Back to Journals » Infection and Drug Resistance » Volume 7
An economic model to compare linezolid and vancomycin for the treatment of confirmed methicillin-resistant Staphylococcus aureus nosocomial pneumonia in Germany
Authors Patel D , Michel A, Stephens J, Weber B, Petrik C, Charbonneau C
Received 31 May 2014
Accepted for publication 16 July 2014
Published 24 October 2014 Volume 2014:7 Pages 273—280
DOI https://doi.org/10.2147/IDR.S68658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Dipen A Patel,1 Andre Michel,2 Jennifer Stephens,1 Bertram Weber,3 Christian Petrik,4 Claudie Charbonneau5
1Health Economic and Outcomes Research, Pharmerit International, Bethesda, MD, USA; 2Klinikum Hanau GmbH, Hanau, Germany; 3Health Technology Assessment and Outcomes Research, 4Anti-infectives, Pfizer, Berlin, Germany; 5Pfizer International Operations, Pfizer France, Paris, France
Background: Across Europe, methicillin-resistant Staphylococcus aureus (MRSA) is considered to be the primary cause of nosocomial pneumonia (NP). In Germany alone, approximately 14,000 cases of MRSA-associated NP occur annually, which may have a significant impact on health care resource use and associated economic costs. The objective of this study was to investigate the economic impact of linezolid compared with that of vancomycin in the treatment of hospitalized patients with MRSA-confirmed NP in the German health care system.
Methods: A 4-week decision tree model incorporated published data and expert opinion on clinical parameters, resource use, and costs (2012 euros) was constructed. The base case first-line treatment duration for patients with MRSA-confirmed NP was 10 days. Treatment success (survival), failure due to lack of efficacy, serious adverse events, and mortality were possible outcomes that could impact costs. Alternate scenarios were analyzed, such as varying treatment duration (7 or 14 days) or treatment switch due to a serious adverse event/treatment failure (at day 5 or 10).
Results: The model calculated total base case inpatient costs of €15,116 for linezolid and €15,239 for vancomycin. The incremental cost-effectiveness ratio favored linezolid (versus vancomycin), with marginally lower costs (by €123) and greater efficacy (+2.7% absolute difference in the proportion of patients successfully treated for MRSA NP). Approximately 85%–87% of the total treatment costs were attributed to hospital stay (primarily in the intensive care unit). Sensitivity analysis yielded similar results.
Conclusion: The model results show that linezolid is a cost-effective alternative to vancomycin for MRSA-confirmed NP, largely attributable to the higher clinical response rate of patients treated with linezolid.
Keywords: cost-effectiveness, linezolid, vancomycin, nosocomial pneumonia, resistant, Staphylococcus aureus
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is an antibiotic-resistant bacterium that threatens individuals in community and health service settings.1,2 In Europe, MRSA is considered to be the primary cause of nosocomial pneumonia (NP).3 In Germany, a national NP surveillance system (Krankenhaus Infections Surveillance System) reported an increasing trend of infection caused by MRSA from 4.9% in 1997–19984 to 27% in 2005.5 However, MRSA infections have shown a slightly decreasing trend in recent years. Using data reported from a mix of laboratories in small (≤200 beds), medium (201–500 beds), and large (>500 beds) German hospitals, the European Antimicrobial Resistance Surveillance Network reported that infections caused by MRSA declined to 20.8% in 2010 and to 16.1% in 2011,6 possibly because of improved hygiene practices in hospitals; however, room still remains for improvement.
