Back to Journals » Journal of Pain Research » Volume 11
An investigation into the efficacy of intra-articular ozone (O2–O3) injection in patients with knee osteoarthritis: a systematic review and meta-analysis
Authors Raeissadat SA, Tabibian E, Rayegani SM , Rahimi-Dehgolan S , Babaei-Ghazani A
Received 26 May 2018
Accepted for publication 2 August 2018
Published 25 October 2018 Volume 2018:11 Pages 2537—2550
DOI https://doi.org/10.2147/JPR.S175441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Seyed Ahmad Raeissadat,1 Elnaz Tabibian,2 Seyed Mansoor Rayegani,3 Shahram Rahimi-Dehgolan,3 Arash Babaei-Ghazani4
1Clinical Development Research Center of Shahid Modarres Hospital, Physical Medicine and Rehabilitation Department and Research Center, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Radiology Department, Medical Imaging Center, Advanced Diagnostic and Interventional Radiology Research Center (ADIR), Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran; 3Physical Medicine and Rehabilitation Department and Research Center, Shohada-e-Tajrish Hospital, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 4Department of Physical Medicine and Rehabilitation, Neuromusculoskeletal Research Center, Iran University of Medical Sciences, Tehran, Iran
Purpose: This study aimed to review and pool the current literature on intra-articular ozone injection in knee osteoarthritis (OA) patients.
Methods: A systematic review of three big databases was performed to identify all English-language randomized clinical trials (RCTs) that evaluated the efficacy of intra-articular ozone injection vs a control injection for knee OA sufferers, using the following two measuring tools: pain VAS and Western Ontario and McMaster Universities Arthritis Index (WOMAC).
Results: A total of 428 patients in five RCTs were included, from which 53% (n=225) were in the ozone group and 47% in the control (hyaluronic acid [HA], dextrose, and air injection) group (n=203). The mean age of the patients in both groups was 64 years. Females were the majority. All studies had at least 2 months of follow-up (F/U). Mean difference (MD) between the groups for VAS in the first month was –0.23 with a P-value of 0.71 (negative value was in favor of ozone), whereas this difference in the third and sixth months reached 1.04 and 1.31, respectively, favoring the control group. These data demonstrated that control injection had a more prolonged pain relief period. A similar trend was seen regarding WOMAC scores; pooled results showed that ozone was slightly better than the control injections during the first month (MD =–7.84 [P=0.15]), but it declined to MD=2.55 and 8.23 at 2- to 3- and 4- to 6-month F/U, respectively, again in favor of control injections. Also, adverse events occurred homogeneously in both ozone (6/150 cases, 4%) and control groups (7/129 cases, 5.4%; P-value=0.31).
Conclusion: Based on the current meta-analysis, intra-articular ozone injection efficacy was significantly superior to placebo and slightly lower to other control injections with non-significant difference. Therefore, ozone could be recommended as an efficient non-surgical treatment, durable for at least 3–6 months, in mild or moderate knee OA management.
