Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Amplitude of Low-Frequency Oscillations in First-Episode Drug-Naive Patients with Major Depressive Disorder: A Resting State Functional Magnetic Resonance Imaging Study
Authors Zhang L, Wei X, Zhao J
Received 19 November 2021
Accepted for publication 3 March 2022
Published 8 March 2022 Volume 2022:18 Pages 555—561
DOI https://doi.org/10.2147/NDT.S348683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Taro Kishi
Lulu Zhang,1 Xinghua Wei,2 Jingping Zhao3
1Department of Psychiatry, Guangzhou First People’s Hospital, Guangzhou, Guangdong, People’s Republic of China; 2Department of Medical Imaging, Guangzhou First People’s Hospital, Guangzhou, Guangdong, People’s Republic of China; 3Department of Psychiatry and Mental Health Institute of the Second Xiangya Hospital, Central South University, Chinese National Clinical Research Center on Mental Disorders, Chinese National Technology Institute on Mental Disorders, Hunan Key Laboratory of Psychiatry and Mental Health, Changsha, Hunan, People’s Republic of China
Correspondence: Lulu Zhang, Department of Psychiatry, Guangzhou First People’s Hospital, 1 Panfu Road, Guangzhou, Guangdong, People’s Republic of China, Tel/Fax +86 2081046473, Email [email protected]
Objective: To observe characteristics of the amplitudes of low-frequency oscillation (LFO) in first-episode drug-naive patients with major depressive disorder (MDD).
Methods: Amplitudes of low-frequency fluctuation (ALFF) and fractional ALFF (fALFF) were computed using resting-state functional magnetic resonance imaging (rs-fMRI) data of 39 first-episode drug-naive patients with MDD and 37 healthy controls.
Results: ALFF and fALFF in the left cerebellum were significantly higher in patients with MDD compared to control group, while ALFF in the right rolandic operculum was significantly lower (all p < 0.001, AlphaSim correction).
Conclusion: Abnormal neurological activity in multiple brain regions in first-episode drug-naive patients with MDD may be involved in the neurobiological mechanisms of MDD and should be considered in future studies.
Keywords: major depressive disorder, first-episode, ALFF, fALFF, fMRI
Introduction
Major depressive disorder (MDD) is a common mental disorder;1 however, its precise underlying mechanisms remain to be elucidated.Resting-state functional magnetic resonance imaging (rs-fMRI) is a promising imaging tool for measuring intrinsic brain activity. Low-frequency oscillation (LFO) amplitudes, which can reflect the spontaneous neural activity of the whole brain, are becoming an important method to study resting state brain function changes.2 The amplitude of low frequency fluctuation (ALFF) and fractional ALFF (fALFF) have been developed to characterize LFO amplitudes. In patients with MDD, altered LFO amplitudes were observed in widely distributed areas, including the medial and inferior frontal gyrus (IFG), lateral temporal lobe, parietal and occipital cortices, and cerebellum.3–9 Although numerous neuroimaging studies have been undertaken to detect intrinsic cerebral activity in MDD using rs-fMRI, inconsistent results hinder our understanding of the exact neuropathology of MDD.3–9 The neuropathological characteristics ofMDDrequire further exploration and verification. Thus, we examined the characteristics of ALFF and fALFF in first-episode drug-naive patients with MDD to better elucidate the alterations in resting brain function in patients with MDD.
Methods
Subjects
Thirty-nine first-episode drug-naive patients with MDD and 37 healthy control (HCs) were recruited to our study. The diagnosis of MDD was made and confirmed based on the Structured Clinical Interview for DSM-IV (SCID) and a total score of the 17-item Hamilton Depression Rating Scale (HAMD17) ≥17. Patients with significant medical or neurological illness or those who were pregnant were excluded. All participants were right-handed. The study protocol was approved by the Medical Ethics Committee of Guangzhou First People’s Hospital, and we carried out the protocol in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients or their legal guardian provided written informed consent before enrollment.