The European Centre for Disease Prevention and Control estimates that 4.1 million patients in the European Union acquire health care-associated infections annually, with ~37,000 associated deaths.7 Approximately 20%–30% of these infections could be prevented with the proper measures. In Germany, 500,000 nosocomial infections occur annually (14,000 MRSA-related) with 10,000–15,000 associated deaths.8 In addition to high mortality, illnesses caused by MRSA consume considerable health care resources and prolong hospitalization.9–11 Due to severe health outcomes, MRSA infections often result in longer inpatient stays and higher associated costs than those with methicillin-susceptible S. aureus infections.12–14 Cases of MRSA NP are associated with a substantial burden of illness.12 In Europe, recent surveillance data estimated over 5,000 annual MRSA-related deaths.3
Currently, approved agents for treatment of MRSA NP include vancomycin, linezolid, and telavancin. Two large, prospective, randomized, double-blind trials demonstrated that linezolid (600 mg every 12 hours) was noninferior in efficacy to fixed-dose vancomycin (1 g twice daily) for treating MRSA NP.15,16 In addition, linezolid has significantly higher survival and clinical cure rates compared with vancomycin.16 Analyzing the same data yielded similar results in patients with MRSA ventilator-associated pneumonia.17
In a recent prospective, randomized, controlled, multicenter study, linezolid showed a higher end-of-study success rate (defined as resolution of clinical signs and symptoms of pneumonia compared with baseline; improvement or lack of progression in chest imaging; and no requirement for additional antibacterial treatment) than vancomycin for the treatment of MRSA NP (57.6% versus 46.6%, linezolid versus vancomycin, respectively; P=0.042).18 In addition, no significant differences were found in 60-day mortality rates or adverse event rates between linezolid and vancomycin.
Few published pharmacoeconomic studies have evaluated treatments for MRSA NP, and none have been from a German perspective that considered varying treatment parameters and allowing for switch of therapy, if needed, to mirror real-world clinical conditions.19–21 The objective of this cost-effectiveness analysis was to evaluate the costs and efficacy of intravenous linezolid compared with intravenous vancomycin in patients with MRSA-confirmed NP.
Materials and methods
Model design
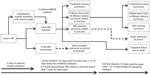
A 4-week decision tree model captured first-line and second-line therapy for patients with MRSA-confirmed NP (Figure 1). This time horizon is typically sufficient to capture intensive care unit (ICU) and general ward stay during first-line and second-line treatment, since first-line and second-line therapies are recommended to be used for up to 14 days each and a previous analysis estimated an average hospital length of stay for MRSA NP of 18–20 days.22
The model population was assumed to be hospitalized patients (aged ≥18 years) with confirmed MRSA NP and similar clinical characteristics as patients in a recent prospective, controlled, multicenter Phase IV clinical trial comparing intravenous linezolid (600 mg every 12 hours) with intravenous vancomycin (15 mg/kg every 12 hours).18
While the hospital undertook laboratory confirmation of the NP pathogen, patients with suspected MRSA NP could be treated with empiric intravenous antibiotic therapy (eg, vancomycin or linezolid) in combination with ceftazidime, imipenem, or piperacillin/tazobactam for one to 3 days; however, the base case model analysis presented here did not include this empiric treatment phase. Following confirmation of MRSA NP, patients started first-line treatment (vancomycin or linezolid) for 10 days. The total model time horizon was up to 4 weeks, which reflected the narrow window of treatment for managing an MRSA NP episode (eg, typical ICU and general ward stays during first-line and second-line treatment). This time horizon was confirmed by the expert opinions of practicing physicians based upon their own clinical experience.
This economic model used only previously published data to simulate a hypothetical patient pathway and no patients were enrolled specifically for this study. Thus, ethics approval and informed consent were not required.
Base case model outcomes and analyses
In the base case scenario, the model was primarily based on clinical trial data (Table 1)18 using linezolid and vancomycin as the main treatment comparators. The base case analysis assumed a treatment duration of 10 days, which was the average length of treatment in the clinical trial.18 Possible treatment outcomes of first-line therapy were: treatment success (defined as resolution of signs and symptoms of NP, improvement or lack of progression in chest imaging, and no additional antibacterial treatment required among survivors); failure due to lack of efficacy among survivors; drug discontinuation due to serious adverse events; and treatment failure due to death (Figure 1).