Keywords: ozone, hyaluronic acid, knee osteoarthritis, systematic review, meta-analysis
Introduction
Knee osteoarthritis (OA) is a prevalent degenerative condition in which functional impairment is caused by mechanical and chemical stress against the joint, resulting in pain and decreased range of motion (ROM). Obese females above the age of 50 years are the most vulnerable group.1 OA is the fourth most common cause of hospital admission in 2009 in the United States with an annual cost of 42.3 billion dollars.2 The prevalence of disease highly varies among different populations: from 19.3% in some rural areas of Iran to 2.8% in the Philippines. About 19% of the Framingham adult population aged 50–60 years showed radiographic signs of knee OA.3 Patients with knee OA usually present with pain, stiffness, swelling and crepitus inside their joints, and it can occasionally result in severe limb deformity. Diagnosis is mainly based on this clinical picture, accompanied by more specific radiological features.4,5
There is no specific cure for knee OA; however, several pharmacological options are available including acetaminophen, oral selective and non-selective NSAIDs, glucosamine, chondroitin sulfate, topical products, and so on, which can alleviate pain and improve function. Although these drugs have proved to be beneficial over a short period of time, there is no evidence showing that such interventions could modify the underlying condition.6–8 Also, there are many nonpharmacological treatments including exercise, orthotics or assistive devices, and physical agent modalities.9,10 The only definite therapeutic option is total knee replacement (arthroplasty), which is reserved for the last stages.5 For nonresponder patients to conservative treatment who are simultaneously not a candidate for arthroplasty, different intra-articular injections could be considered, such as corticosteroids, saline, dextrose, hyaluronic acid (HA), autologous blood, platelet-rich plasma (PRP), Butolinium toxin, and ozone (O2–O3) injection.7,11–14
HA has been approved by the US Food and Drug Administration (FDA) in knee OA treatment since 1997.15 However, other injections are all still under evaluation in order to be accepted as the minimally invasive method of choice. Ozone is a well-known product, which has been used in many fields of dentistry and medicine worldwide. A large number of studies have confirmed the efficacy and safety of ozone therapy in the treatment of herniated lumbar disc, plantar fasciitis, meniscal injuries and other musculoskeletal disorders.16–22 In the recent decade, many orthopedic centers in Europe have begun to treat knee OA patients with intra-articular ozone insufflation.23 Ozone is now available as a solution of O2–O3.5,24
Although the precise biochemical mechanism of ozone intra-articular injection is still unclear, there is increasing evidence confirming ozone efficacy in the treatment of knee OA sufferers.13,25–33 An O2–O3 solution can suppress acute reactive mediators by downregulation of tumor necrosis factor (TNF)α and TNFR2.21,24,33,34 Ozone could also be analgesic mediated by phosphodiesterase-A2 blockage.5,16 The current data suggest that ozone leads to neither acute nor chronic toxicity.21,23 In regard to the high costs and increasing controversy, the aim of this systematic review was to pool current data in order to compare the safety and efficacy of intra-articular ozone vs other similar injections in improving pain and functional status among knee OA sufferers.
Methods
Inclusion and exclusion criteria
Two authors independently (SR-D and ET) searched the following three databases: PubMed, Cochrane Central Register of Controlled Trials and Google Scholar; also, www.clinicaltrials.gov was reviewed for any ongoing registered randomized clinical trials (RCTs). The search was performed with the help of an expert librarian and was limited to English language human studies published up to the end of February 2018. Two reviewers screened all study titles to identify relevant RCTs. After removing duplication, 183 records remained and among them only 26 studies were eligible. Any disagreement was resolved by discussion, and if no consensus was achieved, one of the senior authors (SAR or SMR) made the final decision. After reviewing all eligible full texts, 19 trials were excluded due to non-randomized design or using combination therapy; eventually seven RCTs with a total number of 544 patients were included in the qualitative review (Figure 1). From these, two studies5,34 were not eligible for meta-analysis, thereby five trials with 428 participants were considered for the quantitative review.
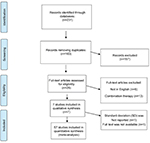  | Figure 1 Standard PRISMA study flow diagram. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis. |
Primary and secondary outcome measures
Studies reporting one of our primary outcome measures were eligible for meta-analysis: pain (based on VAS or Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] subscale of pain) and other WOMAC subscales including joint stiffness and patient’s function. Moreover, for the assessment of adverse events, post-injection flare reactions, septic arthritis and withdrawal because of complications were considered.
Data extraction
Data of all seven included studies were extracted using a standardized form, which include the following: study title, first author and publication year; study design and setting; sample size and demographics of participants; details of interventions in both groups including dose, duration, frequency and number of injections; outcome measures and times of follow-up.
Quality assessment
Two separate reviewers independently evaluated the quality of each study using the PEDro (Physiotherapy Evidence Database) score.35 This scoring system contains 11 domains (Table 1); we assessed risk of bias for each domain of the five included studies using a table. For each domain, a score of “+” or “–/?” indicates a low risk or high risk of bias, respectively. Four of our included trials achieved high quality in the assessment (score>5) and another one was the fair quality (score =5).
