Back to Journals » Journal of Inflammation Research » Volume 15
AMPK/SIRT1 Deficiency Drives Adjuvant-Induced Arthritis in Rats by Promoting Glycolysis-Mediated Monocytes Inflammatory Polarization
Authors Wang DD, He CY, Wu YJ , Xu L, Shi C, Olatunji OJ , Zuo J , Ji CL
Received 14 June 2022
Accepted for publication 11 August 2022
Published 15 August 2022 Volume 2022:15 Pages 4663—4675
DOI https://doi.org/10.2147/JIR.S378090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Dan-Dan Wang,1,2,* Chi-Yi He,3,* Yi-Jin Wu,1,2 Liang Xu,4 Chao Shi,1 Opeyemi Joshua Olatunji,5 Jian Zuo,1,6,7 Cong-Lan Ji8
1Xin’an Medicine Research Center, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu, 241000, People’s Republic of China; 2The Key Laboratory of Anti-Inflammatory and Immune Medicine, Ministry of Education, Institute of Clinical Pharmacology, Anhui Medical University, Hefei, 230032, People’s Republic of China; 3Department of Gastroenterology, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu, 241000, People’s Republic of China; 4Department of Rheumatology, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu, 241000, People’s Republic of China; 5Traditional Thai Medical Research and Innovation Center, Faculty of Traditional Thai Medicine, Prince of Songkla University, Hat Yai, 90110, Thailand; 6Key Laboratory of Non-coding RNA Transformation Research of Anhui Higher Education Institution, Wannan Medical College, Wuhu, 241000, People’s Republic of China; 7Anhui Provincial Engineering Laboratory for Screening and Re-evaluation of Active Compounds of Herbal Medicines in Southern Anhui, Wuhu, 241000, People’s Republic of China; 8School of Pharmacy, Anhui College of Traditional Chinese Medicine, Wuhu, 241000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cong-Lan Ji; Jian Zuo, Email [email protected]; [email protected]
Background: Exact roles of many metabolic regulators in rheumatoid arthritis (RA) are to be clarified. This study aimed to further characterize the impacts of silent information regulator 1 (SIRT1) status changes on this disease.
Methods: Fluctuation pattern of SIRT1 expression in adjuvant-induced arthritis (AIA) rats was monitored using periodically collected white blood cells. Another bath of AIA rats were treated by SIRT1 agonist resveratrol. Blood from these rats was used to separate monocytes and plasma, which were subjected to polymerase chain reaction (PCR), enzyme linked immunosorbent assay (ELISA), and biochemical analyses. Clinical implication of SIRT1 activation was verified by treating AIA rat monocytes with SIRT1 agonist and overexpression vector in vitro.
Results: SIRT1 deficiency occurred in AIA rats, which was accompanied with down-regulation of interleukin 10 (IL-10) and arginase-1 (ARG-1). Resveratrol eased oxidative stress and increased IL-10 production in vivo. Results of ELISA analysis demonstrated that resveratrol attenuated AIA severity in rats. Furthermore, it restored the altered levels of triglyceride, lactate and pyruvate in blood. Resveratrol promoted IL-10 production, and suppressed glycolysis of AIA monocytes cultured in vitro. SIRT1 overexpression similarly reshaped differentiation profile of AIA monocytes, evidenced by changes in metabolism indicators, IL-10 production and AMP-activated protein kinase (AMPK) pathway status. Although overexpressing SIRT1 in normal cells did not affect glycolysis significantly, it attenuated AMPK antagonist-caused abnormality.
Conclusion: SIRT1 deficiency is implicated in AIA-related immune abnormality and metabolism alteration. Activating this signaling with resveratrol would impair the inflammatory polarization of monocytes, and consequently ease the severity of RA.
