Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
An Association Between FNDC5, PGC-1α Genetic Variants and Obesity in Chinese Children: A Case-Control Study
Authors Wang Y, Zhang L, Wu L, Cao R, Peng X, Fu L
Received 3 October 2022
Accepted for publication 15 December 2022
Published 11 January 2023 Volume 2023:16 Pages 47—59
DOI https://doi.org/10.2147/DMSO.S391219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Yuanyuan Wang,1,* Li Zhang,1,* Lu Wu,2 Ruiyao Cao,1 Xingwang Peng,1 Lianguo Fu1
1Department of Children and Adolescent Health, School of Public Health, Bengbu Medical College, Bengbu, People’s Republic of China; 2Graduate School of Wannan Medical College, Wuhu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lianguo Fu, Department of Children and Adolescent Health, School of Public Health, Bengbu Medical College, Bengbu, 233000, People’s Republic of China, Tel +8613195529639, Fax +86-5523175215, Email [email protected]
Background: Fibronectin type III domain containing protein 5 (FNDC5) gene encodes irisin that regulates adipose tissue metabolism. Peroxide-proliferator-activated receptor γ coactivator 1α (PGC-1α) is a powerful promoter of mitochondrial biosynthesis and oxidative metabolism, which plays an important role in inducing heat production and energy consumption of brown fat. PGC1-α expression stimulated an increase in expression of FNDC5.
Purpose: The aims of this study were to analyze the association between FNDC5, PGC-1α genetic variants and overweight or obesity in Chinese children and adolescents.
Methods: A total of 198 children and adolescents with overweight or obesity and 198 children and adolescents with normal weight were screened according to gender and age 1:1. The healthy eating behaviors, moderate-to-vigorous physical activity time were surveyed using food frequency questionnaire and CLASS questionnaire, respectively. Genotypes of FNDC5 and PGC-1α gene were detected using SNaPshot method.
Results: GT genotype of FNDC 5 (rs16835198) increased the risk of overweight or obesity in boys (OR (95% CI): 1.68 (1.00, 2.93)) based on overdominant model; GG genotype of FNDC 5 (rs16835198) decreased the risk of overweight or obesity in girls and boys (OR (95% CI): 0.45 (0.21,0.97), 0.45 (0.24, 0.83), respectively) based on dominant model; TT genotype of FNDC 5 (rs16835198) increased the risk of overweight or obesity in girls based on recessive model (OR (95% CI): 2.46 (1.19, 5.05)), and based on the additive model (OR (95% CI): 3.82 (1.49, 9.80)). There was significant interaction between FNDC 5 (rs16835198) and PGC-1α (rs3755863, rs8192678), healthy eating behaviors, moderate-to-vigorous physical activity time, interaction between PGC-1α (rs8192678) and moderate-to-vigorous physical activity time in the occurrence of overweight or obesity in Chinese children and adolescents.
Conclusion: FNDC5 (rs16835198) played an independent or interactive role with PGC-1α (rs3755863, rs8192678), healthy eating behaviors, moderate-to-vigorous physical activity time in the occurrence of overweight or obesity in Chinese children and adolescents.
