Formulation Intended For Lung Targeting
Statistical optimization is a mathematical method used to solve complex problems, as well as to reduce the amount of time, physical efforts, and costs spent on the experiment. DoE is a mathematical tool used for statistical optimization.43 In our experiment, the numerical optimization technique was employed to optimize process parameters, such as inlet temperature, the polymer concentration, and the feed flow rate. The model contained five center points, four factorial points, and six axial points for a total of 15 experiments. In central composite design (CCD), three variables were used: 0 (intermediate), +1 (highest), and –1 (lowest). This design can optimally reduce the error; the responses can be modeled in a quadratic manner along with the selected variable with which the expected response does not diverge linearly. The experimental design (with associated responses) is shown in Table 1. The particle size from the experimental data point was in the range of 1.12–9.74 µm. All of our experiments were carried out in triplicate (n=3) using random order. All other process parameters, such as the spray nozzle (7 µm), drying air flow rate (160 L/hour), pressure (68 bar), spray (100%), and outlet temperature (20°C) were kept constant throughout the experiment. The polynomial equation obtained to evaluate the response variable is demonstrated in Eq. (1):
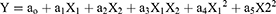
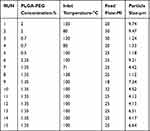 |
Table 1 Factorial Design Matrix Of SPP And Performance Values |
where ao–a5 represent the effects or regression coefficients, and X1–X2 are the studied variables. The resultant equation in terms of coded factors for particle size response shown in Eq. (2):

Effect Of PLGA-PEG Concentration On Particle Size
Particle size is an important characteristic of a targeted drug-delivery system. Many studies demonstrated that particle size plays an important role in biodistribution. In our case, the size requirement of the particles was between 5 and 15 µm. From Figure 1, it is observed that increasing the concentration of PLGA-PEG results in increasing particle sizes. This is due to the presence of greater amounts of solid in a droplet, which increases the size of the particle. Furthermore, such droplets contain lower amounts of liquid to vaporize, which also results in increased particle sizes.44
Effect Of Inlet Temperature On Particle Size
Inlet temperature also plays an important role in particle size. Figure 2 highlights that an increase in inlet temperature shows an increase in particle size. The reason is that particles do not shrink in the early stages while drying, thereby resulting in larger particles.45
Effect Of Feed Flow On Particle Size
Surprisingly, we observed an opposite effect in terms of particle size, as there was an increase in flow rate (Figure 3). An increase in feed flow leads to a decrease in the transit time of particle droplets through the spray-drier. Therefore, the solvent evaporates instantly and leads to a reduction in particle size.46
Numerical Optimization
In this research, a numerical optimization tool was employed to study the low- and high-level constraints and initial design space of the experimental model. Three numerical solutions were obtained using Design-Expert software in quantitative terms (Table 2). Three experiments were carried out under optimal conditions (Figure 4). The obtained particle size (8.24 µm; Figure 5) and predicted size were similar, as determined by the equation; this result was statistically significant. Analysis of variance (ANOVA) for the response surface quadratic model showed that the overall model for particle size production was statistically significant for lung targeting. The R2 coefficient of determination is a statistical measure that was employed, and the obtained value was >70%; thus, all the three variables (inlet temperature, PLGA-PEG concentration, and flow rate) represented functional relationships. The mean particle size obtained during the experiment was suitable for lung targeting. Several other studies have also published similar particle sizes for PLGA-PEG microspheres; specifically, following IV administration, particles with sizes ranging from 5 to 15 µm are entrapped in the capillary network of the lungs.47,48 The obtained formulation can be further used for characterization.