Data Acquisition and Processing
MR Data Acquisition
All MRI images were acquired using a 3-Tesla MRI scanner (Siemens, Erlangen, Germany). Foam pads were used to minimize the head motion and the headphone for reduce noise during scanning. During the resting-state fMRI scan, the participants were asked to keep their eyes closed, relax, remain awake, especially not to think of anything during scanning. The high-resolution T1-weighted images were obtained with a standard magnetization prepared rapid gradient echo (MP-RAGE) sequence [repetition time (TR)/echo time (TE) 2530/2.34 ms, flip angle (FA) 7°, field of view (FOV) 256×224 mm, slice thickness 1.0 mm]. The images of 200 time points were obtained, and lasted for 500s.
MR Data Processing
Most of the functions are based on Statistical Parametric Mapping software package version 8 (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and were running under MATLAB R2013A (MathWorks, Natick, MA). The T1-weighted and rs-fMRI data preprocessing was carried out by using the DPARSF toolbox (http://www.restfmri.net/forum/DPARSF). The preprocessing steps included the removal of the first ten volumes, slice timing, head-motion correction, spatial normalization to the Montreal Neurological Institute (MNI) space, resampling to 3×3×3 mm3, spatial smoothing with a 6-mm Gaussian kernel, and linear detrending.The resulting images were registered as normative templates through spatial standardization.
ALFF and fALFF Calculation
After the preprocessing, the ALFF and fALFF were computed. ALFF was calculated using the Resting-State fMRI Data Analysis Toolkit (REST) version 1.8 (http://resting-fmri.sourceforge.net). We calculated the value for each voxel, which was further divided by the global mean value for standardization. The analysis procedure for the fALFF was performed according to the study of Zou et al.10 After applying a 0.01–0.08-Hz bandpass filter and summing the ALFF values of the fast Fourier transform calculated signal in the range of 0.01–0.08 Hz, the sum of the amplitude across 0.01–0.08 Hz was divided by that across the entire frequency range.
Results
There were no statistical differences in age (20.92±6.22 vs 19.18 ± 0.90, p = 0.093) or sex (11 men/28 women vs.18 men/19 women, p = 0.067) between MDD and HC groups. There was also no statistical difference in number of years of education between the two groups (12.69±2.61 years vs 13.08 ± 0.27 years, p = 0.360).
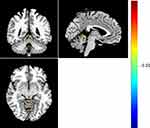
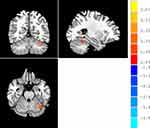
Compared with HC participants, patients with MDD had significantly higher ALFF in the left cerebellum and significantly lower ALFF in the right rolandic opercular cortex (all p < 0.001, AlphaSim correction) (Table 1) (Figures 1–3). It was also found that fALFFin the left cerebellum was significantly higher in patients with MDD compared to that in HCs (p < 0.001, AlphaSim correction) (Table 2) (Figure 4). Spearman rank correlation analysis showed that neither ALFF nor fALFF values of brain regions in patients with MDD significantly correlated with the total HAMD17 scores (all p> 0.05).