Keywords: inflammation, metabolic reprogramming, monocytes, resveratrol, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) affects approximately 1% of the global population. Given its profound negative impacts on labor capability and life expectancy, it becomes a major public health concern.1 Systematic inflammation is the leading factor for both extra-articular and articular manifestations.2 As such, anti-inflammation is a priority for RA therapies, and a better understanding about the mechanism of how RA-related inflammation initiates and progresses is very important. In addition to immune cells, many other possible variables are also involved. Among them, the role of metabolism disruption has been basically confirmed.3 Although the exact mechanism is still largely a mystery, it is undoubted that materials and energy generated by metabolism are required by immune functions.4,5 Hence, it is not surprising to find that cell differentiation is always synchronized with metabolic reprogramming, and many metabolic regulators have been identified as the keys deciding phenotypes of immune cells.6,7 Monitoring metabolic changes would benefit a better understanding of RA pathology and inspire the development of novel therapies.
Previously, we reported that intensified inflammation in rheumatic subjects was accompanied by up-regulation of glycolytic enzymes, and triosephosphate isomerase 1 (TPI1) was identified as a new RA-related inflammatory indicator.8 Meanwhile, it revealed that silent information regulator 1 (SIRT1) expression deficiency was another hallmark of RA-related inflammation based on data collected from collagen-induced arthritis (CIA), which conforms to the changes observed in RA patients.8 Being an intersection of metabolism and immune regulatory signaling networks, SIRT1 promotes oxidative phosphorylation and M2 polarization of monocytes and macrophages.9,10 These orchestrated functions endow SIRT1 with notable anti-inflammatory capacity. SIRT1 therefore functions as an indispensable component required by immune homeostasis. Under the aforementioned context, SIRT1 expression deficiency in rheumatic subjects is understandable when inflammation is evident and glycolysis is accelerated. In fact, the involvement of SIRT1 deficiency in RA has been well documented, and SIRT1 activation is believed to be a potent anti-rheumatic strategy.11 However, there are still several pending issues to be clarified, which limit the potentials of SIRT1-targeting therapies. The fundamental question is whether SIRT1 deficiency is a common phenomenon in rheumatic subjects. Many studies have challenged the idea that SIRT1 expression is always insufficient under RA circumstances, and we also noticed that SIRT1 could be overexpressed in CIA rats at least at certain stages.8
Theoretically, SIRT1 expression deficiency would expedite RA progression by promoting inflammation. As adjuvant-induced arthritis (AIA) is typically induced by Mycobacterium strains, the involvement of innate immunity is significant, and its inflammatory manifestations are severe.12 Comparatively, CIA is mainly characterized by high titer of collagen-reactive antibody and cartilage degeneration.13 According to the experimental purpose, we used AIA models in the current study. Active RA is always accompanied with the aggravated inflammation and accelerated glycolysis. Monocytes and macrophages are the main cells utilizing glucose by means of glycolysis under RA conditions.14 Because SIRT1 is a negative regulator of glycolysis and M1 polarization, we believed that monitoring changes in monocytes/macrophages will shed more light on elucidating the role of SIRT1 in RA. Considering the fact that circulating monocytes have more profound impacts on systematic inflammation than tissue-resident macrophages, we selectively investigated monocytes in AIA rats. To further decipher the clinical implication of SIRT1 regulation on RA therapies, AIA rats and their monocytes were treated by SIRT1 agonist and/or overexpression vector.