Keywords: children, adolescents, FNDC 5, PGC-1α, gene polymorphism
Introduction
Childhood obesity has become one of the most important risk factors for chronic non infectious diseases, such as hyperlipidemia, hypertension, diabetes and metabolic syndrome, and is an important indicator for predicting obesity and risks of health in adulthood.1–3 WHO reported that there were approximately 340 million children and adolescents aged 5–19 with overweight or obese worldwide.4 Liu et al reported that the prevalence of overweight or obesity among Chinese children aged 6–18 years was high with 17.62% and 29.05% in boys, 17.57% and 18.04% in girls, respectively.5 It was well known that overweight or obesity is the result of the interaction between genetic variants and unhealthy environmental factors (such as unhealthy eating behaviors, insufficient physical activity time, etc). Existing studies have shown that serum irisin and its genetic polymorphism are closely related to dyslipidemia and glucose metabolism, and may become a new target for the treatment of obesity and obesity-related metabolic diseases.6
Irisin is a new muscle/adipocytokine discovered in recent years to enhance fat burning and weight loss by increasing energy expenditure, improving glucose tolerance and inducing mitochondrial genetic expression.7 Irisin is encoded by the fibronectin type III domain containing protein 5 (FNDC5) gene.8,9 As a precursor of irisin, FNDC5 is highly expressed in the heart, brain, liver and skeletal muscle, and is essential for maintaining metabolic homeostasis.10,11 It has been reported that the levels of FNDC5 mRNA in adipose tissue and circulating irisin negatively correlated with hyperglycaemia, triglycerides, visceral adiposity and extramyocellular lipid deposition.12 Studies have shown that the wild GG genotype of FNDC5 (rs16835198) is also significantly associated with increased fasting glucose levels in diabetic subjects.13 However, other studies have shown that the TT genotype of FNDC5 (rs16835198) is associated with several metabolic parameters in circulating serum, suggesting that both TT genotype and T allele may increase obesity susceptibility.14 Remarkably, results of these study showed the SNPs of FNDC5 gene correlated with obesity and glucose-lipid metabolism, which may be because it modulate the levels of serum irisin.15,16
Peroxide-proliferator-activated receptor γ coactivator 1α (PGC-1α) is a powerful promoter of mitochondrial biosynthesis and oxidative metabolism, and can induce high expression of brown fat cell uncoupling protein 1 (UCP-1), which plays an important role in inducing heat production and energy consumption of brown fat.17 Two common SNPs of PGC-1α (rs8192678, rs3755863) were associated with obesity,18 which may lead to the development of metabolic syndrome, but the effects may be small and depend on interactions of gene-gene and gene-environment. PGC-1α genetic variants may be one of the genetic causes of obesity in different periods of human life. The effects of genetic variants and interaction between PGC-1α and FNDC5 genes on obesity in children and adolescents are still poorly understood. The purpose of the current study was to reveal the relationship between FNDC5 (rs16835198), PGC-1α (rs8192678, rs3755863), healthy eating behaviors, moderate-to-vigorous physical activity time and overweight or obesity in Chinese children and adolescents based on the case-control study.
Material and Methods
Participants
A total of 198 overweight or obese participants aged 8–14 years were screened from two nine-year schools in Bengbu, Anhui Province, and 198 participants with normal weight were matched according to gender and age 1:1. Sample size calculation formula: 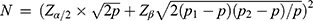 ,
, 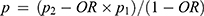 . p1: Risk allele frequency of FNDC5 (rs16835198) in normal weight group was 0.435; p2: Risk allele frequency of FNDC5 (rs16835198) in obesity group was 0.497; OR=1.33;19 Zα/2=1.96, Zβ=1.282. The minimum effective sample size for overweight or obese participants was 162. This study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Bengbu Medical College ([2015] No. 003). All parents or their guardians signed written informed consent.
. p1: Risk allele frequency of FNDC5 (rs16835198) in normal weight group was 0.435; p2: Risk allele frequency of FNDC5 (rs16835198) in obesity group was 0.497; OR=1.33;19 Zα/2=1.96, Zβ=1.282. The minimum effective sample size for overweight or obese participants was 162. This study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Bengbu Medical College ([2015] No. 003). All parents or their guardians signed written informed consent.
Measurement of Morphological Development Indicators
During the measurement of body height and weight, participants had empty stomach and bladder, barefoot, took off their hats, and only wore underwear. Height was measured by a mechanical scale with height metre to the nearest 0.1 cm. Digital body weight scale was used to measure the body weight to the nearest 0.1 kg.