 |
Table 1 Differences in ALFF Between HC and MDD Groups |
 |
Table 2 Differences in fALFF Between HC and MDD Groups |
 |
Figure 1 Warm colors indicate higher ALFF in patients with MDD compared with HC, and cold colors indicate the opposite. |
Discussion
In this study, we found that both ALFF and fALFF were higher in the left cerebellum in patients with MDD than in HC participants, which is in accordance with previous reports that an imbalance in LFOamplitudesis detected in patients with MDD.3–6 It was found that all five primary emotions (happiness, anger, disgust, fear, and sadness) led to activations that were located almost exclusively in the posterior lobe of the cerebellum (lobules VI–IX).11 The cerebellar lobule VIIb, which is a subregion functionally connected to the orbitofrontal, dorsolateral, and medial prefrontal cortex regions, supports executive control. Hariri’s group provided novel evidence that the structural integrity of the cerebellum and cerebello-thalamo-cortical circuit (CTCC) may be a transdiagnostic biomarker of risk for psychopathology.3 Some neuroimaging evidence also shows the involvement of the cerebellum in the pathological process of psychiatric diseases. It was found that there was a significant increase in fALFF in the left CrusIand left cerebellar lobule VI in patients with depression, which suggests that increased cerebellar activity in the resting state may be a state phenomenon of depression.4 Depping et al also found that patients with MDD had higher resting regional cerebellar blood flow in bilateral area VIIa and VIIIb relative to HC participants, and the changes blood flow in cerebellar in MDD were not accompanied by significant cerebellar volume differences.5 Moreover, left cerebellar area VIIa perfusion in MDD was associated with depressive psychopathology.5 In recent studies, it was reported that fALFF was sensitive to changes in post-stroke depressive symptom severity and implicated frontostriatal and cerebellar regions.6
However, there are some contradictory findings. Liu et al found significantly decreased ALFF in the cerebellum of patients with MDD, and suggested that decreased cerebellar ALFF in MDD may indicate suppressed capability to perform proper adjustments of the internal milieu in response to environmental demands as a consequence of disruptions in the cerebro-cerebellar interaction.7 These inconsistencies are thought to be partly due to differences in experimental designs and procedures, heterogeneous clinical populations, and varying inclusion and exclusion criteria.12 Therefore, we recruited first-episode drug-naive patients with MDD in this study to reduce the impact of chronic duration and antidepressant history on brain function. Our findings suggest increased cerebellar resting activity in patients with MDD, and potentially this finding may be related to a disease state phenomenon and abnormality of emotion processing.
Furthermore, we found lower ALFF in the right rolandic operculum in patients with MDD, but no significant difference in fALFFwas found between the two groups in the right rolandic operculum. This result may be related to the small sample size and frequency bands. The underlying structural foundation of frequency-dependent changes was unclear, which will be explored in future studies by performing a combined analysis of multimodal imaging data, and it was found that the cortical volume of the left rolandic operculum positively correlated with the SCL-90-subscale for depressive symptoms.13 Some studies suggest that the bilateral rolandic operculum processes integrated exteroceptive-interoceptive signals that are necessary for bodily self-consciousness.14 The rolandic operculum is thought to be part of the primary gustatory cortex and plays a role in sensory-auditory integration, which is essential for speech production.15 The function of the rolandic operculum is related to sensory perception and cognition, but the mechanism remains unclear, and its role in MDD requires further study. Zhang et al used three types of whole-brain rs-fMRI measures to discriminate MDD and subclinical depression (SD).16 The rs-fMRI measures included (1) regional functional activity features, including ALFF, fALFF, and ReHo; (2) RSFC features; and (3) global network topological features, including node degree (Deg), betweenness centrality (BC), and nodal efficiency (Eff). The most discriminative features in the MDD group vs healthy controls (HCs) were located in the default mode network (DMN) and visual network (VN).16 A recent meta-analysis using fMRI with ALFF and fALFFinpatients with MDD suggested that the decreased intrinsic activity of the cerebellum might be a specific biomarker for current MDD.8 Another meta-analysis explored altered resting-state functional activity in medication-naïve patients with MDD and compared the results with those in healthy controls.9 Patients with MDD had hyperactivity in the left parahippocampal gyrus, left supplementary motor area, left amygdala, left hippocampus, and left middle frontal gyrus, as well as in the left lingual gyrus, left middle occipital gyrus, right cuneus cortex, right MFG, and left cerebellum.9
The present study had some limitations. First, the sample size was relatively small. Second, we only used HAMD17 to assess the depressive symptoms of patients and lacked a comprehensive evaluation of clinical symptoms, such as cognitive deficits. Third, the analytical method was relatively simple. It only found changes in some independent brain regions and did not correlate these changes. However, the causal relationship between changes in function and depression remains unclear. In the future, functional connection and complex network analyses can be combined to identify neural circuits related to MDD. Fourth, while the ALFF and fALFF of the rs-fMRI signal have been proven to be related to spontaneous neural activity, but we could not completely eliminate the effects of physiological noises, such as respiratory and heart rhythm, in our study. Future studies using new scan sequences with a short TR would help avoid the interference of physiological noise. Moreover, monitoring the heartbeat and breathing during scanning will help clarify their impact on brain activity.