  | Table 1 Quality assessment of the included studies using PEDro score Notes: Numbers 1–11 follow PEDro format (https://www.pedro.org.au/wp-content/uploads/PEDro_scale.pdf); PEDro score is calculated from the following different sets of criteria: 1=eligibility criteria specified; 2=patients randomized to groups; 3=concealment of allocation; 4=groups similar at baseline; 5=patients blinded; 6=practitioners administering intervention blinded; 7=assessors blinded; 8=measurements of key outcomes obtained from >85% of the patients; 9=intention to treat analysis; 10=statistical comparisons between groups; and 11=point measures and measures of variability provided. Interpretation: scores 4–5= fair quality; scores >6= high quality; +: criterion clearly satisfied; –: criterion not clearly satisfied; ?: unclear whether criterion was satisfied. Abbreviation: PEDro: Physiotherapy Evidence Database. |
Data analysis
Along with the registration process of this systematic review in the PROSPERO database (with ID No CRD42018088858 available at: www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=88858), the data collected were extracted and analyzed in RevMan 5.3 software (Cochrane Collaboration, Oxford, UK) using calculated pooled effect sizes including RR for dichotomous variables such as presence or absence of adverse events and weighted mean difference (MD) (both raw and standardized MDs [RMD and SMD]), with 95% CI for continuous data. Regarding interpretation, SMD was considered as “large effect size” if the SMD was above 0.8, “moderate” if it was larger than 0.5, and “small” if it was greater than 0.2.36 Also, heterogeneity was assessed using I2 (a value of less than 25% was considered as low heterogeneity and a value of more than 75% as high heterogeneity). The significance level for all tests was 0.05. Based on the I2 value, random and fixed-effect models were used to pool results accordingly. Moreover, a sensitivity analysis was performed after the removal of one placebo study37 to assess the pure comparison of ozone and comparable control interventions and not placebo.
Results
All seven included articles were in English, and all had been published after 2015. Three of them belonged to the Middle East (Iran and Turkey), one Indian study, two Italian, and another trial was from Brazil. Four of them had 4–6 months of follow-up (F/U) and one study continued until 1 year. VAS and WOMAC were the main outcome measuring tools. The Indian study5 did not report exact values of mean and SD and one of the Italian trials34 had no full text available, despite contacting the authors several times. We therefore continued with five RCTs in the quantitative review.
Baseline demographics
Demographics of the trials reviewed in our review have been demonstrated in Table 2. A total of 428 patients in five RCTs were included, of whom, 225 patients (53%) were included in the ozone group and 203 patients (47%) in the control group. The number of participants in each study ranged from 42 to 141 patients. The mean age of the ozone group was 64.5 years, and 80% of whom were females, whereas in the control group the mean age was 64.4 years, and 77% of the participants were women. There was no significant difference between groups in their demographic characteristics.
Summary of studies
Four studies compared ozone vs control interventions (HA and dextrose) and another one37 assessed ozone against placebo (air injection). Except for one trial,37 almost all studies performed 3–4 weekly ozone injections. The mean value and concentration of ozone used varied between 7 and 15 cc of a solution with 15–30 µg/mL ozone. According to three studies13,26,38 that reported Kellgren–Lawrence score (KLS) for severity, ~39% of the participants in both ozone and control groups had grade III knee OA and the rest (61%) had grade II. Another two studies19,37 did not report the exact number of patients in each KLS grade.