Materials and Methods
Chemicals and Reagents
Bacillus Calmette-Guérin (BCG) and incomplete Freund's adjuvant (IFA) were purchased from Rebio Scientific (Shanghai, China). ReverAid First Strand cDNA Synthesis kits, Chemiluminescent HRP substrate kit and all the antibodies used were supplied by Thermo Fisher Scientific (Rockford, IL, USA). Enzyme linked immunosorbent assay (ELISA) kits for interleukin (IL)-10, IL-6, IL-1β, rheumatoid factor (RF), C-reactive protein (CRP) and anti-cyclic citrullinated peptide antibody (anti-CCP) tests and colorimetric quantification kits for malonaldehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), NADP(H) oxidase (NOX), free fatty acid (FFA), lactate, pyruvate, glucose, citric acid, ATP, and triglyceride tests were procured from Multi-Science (Hangzhou, Zhejiang, China) and Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China), respectively. Monocytes separation kits were obtained from Solarbio (Beijing, China). AMP-activated protein kinase (AMPK) agonist 5-Aminoimidazole-4-carboxamide1-β-D-ribofuranoside (AICAR), SIRT1 agonist resveratrol (RSV) and AMPK antagonist compound C (CC) were provided by Yuanye Biotech (Shanghai, China), Sigma-Aldrich (St Louis, MO, USA) and ApexBio (Houston, TX, USA), respectively. Universal qPCR Master Mix and TRNzol Universal total RNA extraction reagent were brought from New England Biolabs (Ipswich, MA, USA) and Tiangen Biotech (Beijing, China), respectively. Gene-specific primers were synthesized by Sangon Biotech (Shanghai, China), and their sequences are shown in Supplementary S1. Lipofectamine 2000 transfection reagent was supplied by Invitrogen (Carlsbad, CA, USA). SIRT1 overexpression plasmid was constructed by General Biol (Chuzhou, Anhui, China) based on pcDNA3.1-EGFP vector. All the reagents used for cell culture were provided by either HyClone (Logan, UT, USA) or Beyotime Biotech (Shanghai, China). Ultrapure water was prepared by a Milli-Q purification system (Millipore, Bedford, MA, USA).
Periodical Assessment of SIRT1 Expression in AIA Rats
All the following animal experiment procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (US National Research Council, 2011) and approved by the Ethics Committee of Wannan Medical College (LLSC-2020-138). To avoid interference brought by gender differences, all the following experiments were conducted on male Wistar rats (supplied by Tianqin Biotechnology, Changsha, Hunan, China). The animals were kept in a specific pathogen free (SPF) laboratory with standard feeding conditions. After 7 days of acclimatization, AIA was induced in the rats according to our previously reported procedures.15 BCG suspension and IFA were grinded in a glass mortar to prepare homogeneous complete Freund’s adjuvant (CFA), and 0.1 mL of this emulsion was intracutaneously injected into the plantar of hind paws at day 0. After 7 days, a booster injection was performed. Secondary inflammation occurred about 1 week later, indicating the successful development of AIA. Body weight and arthritis scores were recorded periodically throughout the observational duration. Arthritis score for each paw was assessed independently according to a previously defined quantification criterion, and their sum was used to evaluate the severity of AIA: 1, slight edema and limited erythema; 2, light edema and erythema; 3, obvious edema and significant erythema; 4, severe edema and extensive erythema.15 Anticoagulation blood was sampled on days 1, 3, 15, 17, 25 and 35 from fossa orbitalis vein of rats, which was subsequently subjected to erythrocyte lysis. White blood cells (WBC) obtained from centrifugation were used in the analysis of gene expression based on polymerase chain reaction (PCR) method. When the rats were sacrificed on day 41, anticoagulation blood was withdrawn from abdominal aorta and used for monocyte preparation with a gradient centrifugation separation kit. NOX activity and FFA concentration in blood plasma were detected by using corresponding kits. A portion of monocytes were used in immunoblotting assays to assess the expression of SIRT1 protein and the remaining cells were used in the experiment in vitro. Hind paws, perirenal fat pad and thymus were dissected from the body and weighed.
Treatment of AIA Rats with RSV
We used RSV, a well-recognized SIRT1 agonist, to treat the rats, and performed the following analyses.16 Induction procedures of AIA were the same as stated above. In this experiment, the rats were divided into three groups (with 6 rats each): normal healthy controls (normal), AIA models (AIA) and RSV-treated AIA rats (RSV). Since the first CFA injection, RSV group was continuously treated with RSV. Above observation found that SIRT1 expression deficiency in AIA rats became not obvious since day 25, and totally disappeared till day 35. Hence, RSV treatment duration was designed as 30 days. RSV showed good anti-rheumatic effects in vivo using the doses in the range of 10–50 mg/kg/d.17,18 To ensure satisfying therapeutic effects, the dose of 50 mg/kg/d was chosen here. Due to its poor oral bioavailability, RSV was given via the intraperitoneal injection. Healthy and model control rats were treated with normal saline. On days 8, 16 and 26, peripheral blood samples were collected. The plasma was used for oxidative stress-related indicators (MDA, SOD and GSH) and IL-10 assessment. When the rats were sacrificed on day 30, hind paws including ankle joints, spleen and perirenal fat pad of rats were cut off and weighed. Expression of pyruvate kinase isozyme type M1 (PKM1) in liver was assessed by PCR method. Ankle joint specimens were fixed in 10% formalin solution, and subjected to HE staining-based histological examination with previously detailed procedures.15 Blood samples were collected into anticoagulant tubes. Inflammatory cytokines, RA-related diagnostic indicators and representative metabolites in plasma were quantified by corresponding kits.