 |
Table 2 Optimization Solution |
Determination Of Encapsulation Efficiency And Percentage Yield
The encapsulation efficiency and percentage yield were found to be 72%±0.8% and 86%±0.4%, respectively. The low encapsulation and yield were due to the millions of uniform droplets, which were formed once atomization came into contact with hot air, which separates the powder from the liquid. The obtained powder sticks to the nebulization chamber, as shown in Figure 6. However, this loss can be minimized by increasing production.49
Particle Size Analysis
In our study, particle size was one of the most important parameters because the targeted drug delivery of microspheres solely depends on it. Generally, microspheres ranging in size from 5 to 15 µm would affect lung deposition. The particle size obtained following the optimization procedure was found to be 8.24 µm (Figure 5), which helps to entrap the microspheres following IV administration.50,51 Additionally, the obtained particles are narrow in size due to the spray nozzle; it ejects millions of uniformly sized droplets that are dried at a constant temperature and produce very narrow-sized particles. Therefore, the drug releases from the formulation will not fluctuate largely in vivo, and it will maintain an adequate therapeutic level. The spray-drying method was found to be superior when compared to the conventional method of microsphere preparation. The reason for this is that the Buchi patented spray-drier is a flourishing drying method that works by removing moisture, which is accomplished by applying heat to the spray solution. In addition, it simultaneously controls the humidity, resulting in a free-flowing powder. The entire process takes place in one single step, ultimately reducing the production cost.52
Surface Morphology
Determining the surface morphology is an important quality control test for spray-dried powder, which affects flow, moisture content, particle size, and bulk density. The powder obtained during this process is found to have shriveled particles, which is similar to the shrinking core when dried at high temperatures (Figure 7). However, this problem can be resolved by optimizing solid feed content. In our experiment, we could not increase the concentration of the solid feed which, in turn, would increase the particle size; however, this is beyond the scope of our study.53–55
In Vitro And Release Kinetics
It has been found that the pure drug (salbutamol) releases 95.4% within the first half hour; however, 28.23% of salbutamol from SPP was released in the first half hour, while the remaining drug released completely (99.1%) within 12 hrs. The drug-release pattern demonstrated an initial burst and was consistent with a biphasic model due to the loosely embedded drug on the particle surface.56 However, the initial burst can be decreased by increasing the polymer concentration. In the case of acute asthma, the loading dose of 4–6 µg kg–1 of salbutamol is given intravenously over a 10-mins period.57 Clinically, the initial burst release is important, as it acts as a loading dose, which helps to reach the minimum therapeutic concentration in the blood to show the drug’s action.58 The release mechanism is studied by fitting the data to various kinetic models (Sigmaplot Ver 9.01) to understand the release mechanism (first order; Higuchi, Baker and Lonsdale, Korsmeyer’s Peppas). The release mechanism involves three processes: swelling, polymer erosion, and dissolution. It has been concluded that a non-Fickian drug-release Peppas pattern is based on the diffusional exponent, n=0.979, as shown in Figure 8.59
The pharmacokinetic studies for the SPP microparticles were fitted using the Pkanalix suite of Lixoft Release 2019 (academic license; Lixoft Corporation, Antony, France), as well as with the aid of the SAEM algorithm. One, two, and three pharmacokinetic models for the SPP formulation were applied to describe the fate of a drug distributed after 120µg/kg following IV administration to the rabbits.35 The plasma concentration was measured for each rabbit at predetermined time intervals; the two-compartment model provides the best fit, as shown in Figure 9. The in vivo pharmacokinetic parameters are illustrated in Table 3. When the pharmacokinetics of the SPP formulation were compared with those of the conventional formulation, the SPP formulation altered the circulation pattern of salbutamol in an animal model in vivo. The half-life following an IV bolus of SPP (t1/2 (α) = 1.97±0.36 h, t1/2(β) = 18.97±2.12 h) was prominently greater than that of the conventional formulation (t1/2 (α) = 0.33±0.17 h, t1/2(β) = 8.4±1.12 h).