One of the two studies not included in our meta-analysis compared three monthly intra-articular injections of 10 mL ozone with 30 µg/mL vs 40 mg methylprednisolone, in two groups (23 knee OA patients of grade I and II KLS in each group, with a mean age of 42 years); after 3 months of F/U, the authors detected 80% success rate for participants in the ozone group against 60% in the corticosteroid group.5
Another study34 excluded from the quantitative review compared the short-term efficacy of five weekly intra-articular injections of HA (n=23), oxygen ozone (n=23), and the combination of both (n=24) among 70 middle-aged knee OA patients based on the Knee injury and Osteoarthritis Outcome Score (KOOS) questionnaire and VAS for pain. Two months of F/U revealed a significant efficacy in all three groups for pain, symptoms, activities of daily living, and quality of life. They finally concluded that the scores in the combination therapy group were higher compared to the HA and ozone groups.34
Hashemi et al (2015).19 As the first included RCT, in the pain clinic of anesthesiology department, the authors divided 80 knee OA patients with grade I and II KLS (mean age =58.2 years and mean BMI =27.5 kg/m2) into two equal groups; participants of one of the two groups received three weekly intra-articular injections of 7 cc ozone at 15 µg/mL. Another group’s intervention was three injections of hypertonic dextrose (12.5% concentration) with 10-day intervals. Within 3 months of F/U, significant improvement was seen in three subscales of WOMAC and VAS for pain among both groups. The authors eventually stated that prolotherapy with dextrose and ozone injection resulted in the same pain relief and functional improvement.19
Lopes de Jesus et al (2017).26 Among the included RCTs, this is the only trial that compared ozone intra-articular injection vs placebo (air injection), which was carried out in Brazil. They divided 98 symptomatic knee OA patients with a mean age of 70 years (grade II and III KLS) into two unequal groups. Ninety-six participants finished the study; among them 61 patients received eight weekly intra-articular injection of 10 cc ozone at 20 µg/mL vs eight weekly intra-articular injections of 10 cc sterile air in another 35 participants. The authors evaluated patients’ improvement based on VAS, WOMAC, and Lequesne index at 1, 2, and 4 months after the last injections. They eventually concluded that although both groups showed relatively similar improvement in short-term F/U, ozone injection was significantly superior to placebo and was still effective by the fourth month after injections.37
Invernizzi et al (2017).38 In another RCT that had the smallest sample size (n=42), Italian researchers compared four weekly intra-articular injections of 10 cc ozone at 20 µg/mL vs four weekly HA injections. They evaluated clinical improvement in pain and other symptoms based on VAS and Oxford knee questionnaire (OKQ) at 1, 2, 3 and 8 weeks after injections. Results showed that although both ozone and HA injections were effective and comparable treatments in knee OA patients, the latter showed longer duration of pain reduction.38
Duymus et al (2016).26 The second RCT among those three trials, which compared HA vs ozone, was performed in Turkey. The authors evaluated the efficacy of four weekly intra-articular injections of 15 cc ozone at 30 µg/mL (n=35) vs a single HA injection (n=34) among moderate knee OA patients with a mean age of 59.9 and BMI =28.0. They followed participants at 1, 3, 6 and 12 months using the WOMAC index and VAS for pain. Researchers found that despite the similar efficacy of both methods within the first month, this trend in the ozone group was not so long-lasting in the following months. Finally, at the sixth month while the clinical efficacy of HA was continued, the ozone effectiveness had disappeared. Thereby, they concluded that HA was more successful than ozone injection.26
Raeissadat et al (2018).20 This RCT as the third comparative trial of HA vs ozone included 141 patients with a mean age =59.6 and BMI =27.5. The authors performed three weekly injections in both groups, 10 cc ozone at 30 µg/mL (n=67) vs 2 cc low-molecular-weight (LMW) HA (n=74). They reassessed patients after 6 months using WOMAC and VAS. The results revealed that both ozone and HA were still effective by the sixth month, and there was no significant difference between clinical effects of HA and ozone.13
Summary of pooled results
VAS pain
Pooled data on pain reduction (based on VAS) at different moments of F/U in comparison to baseline revealed some remarkable results; at the first month after injection, the MD for VAS between ozone and control groups favored the ozone group (three studies and 193 patients, MD =−0.23 [95% CI: –1.46 to 1.00], I2=87%, P=0.7), whereas at 2–3 months, based on four studies and 287 patients, this superiority evidently disappeared and reached MD =0.28 (95% CI: –1.46 to 2.02), favoring the control group. Longer F/U times at 4–6 and 12 months, with sensitivity analysis to remove Lopes de Jesus’s study effect, showed MD =1.31 (95% CI: –2.02 to 4.64) and MD =0.80 (95% CI: 0.43–1.17), respectively. Although this declining trend appears to be definite, only the last meta-analysis revealed a significant difference between two groups (P-value <0.0001). Therefore, it could be summarized that pain reduction (on VAS) in the control group was significantly more effective than that in the ozone group, at long-time F/U periods (Figure 2). Furthermore, as Figure 2E revealed, based on the only placebo study,37 comparison between the VAS MDs of intra-articular ozone vs air injection at 4-month F/U yielded a large36 SMD of 1.49 (95% CI: 1.02–1.96, P=0.00001). Therefore, regarding pain reduction, intra-articular ozone was significantly superior to placebo until 4 months of F/U.