Criteria for Overweight or Obesity
Body mass index (BMI) was calculated as weight (kg)/height (m)2, and overweight or obesity was determined according to BMI Classification Standards for Overweight/Obesity Screening of Chinese School-age Children and adolescents.20
Survey on Dietary Behaviors and Physical Activity Time
Food frequency questionnaire was used to investigate the frequency of healthy eating behaviors, such as breakfast, eggs, milk, fresh vegetables, fruits and nuts. The frequency of each item was assigned 7 points for 7 times per week, 5 points for 4–6 times per week, 2 points for 1–3 times per week, 0.5 points for 2 times per month, 0.25 points for 1 time per month and 0 points for never. The total score of healthy eating behaviors was divided into ≥ P75 and <P75.21
Physical activity time was investigated by CLASS questionnaire,22 and the time of moderate-to-vigorous physical activity time was divided into <60 min and ≥60 min.23
DNA Extraction and SNP Typing Detection
Medical professionals collected 3mL of fasting venous blood from the participants. DNA was extracted using the salting-out method. The national institute of environmental health sciences website (https://snpinfo.niehs.nih.gov/snpinfo/snptag.html) to determine the gene to be detected SNP. The SNP of FNDC5 was rs16835198, the SNPs of PGC-1α were rs8192678, rs3755863. Genotype was performed using the SNaPshot method developed by ABI company. Table 1 contains the sequences of each primer that was used in the qPCR.
 |
Table 1 Sequences of Primers Used for the qPCR Reaction |
Statistical Analysis
IBM SPSS 23.0 software was used for statistical analysis. The data were described using proportion and mean ± standard deviation. t-test was used to compare the difference in the measurement variables between two groups. Chi-square test was used to compare the difference in proportion (%) between groups. Logistic regression model was used to analyze the associations between FNDC5, PGC-1α genetic variants and overweight or obesity in children and adolescents based on different genetic models (overdominant, dominant, recessive and additive model), after adjusting healthy eating behaviors and moderate-to-vigorous physical activity time. Generalized multifactor dimensionality reduction (GMDR) was used to analyze the interactions between FNDC5 (rs16835198), PGC-1α (rs8192678, rs3755863), healthy eating behaviors, moderate-to-vigorous physical activity time in occurrence of overweight or obesity in children and adolescents. P<0.05 was statistically significant.
Results
Basic Information
A total of 396 primary and secondary school students aged 8–14 years were enrolled in this case-control study, including 234 boys (59.09%) and 162 girls (49.91%). The height, weight among overweight or obese children and adolescents were significantly higher than those among normal weight (P<0.05). However, the healthy eating behaviors and moderate-to-vigorous physical activity time were not significantly related to overweight or obesity in children and adolescents (P>0.05), as show in Table 2.
 |
Table 2 Association Between Healthy Eating Behaviors, Moderate-to-Vigorous Physical Activity Time and Overweight or Obesity Among Children and Adolescents |
Association Between FNDC5, PGC-1α Polymorphisms and Overweight/Obesity in Children and Adolescents Based on Different Genetic Models
The distributions of SNPs of FNDC5 and PGC-1α met the Hardy-Weinberg equilibrium. GT, TT genotypes and T allele frequencies of FNDC5 (rs16835198) in overweight or obese children were significantly higher than those in normal weight children (P<0.05). TT genotype and T allele frequencies of FNDC5 (rs16835198) in girls with overweight or obesity were higher than those in girls with normal weight (P<0.05). GT and TT genotype frequencies of FNDC5 (rs16835198) in boys with overweight or obesity were higher than those in boys with normal weight (P<0.05). Based on overdominant model, GT genotype of FNDC5 (rs16835198) increased the risk of overweight or obesity in boys (OR (95% CI):1.68 (1.00, 2.93)). Based on dominant model, GG genotype of FNDC5 (rs16835198) decreased the risk of overweight or obesity in total students, girls and boys (OR (95% CI): 0.45 (0.28,0.73), 0.45 (0.21,0.97) and 0.45 (0.24,0.83), respectively). Based on recessive model, TT genotype of FNDC5 (rs16835198) increased the risk of overweight or obesity in girls (OR (95% CI): 2.46 (1.19, 5.05)). Based on additive model, TT genotype of FNDC5 (rs16835198) increased the risk of overweight or obesity in total students and girls (OR (95% CI): 2.69 (1.48, 4.91) and 3.82 (1.49,9.80), respectively). However, PGC-1α (rs3755863, rs8192678) were not significantly associated with overweight or obesity in children and adolescents based on different genetic models, as shown in Table 3 and Figure 1.