Patients who succeeded on first-line treatment would complete the assigned treatment duration (10 days for base case) and exit the model. With treatment discontinuation due to adverse events or treatment failure, 1.7 and 2.0 additional days of hospital stay were assumed, respectively, during first-line treatment compared with patients who succeeded and exited the model.22 This additional length of stay was based on a post hoc analysis of recent clinical trial data,18 where a bivariate analysis compared length of stay in patients with versus without moderate/severe adverse events and patients with first-line treatment success versus failure. These values were further validated based upon the authors’ expert opinions and their clinical experience.
After failure of first-line treatment for any reason, patients were switched to second-line treatment (eg, patients who failed first-line treatment with linezolid switched to vancomycin and vice versa). The model assumed switch on day 7 and the second-line treatment duration was considered to be 10 days. No third-line treatment was included.
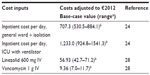
A total payer perspective was considered in the base case analysis, which was comprehensive and comprised all inpatient and outpatient health care (antibiotic and medical) costs. Data on hospital length of stay, inpatient and outpatient resource use, and associated costs and drug costs were obtained from an analysis of the recent clinical trial,18,22,23 published literature, and expert clinical opinion (Tables 1 and 2). Key resources included in the model were days of antibiotic treatment, hospital stay (general ward, isolation, and/or ICU), mechanical ventilator use, and days on intravenous therapy. The costs for a ward day and an ICU day were obtained from the database of the InEK Institute in Germany (German Diagnosis Related Group [DRG] system).24 The database includes cost calculation data for all inpatients treated in 241 German hospitals (including ten university hospitals) in 2011. To obtain ward day costs, the German DRG F49G was used. The cost calculation of the DRG F49G is based on patients with a hospital length of stay of one day (both day and night in a general ward). The ICU day costs used are the arithmetic mean day costs of 45 ICU DRGs. Costs for laboratory tests and physician visits are captured within hospital stay costs.
This study was primarily a cost-effectiveness analysis, and not a cost-utility analysis, because the treatment effect of interest was drug efficacy (ie, proportion of patients successfully treated), instead of quality-adjusted life-years or life-years. The latter two outcomes (quality-adjusted life-years and life-years) were not considered ideal for this analysis, and hence were not included as a model endpoint, because the model uses a short-term duration and the trial data used for this model suggest equal mortality between linezolid and vancomycin.18 As a result, there were negligible differences in quality-adjusted life-years and no difference in life-years between the treatment arms.
The key outcomes from this analysis, which are reported in the results section, are total costs and effectiveness proportion (those successfully treated) for the two treatments, total cost per successfully treated patient for each treatment (calculated as a ratio of total costs and total effectiveness), and incremental cost effectiveness ratio (ICER) calculated as the difference in the costs between treatments divided by the difference in the proportion of successfully treated patients receiving linezolid and vancomycin.
The following key assumptions were made in the base case model:
- every patient received treatment throughout hospitalization and all patients received intravenous therapy during their hospital stay
- in the absence of published data for second-line treatment, the clinical inputs for second-line treatment were the same as for first-line treatment20
- because the model used the 60-day mortality rates (median intent-to-treat population) reported in the clinical trial,18 which represented total mortality and included deaths from first-line and second-line treatment, mortality occurred only at the end of first-line treatment to avoid overestimation attributable to double counting; because the first-line mortality rate between linezolid and vancomycin did not differ in the clinical trial, they were considered the same in this model
- no patient dropouts occurred owing to failure or severe adverse events after first-line treatment
- patients who failed second-line treatment or who had severe adverse events were considered to have completed the full duration of therapy since no third-line therapy was available
- although the mean ICU stay was 10 days, if the treatment duration was 7 days and the patient succeeded after first-line treatment, their ICU stay was then considered to be 7 days; alternatively, if treatment duration was 14 days, then their ICU stay would be 10 days and the remaining 4 days would be in the general ward under isolation.