  | Figure 2 Comparison of VAS-based pain improvement between ozone and control groups. Abbreviations: F/U, follow-up; MD, mean difference; SMD, standardized MD. |
WOMAC pain
Pain reduction on the WOMAC pain subscale also showed similar findings; at the first month, MD for WOMAC pain favored the ozone group (MD =–1.45), but at the second time moment, it reached MD =0.98 favoring the control group. After removing Lopes de Jesus’s study effect, this declining trend became more obvious and at 6-month F/U MD reached 3.28 in favor of the HA/dextrose group. Therefore, after 3 months of F/U, neither VAS nor WOMAC scales did not reveal any significant superiority to ozone injection compared with other intra-articular interventions (Figure 3).
WOMAC joint stiffness
Joint stiffness was the next subscale. At the first month, MD was –0.29 and finally it decreased to 0.17 and 0.76 at 2–3- and 4–6-month F/U, respectively. In summary, the MD for joint stiffness differences between groups showed no remarkable preference for ozone injection even at the first month (Figure 4).
WOMAC function
Patients’ reported function is often the most practical scale to be improved by various treatments. Our findings assume that in comparison to control injections, ozone can be more beneficial in improving the function at the first month. With and without considering the Lopes de Jesus placebo study, MD was –6.14 and –2.6 at the first month after injection in favor of ozone. These MDs at 2–3 and 4–6 months decreased to some values in favor of the control group (MD =3.26 and 6.7 for the whole control group and MD =13.6 and 13.45 for the control group without considering placebo study). Thus, we can conclude that comparative function improvement at longer F/U periods was in favor of control interventions (HA or dextrose), rather than ozone injection (Figure 5).
WOMAC total
Altogether, three subscales revealed a slightly higher efficacy in the ozone group compared to the control group in short-term F/U (within the first month) and significantly lower efficacy in long-term F/U (after 2–3 months and later). MD for the total WOMAC score decreased from –7.84 at the first month to MD =2.55 and 8.23 at 2- to 3- and 4- to 6-month F/U. Finally, this comparison was significantly decreased to MD =7.70 in favor of the control group (HA) at the 12th month (Figure 6). Of note, as demonstrated in Figure 6E, based on the only placebo study,37 comparison between the overall WOMAC MDs of intra-articular ozone vs air injection found a large36 SMD of 0.81 (95% CI: 0.38–1.24, P=0.0002) at the fourth month. So, in regard to overall WOMAC improvement, ozone was a more successful treatment than placebo injection.
Intra-group comparison
We finally calculated changes in VAS and three WOMAC subscales (MD) between baseline level and 3–6 months after injections in the ozone group. The mentioned time was chosen since our aim was to evaluate the long-term efficacy of intra-articular ozone injection, as well as the highest number of participants at that moment (four studies with 203 patients, with this consideration that one of them19 just reported the total WOMAC score, not each subscale’s details). Findings demonstrated that after 3–6 months of F/U, ozone injection would still have a significant effect on pain relief, based on both VAS and WOMAC (MD =3.79 with P=0.01, and MD =4.74 with P=0.04, respectively); however, patients at sixth month had no longer better ROM (MD =1.35 with P=0.12, for joint stiffness) and physical function (MD =11.17 with P=0.06). Of note, in order to compare these subscale changes, we also calculated SMD between baseline and the sixth month scores, showing that ozone injection had a large effect in pain reduction on both VAS and WOMAC (SMD =2.03 with 40% changes, and SMD =1.38 with 24% improvement, respectively), whereas it was associated with lower improvement in ROM and physical functionality (SMD =0.85 and 1.70, respectively, with 17% changes for each subscale). Ozone could therefore be considered as an intervention with at least 6 months of pain relief, among mild-to-moderate knee OA patients, who had no response to other conservative therapies (Figure 7).