In vitro Treatment of Monocytes
We first investigated the direct effects of RSV on AIA monocytes. The cells that were freshly isolated from normal healthy and AIA rats were seeded in 6-well plates at appropriate densities. After the adaptive culture for 4 h, half of AIA monocytes were stimulated with RSV (25 μM) for 12 h. The medium was collected for IL-10 content quantification, and the cells were harvested for PCR analysis. To confirm SIRT1 activation-caused consequences, we overexpressed SIRT1 in AIA monocytes instead of RSV stimulus in the following experiments. The cells seeded in 6-well plates were allowed to grow until 70–90% confluence, and then cultured by transfection mixture, comprising DMEM medium, Lipofectamine 2000 and SIRT1 overexpression plasmid. The medium and cells were collected for ELSIA and PCR analyses. Furthermore, pyruvate and lactate in the medium together with ATP in the cells were quantified by using corresponding kits. Some other cells were used in immunoblotting assays. Next, AIA monocytes were challenged with AMPK antagonist AICAR (250 μM) or agonist CC (3 μM) to assess whether AMPK regulation can cause similar outcomes observed above. Normal monocytes and untreated AIA monocytes were used as controls. After being stimulated for 12 h in vitro, the cells were collected for immunoblotting analysis. Subsequently, some normal monocytes were subjected to CC stimulus, SIRT1 overexpression and the combined treatment. The cells were harvested for immunoblotting analysis, and levels of pyruvate and citric acids in the medium were determined by colorimetric kits.
RT-qPCR Analysis
Tissues or cells were lysed in Trizol reagent on ice. After extraction with chloroform, the aqueous phase was collected and spiked into the same volume of isopropanol. The mixture was vortex and centrifugated. The precipitate obtained was purified by washing with 75% ethanol, and dissolved in DEPC water. RNA within this solution was quantified and used as templates in the PCR step to synthesize cDNA using ReverAid First Strand cDNA Synthesis kits. The manufacturer recommended reaction system and heating program were adopted. The cDNA obtained was subjected to quantitative PCR procedures on a 7500 Real-Time PCR system (Thermo Fisher Scientific, Rockford, IL, USA). Relative expression of genes was assessed based on the cycle threshold (CT) values and β-ACTIN was used as the internal reference.
Immunoblotting Assays
Cells were rinsed with pre-chilled phosphate-buffered saline (PBS) and lysed in RIPA buffer mixture (containing phenylmethanesulfonyl fluoride and phosphatase inhibitors) on ice. After the centrifugation at 12,000 rpm for 10 min under 4°C, the supernatant was collected. The protein contents were determined by bicinchoninic acid kits. The remaining samples were spiked into protein-loading buffer and denatured by boiling. Samples containing equal amount of proteins were loaded on sodium dodecyl sulfate polyacrylamide gels, and separated by electrophoresis. The separated proteins were subsequently transferred onto poly(vinylidene fluoride) (PVDF) membranes. The gels were discarded. According to the position indicator of pre-stained protein marker and molecular weight of each protein, the PVDF membranes were cut into pieces, which were incubated with 5% skim milk, corresponding primary antibodies and secondary antibodies in sequence separately. The signals were finally visualized with the aid of ECL reagent and recorded with an Amersham Imager 600 (GE Healthcare). Semi-quantification results were calculated using Image J (version 1.52a, NIH, Bethesda, MD, USA) using β-ACTIN as the internal reference.