 |
Table 3 Associations Between FNDC5, PGC-1α Genetic Variants and Overweight or Obesity in Children and Adolescents Based on Different Genetic Models |
 |
Figure 1 OR values of FNDC5 (rs16835198) and PGC-1α (rs8192678, rs3755863) associated with overweight or obesity based on different genetic models. |
Interactions of Gene (FNDC5, PGC-1α) Variants, Healthy Eating Behaviors, Physical Activity Time in Occurrence of Overweight or Obesity in Children and Adolescents
Generalized multifactor dimensionality reduction (GMDR) was used to explore the interaction of gene (FNDC5, PGC-1α) variants, healthy eating behaviors, physical activity time in the occurrence of overweight or obesity in children and adolescents. The results showed that the interaction between FNDC5 (rs16835198) and PGC-1α (rs3755863, rs8192678), healthy eating behaviors, moderate-to-vigorous physical activity time was significant in the occurrence of overweight or obesity in children and adolescents (P=0.01). There was significant interaction between PGC-1α (rs8192678) and moderate-to-vigorous physical activity time in the occurrence of overweight or obesity in children and adolescents (P=0.01). As shown in Table 4 and Figure 2.
Discussion
Obesity has been a common and complex multifactorial disease, which leads to excessive fat accumulation under the combined action of environmental and genetic factors.24,25 This study revealed the association between FNDC5 (rs16835198), PGC-1α (rs3755863, rs8192678), eating behaviors, physical activity time and overweight or obesity in children and adolescents. There was significant association between FNDC5 (rs16835198) and overweight or obesity, interaction between FNDC5 (rs16835198) and PGC-1α (rs3755863, rs8192678), healthy eating behaviors, moderate-to-vigorous physical activity time, and interaction between PGC-1α (rs8192678) and moderate-to-vigorous physical activity time in the occurrence of overweight or obesity in children and adolescents.
The results of this study showed that healthy eating behaviors, moderate-to-vigorous physical activity time were not associated with overweight or obesity in sample children and adolescents. Traub et al26 reported that skipping breakfast and increasing screen time were risk factors for overweight or obesity in children and adolescents. Raistenskis et al27 found that obese children had less physical activity than those with normal weight, and sedentary lifestyle was not conducive to the health outcomes of children and adolescents.28 Of course, some studies have shown that the relationships between eating behaviors, physical activity and obesity are not significant.29 In addition, it can also be related to the memory bias of the subjective measurement method of eating behavior and physical activity time. This may also be due to the fact that obese children and adolescents have a higher awareness of healthy eating behaviors and physical activity, and intentionally improve their unhealthy eating behaviors or increase physical activity time to loss weight.
This study found that the SNP of FNDC5 (rs16835198) was significantly associated with overweight or obesity in children and adolescents. T allele frequency of FNDC5 (rs16835198) in overweight or obese children was higher than that in normal weight children. Based on different genetic models, the results showed that TT genotype of FNDC5 (rs16835198) increased the risk of overweight or obesity in children and adolescents. Todendi et al30 reported that TT genotype of FNDC5 (rs16835198) increased risk of obesity in South Brazilian children and adolescents. The studies showed that the obese adults with TT genotype of FNDC5 (rs16835198) had higher levels of total cholesterol (TC), triacylglycerol (TG) and low-density lipoprotein (LDL-C), lower levels of high-density lipoprotein (HDL-C).14 Tang et al31 reported that the interaction between FNDC5 (rs16835198) and BMI could affect fasting insulin level in Chinese population. However, the studies also showed that TT genotype of FNDC5 (rs16835198) was associated with decreased risk of T2DM, and G allele of FNDC5 (rs16835198) was associated with elevated insulin resistance in Egyptians with no effect on renal complications.32,33 We knew that FNDC5 gene encode irisin that regulates adipose tissue metabolism, and plays an important role in energy metabolism and obesity. Studies also demonstrated that irisin is not only a myokine but also an adipokine, with important autocrine and paracrine functions.34 Luo et al3 proposed that lack of irisin was associated with a poor browning response, glucose/lipid derangement, and decreased bone mass in mice. These may be a reasonable explanation for the association between T allele, TT genotype of FNDC5 (rs16835198) and overweight or obesity.