Sensitivity analyses
A univariate sensitivity analysis was utilized to evaluate the impact of uncertainties and robustness of the model and analyses. Model parameters that were considered key were varied individually within predefined sensitivity ranges (Tables 1 and 2), and ICERs were recorded using published sources for ranges when available. If published data were limited or unavailable, an arbitrary range was set based on the investigators’ expert judgment. A tornado diagram was used to present the results, stacking the variables in descending order of their ICER impact.25
A probabilistic sensitivity analysis was programmed in Microsoft Excel and run with all parameters varied simultaneously within their range using 10,000 second-order Monte Carlo simulations. Resource use and cost variables used a gamma distribution and probability variables used a beta distribution.
Results
Base case analysis
Under the model (Tables 1 and 2) base case settings (with no empiric treatment, a 10-day treatment duration and discontinuation or switch of therapy possible after 7 days), the total inpatient (medical plus drug) costs were €15,116 for linezolid and €15,239 for vancomycin (Figure 2). Although the drug costs were €622 higher for linezolid compared with vancomycin, medical costs associated with linezolid were €744 lower with linezolid than with vancomycin. Overall, linezolid “dominated” vancomycin because of the former’s marginally lower costs (by €123 and greater effectiveness [+2.7% absolute difference in proportion of successfully treated patients]). The estimated overall proportion of successfully treated patients (over first-line and second-line treatment) was 62.9% and 60.2% for linezolid and vancomycin, respectively, and the total cost per successfully treated patient was €24,039 (linezolid) and €25,318 (vancomycin).
The model calculated that about 85%–87% of total treatment costs were attributed to hospital stay (primarily ICU stay costs; Figure 2). General ward costs were higher for vancomycin when compared with linezolid because although the length of hospital stay with each treatment was comparable, a higher percentage of vancomycin patients failed first-line therapy and transitioned to second-line treatment, resulting in a longer overall hospital stay.
Alternative scenario analyses
The one-way sensitivity analysis identified variables with the greatest impact on the model results (Figure 3). The lowest ICER was approximately −€81,000 (ICU stay with linezolid at its lower value of 6.1), suggesting a dominant scenario for linezolid. The highest ICER was approximately €102,000 (based on clinical efficacy of vancomycin at its higher value of 52.9%) and approximately €65,000 when the ICU stay for vancomycin was at a low of 6.6 days. Since these ICER can be considered greater than the acceptable willingness to pay (WTP) threshold, vancomycin appears to be the cost-effective option under these scenarios.
Since a WTP threshold has not been established for successfully treating one patient, we tested different WTP values in the probabilistic sensitivity analysis cost-effectiveness acceptability curve (Figure 4), that shows percentages for linezolid being cost-effective compared with vancomycin at the different WTP thresholds. At a WTP of €0, linezolid had a 53.9% chance of being cost-effective and at a WTP of €120,000, its chance of cost effectiveness rises to 94.1%.
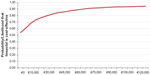  | Figure 4 Probability that linezolid is cost-effective. |
Discussion
A cost-effectiveness analysis based on a short-term, decision-tree modeling approach compared linezolid with vancomycin for treatment of MRSA NP in hospitalized adults. Linezolid was found to be a less costly and more efficacious treatment alternative compared with vancomycin in all of the tested scenarios, primarily because of the higher clinical response rate for linezolid. The higher drug acquisition cost of linezolid was offset by lower costs because of treatment failure and serious adverse events, as well as fewer days spent in hospital when accounting for combined first-line and second-line therapies. Inpatient hospital stay costs accounted for the largest proportion of overall costs.
In the majority of one-way sensitivity analysis scenarios and under varying WTP thresholds in probabilistic sensitivity analysis, linezolid was the cost-effective treatment option. However, vancomycin was found to be cost-effective for its low ICU stay and high efficacy rate, which seem to be the most sensitive variables in the sensitivity analysis and have the greatest impact on the ICER. This finding was not surprising given that the per diem cost of an ICU stay can be quite expensive in Germany and can account for the largest proportion of total treatment costs.