Adverse events
The adverse events were also similar between two groups (Table 3). None of the seven RCTs have reported any major complications. Three studies13,37,38 that reported the exact number of complications revealed no significant difference between safety of ozone and control intra-articular injections (occurrence rate =4.0% and 5.4%, respectively, with P-value=0.31), and according to the findings of the study by Lopes de Jesus et al,37 ozone had minor reactions no more than placebo (sterile air) injections. Other two studies did not mention the number of patients with minor complications.19,26
  | Table 3 AEs after injection Note: P-value=0.31. Abbreviation: AE, adverse event. |
Discussion
This review aimed to investigate the efficacy and safety of intra-articular ozone injection through comparing with other similar interventions, such as HA or dextrose injection as the control group. The existing body of evidence had well demonstrated that ozone injection was evidently effective for short-term management (1–3 months) of mild-to-moderate knee OA patients (grade I–III KLS). But the main challenge was on longer periods of time in which different studies had declared heterogeneous results. As depicted in Figures 2–6, the present pooled data gathered from seven included RCTs confirm the short-term efficacy of intra-articular ozone, better than placebo (air)37 and corticosteroids,5 but equal to other well-documented control injections such as dextrose19 or HA.13,26,34,38 However, at 3–6 months after injections, the therapeutic efficacy of ozone decreased to a level, slightly lower than that of other injections. The declining trend of ozone efficacy, in comparison to the control group, appears to be definite in all WOMAC subscales and VAS scores. Evidently, the therapeutic trajectory of comparative ozone efficacy vs the control group has gradually changed from negative values (favoring ozone) toward positive amounts (in favor of the control group), but only two of the comparative meta-analyses revealed a significant difference between two groups at the 12th month of F/U, MD =0.80 and 7.70 with P-values <0.0001 for VAS and total WOMAC, respectively; it means that, before the mentioned time, there was no statistically significant superiority for none of these two groups (Figures 3–5); exactly in contrast to the conclusion of the study by Duymus et al, which had declared that at 3-month F/U ozone had significantly lower efficacy compared to HA, and at the sixth month after injection while the clinical efficacy of HA continued, ozone effectiveness had disappeared.26
As mentioned earlier, comparing three WOMAC subscales revealed that the maximum changes (expressed as MD between baseline and 3–6 months post-injection values) were seen for pain subscale (MD =4.74, SMD =1.38 with 24% reduction), approximately similar to VAS improvement (MD =3.79, SMD =2.03 with 40% reduction), whereas the improvement was remarkably lower for joint stiffness section (MD =1.35, SMD =0.85, 17% change) and physical function (MD =11.17, SMD =1.7, 16% change). Therefore, it could be concluded that after 3–6 months of F/U, ozone injection would still provide significant efficacy in pain relief. However, after 6 months, ozone therapy was not associated with significant improvement of ROM and functionality (Figure 7). Also, as Figures 2E and 6E reveal, it is noteworthy that intra-articular ozone efficacy on all symptoms relief was significantly higher than placebo injection up to 4 months of F/U (SMD =1.49 and 0.81 for VAS and overall WOMAC, respectively). Eventually, from a safety point of view, two methods had similar minor adverse events (occurrence rate of 4.0% vs 5.4% for ozone and control groups, respectively). In fact, ozone was as safe as other intra-articular injections (HA or dextrose) and none of them had major complications (Table 3).
Limitations
An important limitation of this meta-analysis was the large variation among different studies’ protocols and settings that will certainly result in various patients’ responsiveness to treatments. Due to this heterogeneity, we applied the random-effect model for meta-analysis, but there have still been many considerations to be emphasized. In this systematic review, females were the majority in both treatment and control groups (80% and 77%, respectively), with an overall mean age of about 64.5 years (from 57.3 to 70.5). The grade of OA among studies was another issue that should be addressed; except for Hashemi et al’s study,19 which just included patients with mild grades (I and II), the other four RCTs were grade II and III KLS and altogether ~40% of the participants had grade III knee OA. In addition to these variations in demographics and selection parameters, the included studies used certain models of ozone generator machines and HA brands with different molecular weights (MWs) and other biochemical characteristics that could lead to additional heterogeneity. For example, in one13 of three HA studies, a single injection of high MW-HA was compared to four weekly rounds of ozone injection; another two HA RCTs compared equal sessions of low MW-HA (three injections in Raeissadat et al’s study13 and four in Invernizzi et al’s study47). Furthermore, people in different countries are not the same in their anthropometric and psycho-socioeconomic factors and even the coherence to advised exercise program, which has certainly a crucial role in any treatment effectiveness and durability. Included RCTs were from India,5 Brazil,37 Italy,34,38 and the Middle East13,19,26 (Table 2); therefore, present results could probably be extrapolated to knee OA patients worldwide. Future reviews will be more comprehensive if larger high-quality RCTs would evaluate similar outcome-measuring tools among their well-selected patients, at longer periods, to better describe the long-term efficacy of this emerging treatment.