In this study, the results of generalized multifactor dimensionality reduction (GMDR) showed that there was significant interaction between FNDC5 (rs16835198) and PGC-1α (rs3755863, rs8192678) in the occurrence of overweight or obesity in children and adolescents. Boström et al35 showed that in mouse that PGC1-α expression in muscle stimulated an increase in expression of FNDC5. The level of FNDC5 mRNA is increased in skeletal muscle in some exercise paradigms.36 As a key regulator of energy metabolism, PGC-1α can not only regulate glucose metabolism in liver and muscle, but also regulate lipid oxidation and adipocyte differentiation. Studies suggest that PGC-1α can be a potential therapeutic target for various metabolic diseases.37 Lin et al38 demonstrated that A allele of PGC-1α (rs8192678) was an independent risk factor of nonalcoholic fatty liver disease in obese children and adolescents. These may be a reasonable explanation for the interaction between FNDC5 (rs16835198) and PGC-1α (rs3755863, rs8192678) in the occurrence of overweight or obesity.
The results of this study showed that there was significant interaction between FNDC5 (rs16835198) and healthy eating behaviors, moderate-to-vigorous physical activity time in the occurrence of overweight or obesity in children and adolescents, and the TT genotype of FNDC5 (rs16835198) had the most significant effect. This shows that the children with TT genotype of FNDC5 (rs16835198) are more likely to be obese if they have insufficient healthy eating behavior or moderate to high intensity physical activity. The FNDC5 (rs16835198) variant may effect encoding of irisin, thus affecting role of irisin in energy metabolism. Studies showed that exercise increased levels of irisin in children, which may be more instrumental in mediating lipid metabolism.39
The interaction between PGC-1α (rs8192678) and moderate-to-vigorous physical activity time was significant in the occurrence of overweight or obesity in children and adolescents, and the GA genotype of PGC-1α (rs8192678) had the most significant effect. This means that the children with GA genotype of PGC-1 (rs8192678) and insufficient physical activity are more likely to be obese. In a meta-analysis of the PGC-1α (rs8192678) variant, the A allele and AA genotype were suggested to be beneficial for athletic performance.40 The study showed that the adolescents with A allele of PGC-1α (rs8192678) had higher TG than those with G allele of PGC-1α (rs8192678).41 Some studies showed that PGC-1α gene regulated the induction of muscle adaptation training.42 Endurance training can increase expression of PGC-1α mRNA.43 However, the association between PGC-1α (rs8192678) and physical performance was not reproducible in different races and populations.44 Eynon et al45 found that the GG genotype of PGC-1α (rs8192678) was conducive to the increase in aerobic capacity. Yang et al reported that the G allele of PGC-1α (rs8192678) was associated with endurance performance, while the A allele of PGC-1α (rs8192678) was associated with power performance in Chinese Han Population.46
There were also some limitations. First, the subjective methods were used to measure healthy eating behaviors and physical activity time, which might have recall bias. Second, children suffering from obesity may increase their awareness of healthy eating behaviors and physical activities, so that they can change unhealthy eating behaviors or strengthen physical exercise. Third, we only studied Chinese children and adolescents, the generalizability to other ethnic groups was limited. Finally, the exact molecular mechanism of association between FNDC5 (rs16835198) and childhood overweight or obesity remained to be elucidated.
Conclusion
This study showed that FNDC5 (rs16835198) was significantly independently associated with overweight or obesity, and played an interactive role with PGC-1α (rs3755863, rs8192678), healthy dietary behaviors and moderate-to-vigorous physical activity time in the occurrence of overweight/obesity in Chinese children.
Abbreviations
GMD, Rgeneralized multi-factor dimensionality reduction method; FNDC5, fibronectin type III domain containing protein 5 gene; SNP, single nucleotide polymorphism; PGC-1α, Peroxide-proliferator-activated receptor γ coactivator 1α; UCP-1, uncoupling protein1; Tr.BA, Training Bal.Acc; Te.BA, Testing Bal.Acc; CVC, CV Consistency.