 |
Table 3 Pharmacokinetic Parameters Of SPP And Salbutamol Injection Following Intravenous Administration |
The obtained data show the sustained release and targeting efficacy of SPP, and the concentration of the drug in the lung is higher (1,410.1±10.11 µg/g, 15 mins) when compared to the conventional formulation (92±0.56 µg/g, 10 mins). A clinical study shows that the initial high salbutamol concentration in the lungs can help to widen the airways and relieve the symptoms of asthma.60
Particle size plays an important role in drug distribution. The results proved that the SPP formulation is higher in the lungs when compared to the conventional formulation. This is due to the narrow particle size of microspheres, which is between 5 and 15 µm. These microspheres are obtained from spray-drying techniques, and the results of this study are also supported by other researchers.61,62 Both drug-distribution schemes (ie, with SPP and conventional approaches) indicate that the organ/tissue is exposed to the drug to a greater extent if the Re (drug exposure) value is >1. The lung-targeting efficacy (Te) of the SPP formulation and the conventional formulation are shown in Table 4. The SPP results in this study showed the superior value of Re – ie, 11.2 for the lungs, 0.51 for the liver, 0.23 for plasma, and 2.1 for the spleen (Figure 10). This indicates that SPP formulation exposure to the lung is drastically higher when compared to the liver and spleen. The results showed that the microspheres are more concentrated at the target site (ie, the lungs).
 |
Table 4 Lung-Targeting Parameters Of SPP And Salbutamol Bolus (Control) |
In Vitro Cytotoxicity Studies
The salbutamol-loaded PLGA-PEG microspheres (SPP) appear to be non-toxic and well tolerated by human alveolar basal epithelium A549 in vitro. A maximum percentage of viable cells was observed (93.63%±7.9% and 98.53%±6.6%) for PLGA-PEG and SPP, respectively, even at higher concentrations of 100 µg/mL. Moreover, there was no significant difference between the polymer and drug-loaded SPP in terms of percent cell viability (Figure 11). The relative growth rate of PLGA-PEG with salbutamol microspheres was 2.5%, indicating very low cytotoxicity. Overall, the MTT assay revealed that the SPP microspheres (SPP) synthesized in the present study had high biocompatibility. Similar polymers were reported to have a good safety profile when tested on human bronchial epithelial 16HBE14o cell lines.63
DNA Fragmentation Assay
The DNA fragment assay is an indicator of apoptosis; this method was carried out to determine the influence of SPP microparticles on DNA damage in human alveolar basal epithelium by incubating 100 µg of the SPP microparticles with cells for 24 hrs. The results showed that the SPP microparticles did not have fragmented DNA when compared to the control group (Figure 12).
Stability Testing
Stability testing is carried out to prove that a given formulation is stable under different environmental factors, such as varying degrees of humidity and temperature. The drug-loaded PLGA-PEG microspheres (SPP) were filled and packed tightly in amber-color vials and were exposed to stability testing according to the ICH Q1AR2 guidelines.42 The packed vials were kept under three different conditions: 5°C±2°C, 25°C ±2°C, and 40°C±2°C/75%±5% relative humidity for 1 year. The formulations were further analyzed for their physical appearance, drug content, and particle size. The drug content varied between 2.5% and 4%, which means that the results fell within a safe range. The end-product was stable, and no changes were observed under 5°C±2°C and 25°C±2°C. However, at 40°C±2°C/75%±5% relative humidity, the formulation demonstrated liquefaction, as shown in Figure 13. Based on the report generated from Sigmaplot (version 9.01), salbutamol lost its 10% activity and retained 90% of its potency (t90=29.3 months).