In summary, our findings suggest that abnormal neurological activity in multiple brain regions in first-episode drug-naive patients with MDD may be involved in the neurobiological mechanisms of MDD, particularly the increased ALFF and fALFF in the left cerebellum. This suggests that abnormal spontaneous brain activity in the resting state may become a neurobiological substrate of MDD and should be considered in future studies.
Funding
This study was supported by grants from the Natural Science Foundation of Guangdong Province (Grant No. 2017A030313809) and the Science and Technology Program of Guangzhou (Grant No. 202002030262).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
2. Wang L, Kong Q, Li K, et al. Frequency-dependent changes in amplitude of low-frequency oscillations in depression: a resting-state fMRI study. Neurosci Lett. 2016;614:105–111. doi:10.1016/j.neulet.2016.01.012
3. Hariri AR. The emerging importance of the cerebellum in broad risk for psychopathology. Neuron. 2019;102(1):17–20. doi:10.1016/j.neuron.2019.02.031
4. Guo W, Liu F, Liu J, et al. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:13–18. doi:10.1016/j.pnpbp.2013.06.009
5. Depping MS, Wolf ND, Vasic N, et al. Aberrant resting-state cerebellar blood flow in major depression. J Affect Disord. 2018;226:227–231. doi:10.1016/j.jad.2017.09.028
6. Goodin P, Lamp G, Vidyasagar R, et al. Correlated resting-state functional MRI activity of frontostriatal, thalamic, temporal, and cerebellar brain regions differentiates stroke survivors with high compared to low depressive symptom scores. Neural Plast. 2019;2019:2357107. doi:10.1155/2019/2357107
7. Liu J, Ren L, Womer FY, et al. Alterations in amplitude of low frequency fluctuation in treatment‐naïve major depressive disorder measured with resting‐state fMRI. Hum Brain Mapp. 2014;35(10):4979–4988. doi:10.1002/hbm.22526
8. Zhou M, Hu X, Lu L, et al. Intrinsic cerebral activity at resting state in adults with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:157–164. doi:10.1016/j.pnpbp.2017.02.001
9. Ma X, Liu J, Liu T, et al. Altered resting-state functional activity in medication-naïve patients with first-episode major depression disorder vs. healthy control: a quantitative meta-analysis. Front Behav Neurosci. 2019;13:89. doi:10.3389/fnbeh.2019.00089
10. Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi:10.1016/j.jneumeth.2008.04.012
11. Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. NeuroImage. 2012;61(4):805–811. doi:10.1016/j.neuroimage.2012.03.044
12. Müller VI, Cieslik EC, Serbanescu I, et al. Altered brain activity in unipolar depression revisited meta analyses of neuroimaging studies. JAMA Psychiatry. 2017;74(1):47–55. doi:10.1001/jamapsychiatry.2016.2783
13. Besteher B, Gaser C, Langbein K, et al. Effects of subclinical depression, anxiety and somatization on brain structure in healthy subjects. J Affect Disord. 2017;215:111–117. doi:10.1016/j.jad.2017.03.039
14. Zhao Y, Lambon Ralph MA, Halai AD. Relating resting-state hemodynamic changes to the variable language profiles in post-stroke aphasia. Neuroimage Clin. 2018;20:611–619. doi:10.1016/j.nicl.2018.08.022
15. Mălîia MD, Donos C, Barborica A, et al. Functional mapping and effective connectivity of the human operculum. Cortex. 2018;109:303–321. doi:10.1016/j.cortex.2018.08.024
16. Zhang B, Liu S, Liu X, et al. Discriminating subclinical depression from major depression using multi-scale brain functional features: a radiomics analysis. J Affect Disord. 2022;297:542–552. doi:10.1016/j.jad.2021.10.122
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.