Data Sharing Statement
All data generated or analyzed during this study are not publicly available to maintain the privacy of the individuals’ identities. The dataset supporting the conclusions is available upon request to the corresponding author.
Ethics Approval and Consent to Participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Medical Research Ethics Committee of Bengbu Medical College ([2015] NO.003). Written informed consent was obtained from parents or their guardians.
Acknowledgments
The authors would thank the students and teachers who participated in the current study. Yuanyuan Wang and Li zhang are co-first authors for ths study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This project was supported by National Natural Science Foundation of China (Grant numbers [81502823]), 512 Talent Cultivation Plan of Bengbu Medical College (Grant numbers [by51201204]).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao NN, Dong GP, Wu W, et al. Fto gene polymorphisms and obesity risk in Chinese population: a meta-analysis. World J Pediatr. 2019;15(4):382–389. doi:10.1007/s12519-019-00254-2
2. Aggarwal B, Jain V. Obesity in children: definition, etiology and approach. Indian J Pediatr. 2018;85(6):463–471. doi:10.1007/s12098-017-2531-x
3. Liu M, Cao B, Liu M, et al. High prevalence of obesity but low physical activity in children aged 9–11 years in Beijing. Diabetes Metab Syndr Obes. 2021;14:3323–3335. doi:10.2147/DMSO.S319583
4. Zempsky WT, Bhagat PK, Siddiqui K. Practical challenges-use of paracetamol in children and youth who are overweight or obese: a narrative review. Paediatr Drugs. 2020;22(5):525–534. doi:10.1007/s40272-020-00417-z
5. Liu M, Cao B, Luo Q, et al. Associations between sleep duration, wake-up time, bedtime, and abdominal obesity: results from 9559 Chinese children aged 7–18 years. Front Endocrinol. 2021;12:1330. doi:10.3389/fendo.2021.735952
6. Kitada M, Ogura Y, Suzuki T, et al. A low-protein diet exerts a beneficial effect on diabetic status and prevents diabetic nephropathy in wistar fatty rats, an animal model of type 2 diabetes and obesity. Nutr Metab. 2018;15:20. doi:10.1186/s12986-018-0255-1
7. Yin C, Hu W, Wang M, et al. Irisin as a mediator between obesity and vascular inflammation in Chinese children and adolescents. Nutr Metab Cardiovasc Dis. 2020;30(2):320–329. doi:10.1016/j.numecd.2019.09.025
8. Polyzos SA, Anastasilakis AD, Efstathiadou ZA, et al. Irisin in metabolic diseases. Endocrine. 2018;59(2):260–274. doi:10.1007/s12020-017-1476-1
9. Önalan Etem E, Diş Ö, Tektemur A, Korkmaz H, Buran Kİ. Common single nucleotide polymorphisms in the fndc5 gene and serum irisin levels in acute myocardial infarction. Anatol J Cardiol. 2021;25(8):528–535. doi:10.5152/AnatolJCardiol.2021.36214
10. Cao RY, Zheng H, Redfearn D, Yang J. Fndc5: a novel player in metabolism and metabolic syndrome. Biochimie. 2019;158:111–116. doi:10.1016/j.biochi.2019.01.001
11. Li H, Zhang Y, Wang F, et al. Effects of irisin on the differentiation and browning of human visceral white adipocytes. Am J Transl Res. 2019;11(12):7410–7421. doi:10.1152/ajpendo.00094.2016
12. Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(5):1091–1107. doi:10.1113/jphysiol.2013.264655
13. Tanisawa K, Taniguchi H, Sun X, et al. Common single nucleotide polymorphisms in the fndc5 gene are associated with glucose metabolism but do not affect serum irisin levels in Japanese men with low fitness levels. Metabolism. 2014;63(4):574–583. doi:10.1016/j.metabol.2014.01.005