Conclusion
Asthma is a severe, chronic disease that results in the deaths of millions of people. In this paper, a salbutamol-loaded, PLGA-PEG-optimized, microsphere-based solution was presented as a cure for asthma in public health. This formulation enables the distribution of narrow and shriveled particles with a small SPP sample volume, a higher yield, and good drug content. The microspheres were found to release maximal amounts of the drug for up to 12 hrs in vitro. These results were verified by releasing the drug in an animal model. We determined that microspheres hold great potential to revolutionize the public health domain by delivering an active compound. The mean particle size produced in our experiments was 8.24 µm, which is an ideal size for deposition in the capillary bed of the lungs. The maximum drug encapsulation and percentage yield were 72%±0.8% and 86%±0.4%, respectively. These results highlight that the sustained release and targeting efficacy of SPP and the drug concentration in the lung was higher (1,410.1±10.11 µg/g, 15 mins) when compared with the conventional formulation (92±0.56 µg/g, 10 mins). The proposed product is also stable under conditions of 5°C±2°C, 25°C ±2°C, and 40°C±2°C/75%±5% relative humidity. Further, the MTT and apoptosis assays revealed that PLGA-PEG-loaded salbutamol produced the lowest cytotoxicity and demonstrated biocompatibility in alveolar cells. These results highlighted how the SPP microparticles could be utilized as a potential drug-delivery system for asthma patients. This is a major step forward in the public health domain on the quest to cure asthma.
 |
Figure 1 Design Expert 3D response surface showing the mutual effect of PLGA-PEG concentration and feed flow. |
 |
Figure 2 Design Expert 3D response surface showing the mutual effect of PLGA-PEG concentration and inlet temperature. |
 |
Figure 3 Design Expert 3D response surface showing the mutual effect of feed flow and inlet temperature. |
 |
Figure 4 Ramp function graph showing the optimized results for particle size. |
 |
Figure 5 Particle size distribution of SPP, as determined using the Nano-ZS (Malvern Instruments, Malvern, UK). |
 |
Figure 6 Electrostatic particle collector showing particle adhesion to the wall, indicating loss during collection. |
 |
Figure 7 Scanning electron microscopy image of SPP microspheres. |
 |
Figure 8 SPP release kinetics model fitting over time (hours) against the percentage cumulative drug release of SPP (n=3). Error bars indicate the standard deviation. |
 |
Figure 9 Plasma concentration–time profiles of the SPP formulation following intravenous administration. |
 |
Figure 10 (A) In vivo time-course of the drug concentration of salbutamol in various tissues and its SPP (B) formulation in various tissues. |
 |
Figure 11 The cell cytotoxicity results of the SPP formulation at different concentrations following exposure of the alveolar basal epithelium A549. |
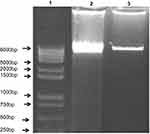 |
Figure 12 SPP-induced DNA fragmentation (apoptosis) in alveolar basal epithelium A549 (n=3) experiments was performed (lane 1: DNA ladder; lane 2: control; lane 3: SPP). |
 |
Figure 13 Stability studies of the SPP formulation showing t90=29.3 months. |
Data Availability
The scanning electron microscopy, particle size determination, in vitro release, optimization, in vivo release, in vitro cytotoxicity studies, DNA fragmentation assay and stability data used to support the findings of this study are included within the manuscript.
Acknowledgements
We thank the Deanship of Scientific Research, King Faisal University, Al-Ahsa, Saudi Arabia for funding this project (grant number: 1811018). The authors wish to thank Mr. Tameem M. Alyahian and Mr. Naif Ali Alunssif, who helped to procure chemicals, prepare the formulation, and handle the instruments. English-language editing of this manuscript was provided by Journal Prep Services.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. WHO. Global Status Report on Noncommunicable Diseases 2014. World Health Organization; 2014.
2. Network BTSSIG. British guideline on the management of asthma. Thorax. 2008;63:iv1. doi:10.1136/thx.2008.097741
3. Chatterjee A, Kubendran S, King J, DeVol R Chronic disease and wellness in america, measuring the economic burden in a changing nation. Technical Report; 2014.
4. McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health‐related quality of life burden in severe asthma. Med J Aust. 2018;209(S2):S28–S33. doi:10.5694/mja2.2018.209.issue-S2
5. Lakshminarayanan S. Role of government in public health: current scenario in India and future scope. J Family Community Med. 2011;18(1):26–30. doi:10.4103/1319-1683.78635
6. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi:10.3389/fped.2019.00246
7. Kumar A, Zhang X, Liang X-J. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol Adv. 2013;31(5):593–606. doi:10.1016/j.biotechadv.2012.10.002
8. Tripathi K. Essentials of medical pharmacology, Jaypee Brothers. Med Pub Ltd New Delhi Edn. 2003;5:93–94.
9. Harsha NS, Rani RS. Drug targeting to lungs by way of microspheres. Arch Pharm Res. 2006;29(7):598–604. doi:10.1007/BF02969272
10. Billington CK, Penn RB, Hall IP. β(2) Agonists. Handb Exp Pharmacol. 2017;237:23–40.
11. Nakpheng T, Songkarak S, Suwandecha T, Sritharadol R, Chunhachaichana C, Srichana T. Evidences for salbutamol metabolism by respiratory and liver cell lines. Drug Metab Pharmacokinet. 2017;32(2):127–134. doi:10.1016/j.dmpk.2016.11.006
12. Rowland M. Influence of route of administration on drug availability. J Pharm Sci. 1972;61(1):70–74. doi:10.1002/jps.2600610111
13. Sellers WFS. Inhaled and intravenous treatment in acute severe and life-threatening asthma. Br J Anaesth. 2012;110(2):183–90.
14. Shakeri-Zadeh A, Shiran M-B, Khoee S, Sharifi AM, Ghaznavi H, Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29(4):548–556. doi:10.1177/0885328214536940
15. Shakeri-Zadeh A, Khoee S, Shiran M-B, Sharifi AM, Khoei S. Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumor irradiation by ultrasound on CT26 tumors in BALB/c mice. J Mater Chem B. 2015;3(9):1879–1887. doi:10.1039/C4TB01708K
16. Swider E, Koshkina O, Tel J, Cruz LJ, de Vries IJM, Srinivas M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38–51. doi:10.1016/j.actbio.2018.04.006
17. Radha GV, Rani TS, Sarvani B. A review on proniosomal drug delivery system for targeted drug action. J Basic Clin Pharm. 2013;4(2):42–48. doi:10.4103/0976-0105.113609
18. Kutscher HL, Chao P, Deshmukh M, et al. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release. 2010;143(1):31–37. doi:10.1016/j.jconrel.2009.12.019
19. Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond). 2016;11(6):673–692. doi:10.2217/nnm.16.5
20. Virmani T, Gupta J. Pharmaceutical application of microspheres: an approach for the treatment of various diseases. Int J Pharm Sci Res. 2017;8:3252–3260.
21. Hong X, Wei L, Ma L, Chen Y, Liu Z, Yuan W. Novel preparation method for sustained-release PLGA microspheres using water-in-oil-in-hydrophilic-oil-in-water emulsion. Int J Nanomedicine. 2013;8:2433–2441. doi:10.2147/IJN.S45186
22. National Research Council Committee on Prudent Practices for Handling SDCL. Prudent Practices in the Laboratory: Handling and Disposal of Chemicals. National Academy Press; 1995.
23. Branchu S, Forbes RT, York P, Petrén S, Nyqvist H, Camber O. Hydroxypropyl-beta-cyclodextrin inhibits spray-drying-induced inactivation of beta-galactosidase. J Pharm Sci. 1999;88(9):905–911. doi:10.1021/js9804819
24. Freitas S, Merkle HP, Gander B. Ultrasonic atomisation into reduced pressure atmosphere—envisaging aseptic spray-drying for microencapsulation. J Control Release. 2004;95(2):185–195. doi:10.1016/j.jconrel.2003.11.005
25. Arpagaus C. A novel laboratory-scale spray dryer to produce nanoparticles. Drying Technol. 2012;30(10):1113–1121. doi:10.1080/07373937.2012.686949
26. Littringer EM, Zellnitz S, Hammernik K, Adamer V, Friedl H, Urbanetz NA. Spray drying of aqueous salbutamol sulfate solutions using the nano spray dryer B-90—the impact of process parameters on particle size. Drying Technol. 2013;31(12):1346–1353. doi:10.1080/07373937.2013.793701
27. Nagaraja SH, Al-Dhubiab BE, Tekade RK, et al. Novel preparation and effective delivery of mucoadeshive nanoparticles containing anti-diabetic drug. Indian J Pharm Edu Res. 2019;53(2):S43–S49. doi:10.5530/ijper.53.2s.47
28. Khare P, Jain S. Influence of rheology of dispersion media in the preparation of polymeric microspheres through emulsification method. AAPS PharmSciTech. 2009;10(4):1295–1300. doi:10.1208/s12249-009-9315-1
29. Wu G, Chen L, Li H, Deng C-L, Chen X-F. Comparing microspheres with different internal phase of polyelectrolyte as local drug delivery system for bone tuberculosis therapy. Biomed Res Int. 2014;2014:297808.