These results are consistent with those of other previously published economic analyses of the treatment of MRSA NP using linezolid or vancomycin.20,21 Mullins et al applied a retrospective decision analytic model to pooled efficacy data from two clinical trials and health plan hospital claims data and determined hospital costs for suspected NP in patients in the USA.21 Factoring in median daily hospital charges and mean treatment durations, total hospitalization charges were estimated at $32,636 for linezolid compared with $32,024 for vancomycin. The ICER for linezolid per life saved was $3,600; however, efficacy estimates were based on a small patient sample with MRSA NP (n=160) and examined cost-effectiveness for only first-line treatment with linezolid or vancomycin.
A German cost-effectiveness analysis20 used a decision analytic model based on published clinical data16 to show higher clinical cure rates and survival with linezolid but at small incremental costs compared with vancomycin, resulting in an acceptable ICER of cost per death avoided and cost per patient cured.
From a clinical standpoint, linezolid demonstrated higher efficacy than vancomycin for treatment of MRSA NP, with fewer patients requiring a switch to second-line therapy. The longer hospital stays associated with switching from vancomycin as first-line to second-line treatment required additional resource use, including physician and health care professional time that could have been spent treating other patients.
This economic analysis included patients who received optional empiric therapy (2 days) followed by first-line and second-line (if needed) treatment once MRSA was confirmed, with the empiric treatment costs not included in the presented scenarios. Therefore, costs were not considered in patients without MRSA infection. In clinical practice, empiric antibiotic treatment is initiated as soon as MRSA is suspected, and success of antibiotic treatment (and related costs of empiric therapy) is determined by how well MRSA is predicted and by the proportion of patients with MRSA in the treated population. Therefore, this analysis did not include costs of initial empiric therapy and harms from: not covering methicillin-susceptible S. aureus and only using MRSA coverage; choosing vancomycin, which could lead to the possibility of renal toxicity developing in patients without MRSA; and not starting empiric therapy with either drug and having a delay in starting appropriate therapy until after culture results have been confirmed. Although these clinical aspects were not addressed in this model, they are important to explore in future studies.
This study had limitations. The model base case scenario considered conditions under which the clinical trial was performed,18 which may differ in real-life clinical practice in Germany. In the absence of published data for a few of this model’s parameters, the expert opinions of the investigators were used. However, we feel that the investigators have extensive clinical expertise and experience treating MRSA NP and trust that the model will still produce relevant data based on their judgment. The model included only first-line and second-line treatments consistent with other published models,20 which is justifiable because the majority of resources were used and outcomes were witnessed within the first two lines of therapy. The study estimated direct costs only and did not include indirect costs related to lost productivity as a result of the length of hospital stay, convalescence, or early mortality. The study used 60-day mortality data,18 which were the best available trial data for this 4-week model. An argument could be made that 30-day data would be more appropriate; however, based on the survival curve, the difference between 30 and 60 days was found to be small. Quality-adjusted life-years were not used as the treatment efficacy measure in this model; rather, “proportion of successfully treated patients” was used. This is potentially limiting, since no clearly defined ICER threshold per successfully treated patient has been established. However, we believe that successful treatment is a clinically important efficacy measure for NP, and is relevant for this model.
Conclusion
This health economic model has been adapted for a German health care system and indicates that initiating treatment with linezolid is less costly and more efficacious when compared with vancomycin for MRSA NP. The cost savings were largely because of lower treatment failure rates, resulting in fewer days of hospitalization. Future analyses should test the generalizability of these results in other countries and should model the empiric treatment phase to better understand the implications of using specific treatments before MRSA confirmation on overall costs and outcomes.
Acknowledgments
The authors thank Gaurang Bhatt, formerly of Pfizer Inc., and Xin Gao, of Pharmerit International, for their work on early development of this model. This study was sponsored by Pfizer Inc. Pharmerit North America LLC received research funding from Pfizer Inc. for development of the model. Editorial support was provided by Ray Beck, Jr, of Engage Scientific Solutions and was funded by Pfizer Inc.
Disclosure
DAP and JS are employees of Pharmerit but received no funding for development of the manuscript. BW, CP, and CC are employees of Pfizer Inc. AM has received honoraria from Pfizer for speaking engagements but declares no other potential financial competing interests.