14. Abdu Allah AM, Hammoudah SA, Abd El Gayed EM, El-Attar LM, Shehab-Eldin WA. Obesity and its association with irisin level among individuals with fndc5/irisin gene variants rs16835198 and rs726344. Protein Pept Lett. 2018;25(6):560–569. doi:10.2174/0929866525666180508120653
15. Al-Daghri NM, Mohammed AK, Al-Attas OS, et al. SNPs in fndc5 (Irisin) are associated with obesity and modulation of glucose and lipid metabolism in Saudi subjects. Lipids Health Dis. 2016;15:54. doi:10.1186/s12944-016-0224-5
16. Metwally M, Bayoumi A, Romero-Gomez M, et al. A polymorphism in the irisin-encoding gene (fndc5) associates with hepatic steatosis by differential miRNA binding to the 3’utr. J Hepatol. 2019;70(3):494–500. doi:10.1016/j.jhep.2018.10.021
17. Dang C, Han B, Li Q, Han R, Hao J. Up-regulation of pgc-1α in neurons protects against experimental autoimmune encephalomyelitis. FASEB j. 2019;33(12):14811–14824. doi:10.1096/fj.201901149RR
18. Csép K, Szigeti E, Vitai M, Korányi L. The ppargc1a - gly482ser polymorphism (rs8192678) and the metabolic syndrome in a central Romanian population. Acta Endocrinol. 2017;13(2):161–167. doi:10.4183/aeb.2017.161
19. Gao L, Wang L, Yang H, Pan H, Zhu H. Mc4r single nucleotide polymorphisms were associated with metabolically healthy and unhealthy obesity in Chinese northern Han populations. Int J Endocrinol. 2019;2019:1–9. doi:10.1155/2019/4328909
20. Ji CY. Report on childhood obesity in China (1)--body mass index reference for screening overweight and obesity in Chinese school-age children. Biomed Environ Sci. 2005;18(6):390–400. doi:10.1111/j.1467-842X.2005.tb00258.x
21. Yuan Y, Xie H, Sun L, et al. A novel indicator of children’s lipid accumulation product associated with impaired fasting glucose in Chinese children and adolescents. Diabetes Metab Syndr Obes. 2020;13:1653–1660. doi:10.2147/dmso.S238224
22. Huang YJ, Wong SH, Salmon J. Reliability and validity of the modified Chinese version of the children’s leisure activities study survey (class) questionnaire in assessing physical activity among Hong Kong children. Pediatr Exerc Sci. 2009;21(3):339–353. doi:10.1123/pes.21.3.339
23. Silva DAS, Chaput JP, Tremblay MS. Participation frequency in physical education classes and physical activity and sitting time in Brazilian adolescents. PLoS One. 2019;14(3):e0213785. doi:10.1371/journal.pone.0213785
24. Boaghi A, Pop RM, Vasilache SL, et al. Plasma rbp4 level in association with body composition, metabolic profile, stra6 and rbp4 gene polymorphisms in obese Romanian children. Diabetes Metab Syndr Obes. 2020;13:4643–4650. doi:10.2147/DMSO.S273146
25. Chirita-Emandi A, Serban CL, Paul C, et al. Chdh-pnpla3 gene-gene interactions predict insulin resistance in children with obesity. Diabetes Metab Syndr Obes. 2020;13:4483–4494. doi:10.2147/DMSO.S277268
26. Traub M, Lauer R, Kesztyüs T, et al. Skipping breakfast, overconsumption of soft drinks and screen media: longitudinal analysis of the combined influence on weight development in primary schoolchildren. BMC Public Health. 2018;18(1):363. doi:10.1186/s12889-018-5262-7
27. Raistenskis J, Sidlauskiene A, Strukcinskiene B, Uğur Baysal S, Buckus R. Physical activity and physical fitness in obese, overweight, and normal-weight children. Turk J Med Sci. 2016;46(2):443–450. doi:10.3906/sag-1411-119
28. Ensenyat A, Serra-Paya N, Sagarra-Romero L. Objectively measured sedentary behaviour in overweight and obese prepubertal children: challenging the school. Int J Environ Health Res. 2020;30(5):533–544. doi:10.1080/09603123.2019.1609656
29. Dent R, McPherson R, Harper ME. Factors affecting weight loss variability in obesity. Metabolism. 2020;113:154388. doi:10.1016/j.metabol.2020.154388