30. Jones AK, Bejugam NK, Nettey H, Addo R, D’Souza MJ. Spray-dried doxorubicin-albumin microparticulate systems for treatment of multidrug resistant melanomas. J Drug Target. 2011;19(6):427–433. doi:10.3109/1061186X.2010.504270
31. Zhang X, Chen F, Ni J. A novel method to prepare magnetite chitosan microspheres conjugated with methotrexate (MTX) for the controlled release of MTX as a magnetic targeting drug delivery system. Drug Deliv. 2009;16(5):280–288. doi:10.1080/10717540902989555
32. Venkatesan P, Manavalan R, Valliappan K. Preparation and evaluation of sustained release loxoprofen loaded microspheres. J Basic Clin Pharm. 2011;2(3):159–162.
33. Harsha S, Al-Khars M, Al-Hassan M, et al. Pharmacokinetics and tissue distribution of spray-dried carboplatin microspheres: lung targeting via intravenous route. Arch Pharm Res. 2014;37(3):352–360. doi:10.1007/s12272-013-0151-1
34. Fan Y, Shan-Guang W, Yu-Fang P, Feng-Lan S, Tao L. Preparation and characteristics of erythromycin microspheres for lung targeting. Drug Dev Ind Pharm. 2009;35(6):639–645. doi:10.1080/03639040802512243
35. Perreault S, Ong H, Du Souich P. Salbutamol disposition and dynamics in conscious rabbits: influence of the route of administration and of the dose. J Pharmacokinet Biopharm. 1992;20(5):461–476. doi:10.1007/BF01061466
36. Soriano-Ursúa MA, Correa-Basurto J, Romero-Huerta J, Elizalde-Solis O, Galicia-Luna LA, Trujillo-Ferrara JG. Pharmacokinetic parameters and a theoretical study about metabolism of BR-AEA (a salbutamol derivative) in rabbit. J Enzyme Inhib Med Chem. 2010;25(3):340–346. doi:10.3109/14756360903179450
37. Zhao RZ, Yuan D, Liu SJ, Chen YJ, Liu LJ, Zhao Y. Liver targeting effect of vinegar-baked Radix Bupleuri on rhein in rats. J Ethnopharmacol. 2010;132(2):421–428. doi:10.1016/j.jep.2010.08.021
38. Gupta PK, Hung CT. Quantitative evaluation of targeted drug delivery systems. Int J Pharm. 1989;56(3):217–226. doi:10.1016/0378-5173(89)90018-5
39. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
40. Zhivotosky B, Orrenius S. Assessment of apoptosis and necrosis by DNA fragmentation and morphological criteria. Current Protoc Cell Biol. 2001;Chapter 18:
41. Basnakian AG, James SJ. A rapid and sensitive assay for the detection of DNA fragmentation during early phases of apoptosis. Nucleic Acids Res. 1994;22(13):2714–2715. doi:10.1093/nar/22.13.2714
42. Tyagi LK, Kori ML, Study S. In-vivo evaluation of lornoxicam loaded ethyl cellulose microspheres. Int J Pharm Sci Drug Res. 2014;6(1):26–30.