References
Goetghebeur M, Landry PA, Han D, Vicente C. Methicillin-resistant Staphylococcus aureus: a public health issue with economic consequences. Can J Infect Dis Med Microbiol. 2007;18(1):27–34. | |
Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21(8):510–515. | |
Kock R, Becker K, Cookson B, et al. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 2010;15(41):19688. | |
Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32 Suppl 2:S114–S132. | |
European Centre for Disease Prevention and Control. European Antimicrobial Resistance Surveillance Network (EARS-Net). Available from: http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net/Pages/index.aspx. Accessed April 30, 2014. | |
European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2011. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm, Sweden: European Centre for Disease Prevention and Control; 2012. Available from: http://www.ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-surveillance-europe-2011.pdf. Accessed November 8, 2013. | |
European Centre for Disease Prevention and Control. Healthcare-associated infections. Available from: http://www.ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/pages/index.aspx. Accessed November 20, 2013. | |
Gastmeier P, Geffers C. [Nosocomial infections in Germany. What are the numbers, based on the estimates for 2006?]. Dtsch Med Wochenschr. 2008;133(21):1111–1115. German. | |
Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am J Respir Crit Care Med. 1996;153(1):343–349. | |
Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94(3):281–288. | |
Rosenthal VD, Guzman S, Migone O, Safdar N. The attributable cost and length of hospital stay because of nosocomial pneumonia in intensive care units in 3 hospitals in Argentina: a prospective, matched analysis. Am J Infect Control. 2005;33(3):157–161. | |
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–174. | |
Eckmann C, Lawson W, Nathwani D, et al. Antibiotic treatment patterns across Europe in patients with complicated skin and soft-tissue infections due to meticillin-resistant Staphylococcus aureus: a plea for implementation of early switch and early discharge criteria. Int J Antimicrob Agents. 2014;44(1):56–64. | |
Reed SD, Friedman JY, Engemann JJ, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2005;26(2):175–183. | |
Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32(3):402–412. | |
Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124(5):1789–1797. | |
Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32(6):1396–1405. | |
Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–629. | |
Ott E, Bange FC, Reichardt C, et al. Costs of nosocomial pneumonia caused by meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2010;76(4):300–303. | |
De Cock E, Krueger WA, Sorensen S, et al. Cost-effectiveness of linezolid vs vancomycin in suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia in Germany. Infection. 2009;37(2):123–132. | |
Mullins CD, Kuznik A, Shaya FT, et al. Cost-effectiveness analysis of linezolid compared with vancomycin for the treatment of nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Ther. 2006;28(8):1184–1198. | |
Solem C, Niederman MS, Chastre J, et al. IN4 Healthcare resource utilization (HCRU) and costs among patients treated for nosocomial pneumonia (NP) caused by methicillin-resistant Staphylococcus aureus (MRSA): Secondary analysis of a multi-center, randomized, controlled study. Value Health. 2012;15(4):A10. | |
Niederman M, Chastre JE, Solem C, et al. Incidence of renal failure and associated economic burden among patients with nosocomial pneumonia caused by methicillin resistant Staphylococcus aureus (MRSA-NP) treated with linezolid or vancomycin: a secondary analysis of a multi-center randomized double-blind clinical trial. Paper presented at IDWeek 2012, San Diego, CA, USA, October 17–21, 2012. | |
G-DRG System 2013. [Institute for Hospital Reimbursement (InEK)]. Available from: http://www.g-drg.de/cms/G-DRG-System_2013. Accessed December 2, 2013. | |
Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012;15(6):835–842. | |
Wunderink RG, Mendelson MH, Somero MS, et al. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest. 2008;134(6):1200–1207. | |
Rello J, Nieto M, Solé J, et al. Spanish economic analysis of resource-use burden among patients with nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA-NP) treated with linezolid or vancomycin, with a special focus on patients developing renal failure. Paper presented at the 23rd European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, April 27–30, 2013. | |
IMS Health database. http://www.imshealth.com/portal/site/ims. Accessed October 2, 2013. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.