Conclusion
Based on the current meta-analysis, intra-articular ozone injection efficacy was superior to placebo and equal to other control injections; therefore, ozone could be recommended as an efficient non-surgical treatment, durable for at least 3–6 months, in mild or moderate knee OA management, particularly among middle-aged women.
Acknowledgments
This article has been extracted from the thesis written by Dr. Shahram Rahimi-Dehgolan in School of Medicine, Shahid Beheshti University of Medical Sciences (Registration No:85). Authors would like to express their deep sense of gratitude to Mrs. Mehrnaz Mehrabi for her kind contribution and support, as the coordinator of Clinical Development Research Center of Shahid Modarres Hospital. The abstract of this paper was accepted for poster presentation in the latest congress of International Society of Physical and Rehabilitation Medicine (ISPRM 2018); and was previously published in “Poster Abstracts” as a supplementary issue of Annals of Physical Medicine and Rehabilitation Journal: http://www.jisprm.org/article.asp?issn=2349-7904;year=2018;volume=1;issue=5;spage=103;epage=557;aulast=.
Disclosure
The authors report no conflicts of interest in this work.
References
Richmond J, Hunter D, Irrgang J, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92(4):990–993. | ||
Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1): S13–S19. | ||
Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. | ||
Tehrani-Banihashemi A, Davatchi F, Jamshidi AR, Faezi T, Paragomi P, Barghamdi M. Prevalence of osteoarthritis in rural areas of Iran: a WHO-ILAR COPCORD study. Int J Rheum Dis. 2014;17(4):384–388. | ||
Mishra SK, Pramanik R, Das P, et al. Role of intra-articular ozone in osteo-arthritis of knee for functional and symptomatic improvement. Ind J Phys Med Rehabilit. 2011;22(2):65–69. | ||
Maricar N, Callaghan MJ, Felson DT, O’Neill TW. Predictors of response to intra-articular steroid injections in knee osteoarthritis – a systematic review. Rheumatology. 2013;52(6):1022–1032. | ||
Khoshbin A, Leroux T, Wasserstein D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037–2048. | ||
Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2): CD002946. | ||
Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. | ||
Raeissadat SA, Rayegani SM, Sedighipour L, Bossaghzade Z, Abdollahzadeh MH, Nikray R, Mollayi F. The efficacy of electromyographic biofeedback on pain, function and maximal thickness of vastus medialis oblique muscle in patients with knee osteoarthritis: a randomized clinical trial. J Pain Res. In Press 2018. | ||
Rayegani SM, Enayati E, Raeissadat SA, Rahimi-Dehgolan S, Babaei-Ghazani A. The new role of intra-articular Botulinium toxin-A injection on pain and function of patients with knee osteoarthritis. J Lasers Med Sci. In Press Article 20148. | ||
Raeissadat SA, Babaee M, Rayegani SM, et al. An overview of platelet products (PRP, PRGF, PRF, etc.) in the Iranian studies. Future Sci. OA. 2017;3(4):FSO231. | ||
Raeissadat SA, Rayegani SM, Forogh B, Hassan Abadi P, Moridnia M, Rahimi-Dehgolan S. Intra-articular ozone or hyaluronic acid injection: which one is superior in patients with knee osteoarthritis? A 6-month randomized clinical trial. J Pain Res. 2018;11:111–117. | ||
Raeissadat SA, Rayegani SM, Ahangar AG, Abadi PH, Mojgani P, Ahangar OG. Efficacy of intra-articular injection of a newly developed plasma rich in growth factor (PRGF) versus hyaluronic acid on pain and function of patients with knee osteoarthritis: a single-blinded randomized clinical trial. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:1179544117733452. | ||
Adams ME, Atkinson MH, Lussier AJ, et al. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthr Cartil. 1995;3(4):213–225. | ||
Al-Jaziri AA, Mahmoodi SM. Painkilling effect of ozone-oxygen injection on spine and joint osteoarthritis. Saudi Med J. 2008;29(4):553–557. | ||