43. Mistry A, Ravikumar P. Development and evaluation of azelaic acid based ethosomes for topical delivery for the treatment of acne. Indian J Pharm Educ. 2016;50:S232–S243. doi:10.5530/ijper
44. Patel BB, Patel JK, Chakraborty S, Shukla D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm J. 2015;23(4):352–365. doi:10.1016/j.jsps.2013.12.013
45. Nijdam JJ, Langrish TAG. The effect of surface composition on the functional properties of milk powders. J Food Eng. 2006;77(4):919–925. doi:10.1016/j.jfoodeng.2005.08.020
46. Bilancetti L, Poncelet D, Loisel C, Mazzitelli S, Nastruzzi C. A statistical approach to optimize the spray drying of starch particles: application to dry powder coating. AAPS PharmSciTech. 2010;11(3):1257–1267. doi:10.1208/s12249-010-9492-y
47. Bozdag S, Calis S, Kas HS, Ercan MT, Peksoy I, Hincal AA. In vitro evaluation and intra-articular administration of biodegradable microspheres containing naproxen sodium. J Microencapsul. 2001;18(4):443–456. doi:10.1080/02652040010018641
48. Ramaiah B, Nagaraja SH, Kapanigowda UG, Boggarapu PR, Subramanian R. High azithromycin concentration in lungs by way of bovine serum albumin microspheres as targeted drug delivery: lung targeting efficiency in albino mice. DARU J Pharm Sci. 2016;24:14. doi:10.1186/s40199-016-0153-x
49. Li X, Anton N, Arpagaus C, Belleteix F, Vandamme TF. Nanoparticles by spray drying using innovative new technology: the Büchi nano spray dryer B-90. J Control Release. 2010;147(2):304–310. doi:10.1016/j.jconrel.2010.07.113
50. Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci. 2011;73(4):355–366. doi:10.4103/0250-474X.95607
51. Harsha S. Dual drug delivery system for targeting H. pylori in the stomach: preparation and in vitro characterization of amoxicillin-loaded Carbopol® nanospheres. Int J Nanomedicine. 2012;7:4787–4796. doi:10.2147/IJN.S34312
52. Arpagaus C. Nano spray dryer B-90: literature review and applications. Büchi Inf Bull. 2011;63:8.
53. Anandharamakrishnan C, Ishwarya SP. Spray Drying Techniques for Food Ingredient Encapsulation. Wiley; 2015.
54. Miller DA, Gil M, Williams Iii ROO, Watts ABB, Miller DAA. Spray-Drying Technology Formulating Poorly Water Soluble Drugs. Vol. 3. New York: Springer; 2012:363–442.
55. De Jaeghere F, Allémann E, Doelker E, et al. pH-Dependent dissolving nano- and microparticles for improved peroral delivery of a highly lipophilic compound in dogs. Aaps J. 2001;3(1):92–99. doi:10.1208/ps030108
56. Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27(1):1–12. doi:10.1007/BF02980037
57. Skinner DV. Cambridge Textbook of Accident And Emergency Medicine. Cambridge University Press. 1997.
58. Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2–3):121–136. doi:10.1016/S0168-3659(01)00248-6
59. Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7(4):429–444. doi:10.1517/17425241003602259
60. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600–612. doi:10.1046/j.1365-2125.2003.01893.x
61. Wang H, Xu Y, Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation. Int J Mol Sci. 2014;15(3):3519–3532. doi:10.3390/ijms15033519
62. Yang F, Kang J, Yang F, Zhao Z, Kong T, Zeng Z. Preparation and evaluation of enrofloxacin microspheres and tissue distribution in rats. J Vet Sci. 2015;16(2):157–164. doi:10.4142/jvs.2015.16.2.157
63. Tawfeek HM. Evaluation of PEG and mPEG-co-(PGA-co-PDL) microparticles loaded with sodium diclofenac. Saudi Pharm J. 2013;21(4):387–397. doi:10.1016/j.jsps.2012.11.006
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