30. Todendi PF, Klinger EI, Geraldo ACR, et al. Genetic risk score based on fat mass and obesity-associated, transmembrane protein 18 and fibronectin type iii domain containing 5 polymorphisms is associated with anthropometric characteristics in south Brazilian children and adolescents. Br J Nutr. 2019;121(1):93–99. doi:10.1017/s0007114518002738
31. Tang S, Zhang R, Jiang F, et al. An interaction between a fndc5 variant and obesity modulates glucose metabolism in a Chinese han population. PLoS One. 2014;9(11):e109957. doi:10.1371/journal.pone.0109957
32. Khidr EG, Ali SS, Elshafey MM, Fawzy OA. Association of irisin and fndc5 rs16835198 g>t gene polymorphism with type 2 diabetes mellitus and diabetic nephropathy. An Egyptian pilot study. Gene. 2017;626:26–31. doi:10.1016/j.gene.2017.05.010
33. Staiger H, Böhm A, Scheler M, et al. Common genetic variation in the human fndc5 locus, encoding the novel muscle-derived ‘browning’ factor irisin, determines insulin sensitivity. PLoS One. 2013;8(4):e61903. doi:10.1371/journal.pone.0061903
34. Roca-Rivada A, Castelao C, Senin LL, et al. Fndc5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8(4):e60563. doi:10.1371/journal.pone.0060563
35. Boström P, Wu J, Jedrychowski MP, et al. A pgc1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi:10.1038/nature10777
36. Pekkala S, Wiklund PK, Hulmi JJ, et al. Are skeletal muscle fndc5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591(21):5393–5400. doi:10.1113/jphysiol.2013.263707
37. Wang HT, Zhang YC, Xu MY, et al. Research progresses on pgc-1α, a key energy metabolic regulator. Sheng Li Xue Bao. 2020;72(6):804–816.
38. Lin YC, Chang PF, Chang MH, Ni YH. A common variant in the peroxisome proliferator-activated receptor-γ coactivator-1α gene is associated with nonalcoholic fatty liver disease in obese children. Am J Clin Nutr. 2013;97(2):326–331. doi:10.3945/ajcn.112.046417
39. Buccoliero C, Oranger A, Colaianni G, et al. The effect of irisin on bone cells in vivo and in vitro. Biochem Soc Trans. 2021;49(1):477–484. doi:10.1042/BST20200978
40. Chen Y, Wang D, Yan P, et al. Meta-analyses of the association between the ppargc1a gly482ser polymorphism and athletic performance. Biol Sport. 2019;36(4):301–309. doi:10.5114/biolsport.2019.88752
41. Queiroz EM, Cândido AP, Castro IM, et al. Igf2, lepr, pomc, pparg, and ppargc1 gene variants are associated with obesity-related risk phenotypes in Brazilian children and adolescents. Braz J Med Biol Res. 2015;48(7):595–602. doi:10.1590/1414-431x20154155
42. Ahmetov II, Mozhayskaya IA, Flavell DM, et al. Pparalpha gene variation and physical performance in Russian athletes. Eur J Appl Physiol. 2006;97(1):103–108. doi:10.1007/s00421-006-0154-4
43. Franks PW, Barroso I, Luan J, et al. Pgc-1alpha genotype modifies the association of volitional energy expenditure with [ov0312]o2max. Med Sci Sports Exerc. 2003;35(12):1998–2004. doi:10.1249/01.Mss.0000099109.73351.81
44. He Z, Hu Y, Feng L, et al. Is there an association between ppargc1a genotypes and endurance capacity in Chinese men? Scand J Med Sci Sports. 2008;18(2):195–204. doi:10.1111/j.1600-0838.2007.00648.x
45. Eynon N, Meckel Y, Sagiv M, et al. Do ppargc1a and pparalpha polymorphisms influence sprint or endurance phenotypes? Scand J Med Sci Sports. 2010;20(1):e145–e150. doi:10.1111/j.1600-0838.2009.00930.x
46. Yang R, Jin F, Wang L, et al. Prediction and identification of power performance using polygenic models of three single-nucleotide polymorphisms in Chinese elite athletes. Front Genet. 2021;12:726552. doi:10.3389/fgene.2021.726552
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