Babaei-Ghazani A, Karimi N, Forogh B, et al. Comparison of ultrasound-guided local ozone (O2-O3) injection vs corticosteroid injection in the treatment of chronic plantar fasciitis: a randomized clinical trial. Pain Med. 2018. | ||
Karimzadeh A, Raeissadat SA, Erfani-Fam S, Sedighipour L, Babaei-Ghazani A. Autologous whole blood versus corticosteroid local injection in treatment of plantar fasciitis: A randomized, controlled multicenter clinical trial. Clin Rheumatol. 2017;36(3):661–669. | ||
Hashemi M, Jalili P, Mennati S, Sh M, et al. The effects of prolotherapy with hypertonic dextrose versus prolozone (intraarticular ozone) in patients with knee osteoarthritis. Anesth Pain Med. 2015;5(5):e27585. | ||
Raeissadat SA, Rayegani SM, Sadeghi F, Rahimi-Dehgolan S. Comparison of ozone and lidocaine injection efficacy vs dry needling in myofascial pain syndrome patients. J Pain Res. 2018;11:1273–1279. | ||
Velio Alvaro Bocci VA. Scientific and medical aspects of ozone therapy: state of the art. Arch Med Res. 2006;37(4):425–435. | ||
Wang B, Dong GZ, Yx J, Yan CS. Case–control study on therapeutic effects of ozone and triamcinolone acetonide on the treatment of meniscal injury. Zhongguo Gu Shang. 2014;27(4):295–298. | ||
Borrelli E, Alexandre A, Iliakis E, Alexandre A, Bocci V. Disc herniation and knee arthritis as chronic oxidative stress diseases: the therapeutic role of oxygen ozone therapy. J Arthritis. 2015;2015. | ||
Manoto SL, Maepa MJ, Motaung SK. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J Biol Sci. 2018;25(4):672–679. | ||
Baltzer AWA, Moser C, Jansen SA, Krauspe R. Serum A conditioned (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr Cartil. 2009;17(2):152–160. | ||
Duymus TM, Mutlu S, Dernek B, et al. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):485–492. | ||
Curran MP. Hyaluronic acid (Supartz®): a review of its use in osteoarthritis of the knee. Drugs Aging. 2010;27(11):925–941. | ||
Jüni P, Reichenbach S, Trelle S, et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;56(11):3610–3619. | ||
Kilincoglu V, Yeter A, Servet E, Kangal M, Yildirim M. Short term results comparison of intraarticular platelet-rich plasma (PRP) and hyaluronic acid (HA) applications in early stage of knee osteoarthritis. Int J Clin Exp Med. 2015;8(10):18807–18812. | ||
Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–1501. | ||
Momen Zadeh S, Pour Farrokh M, Hashemi M, Barikani A. Comparison of intra-articular oxygen-ozone and hyaluronic acid prolotherapy on pain and disability of osteoarthritis patients. Res Med. 2014;38(1):32–36. | ||
Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86-A(3):538–545. | ||
Chen H, Yu B, Lu C, Lin Q. The effect of intra-articular injection of different concentrations of ozone on the level of TNF-α, TNF-R1, and TNF-R2 in rats with rheumatoid arthritis. Rheumatol Int. 2013;33(5):1223–1227. | ||
Giombini A, Menotti F, di Cesare A, et al. Comparison between intraarticular injection of hyaluronic acid, oxygen ozone, and the combination of both in the treatment of knee osteoarthrosis. J Biol Regul Homeost Agents. 2016;30(2):621–625. | ||
Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. | ||
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. | ||
Lopes de Jesus CC, dos Santos FC, de Jesus L, Monteiro I, Sant’ana M, Trevisani VFM. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: a randomized, double-blinded, placebo-controlled study. PLoS ONE. 2017;12(7): e0179–e0185. | ||
Invernizzi M, Stagno D, Carda S, Grana E, Picelli A. Safety of intra-articular oxygen-ozone therapy compared to intra-articular sodium hyaluronate in knee osteoarthritis: a randomized single blind pilot study. Int J Phys Med Rehabil. 2017;5:385. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