Statistical Analysis
The data were presented as mean ± standard deviation. Statistical differences among groups were analyzed by GraphPad Prism 8.0 (GraphPad Software, Cary, NC, USA) using two-way analysis of variance coupled with Tukey post hoc test.
Results
SIRT1 Deficiency in AIA Rats Was Reinforced When Inflammation Was Intensified
The progress pattern of AIA in rats is displayed in Figure 1A. It was revealed that secondary inflammation was obviously evident since day 16 and reached the peak about day 20. Similar to previous observation, AIA caused obvious loss in body weight gain (Figure 1A) and fat storage (Figure 1B).15,19 The paw swelling in AIA rats was still vivid at the point of sacrifice, and meanwhile, the weight of thymus was reduced a bit (Figure 1B). Activity of the inflammatory indicator NOX was elevated in AIA rats, and these animals were also detected with higher levels of blood FFA compared to normal controls (Figure 1C). All the evidences confirmed the successful development of AIA in rats, and preliminarily revealed the altered metabolism profile in vivo. Using periodically collected blood, we performed PCR to analyze the expression of some inflammatory-related genes in WBC. The results demonstrated that intensified inflammation occurred on day 17, as suggested by down-regulation of IL-10 and ARG-1, as well as up-regulation of IL-1β. Meanwhile, the expression deficiency of SIRT1 as well as its upstream nicotinamide phosphoribosyltransferase (NAMPT) became notable (Figure 2A). Although PPAR-γ expression in AIA rats was generally insufficient too, an opposite change was observed at this time point. Using the data obtained from all the 6 time points, we conducted correlation analysis (Figure 2B). As anticipated, SIRT1 positively correlated to IL-10 (Figure 2C). Above data suggested that before sacrifice, down-regulation of SIRT1 had already become not obvious in WBC. But immunoblotting assay performed on the isolated monocytes indicated that SIRT1 expression deficiency persisted until sacrifice (Figure 2D). This discrepancy could be caused by different samples used in the two analyses. SIRT1 was assumed to fluctuate in line with the status of monocytes. The changes could be narrowed in the PCR analysis because it was based on total WBC population, where the distribution of monocytes is limited.
RSV Attenuated Both Immune and Metabolism Abnormalities in AIA Rats
The aforementioned experiments revealed that SIRT1 deficiency in AIA rats was related to inflammation indeed. We consequently treated AIA rats with RSV to test whether activating SIRT1 would benefit the recovery of AIA. The experimental arrangement is shown in Figure 3A. Obviously, RSV hampered AIA progress, but it did not prevent the trend of body weight gain decrease (Figure 3B). It is widely believed that RSV exerts anti-inflammatory effects by acting as an anti-oxidative reagent.20,21 Therefore, we monitored dynamic changes of some oxidative indicators in blood before inflammation resolution, and found that RSV eased oxidative stress in the treated AIA rats, and the most significant anti-oxidative effects were observed on day 16 during the fast progress stage of AIA. The increase in MDA level and decline in SOD activity in AIA rats at that moment were obvious, and RSV treatment restored these changes (Figure 3C). Differences about GSH levels among groups did not become significant until day 26. RSV therapy-caused IL-10 secretion changes were much more notable than those of oxidative indicators (Figure 3C). Because some samples were used out, only 4 data from each group were kept in this analysis.
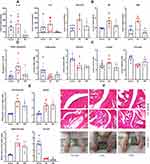
In fact, RSV did not only increase IL-10, but reshaped the immune environment of AIA rats as a whole. Because IL-1β is an important indicator of inflammation, we determined its levels on day 16 when inflammation fast progressed in AIA rats, and found that RSV significantly reduced this inflammatory cytokine (Figure 4A). RSV-brought impacts on levels of many other immune indicators were still persistent until day 30 when the rats were sacrificed. Despite the fact that the increase in IL-6 in AIA rats became not very significant, RSV treatment still profoundly reduced its mean level from 1764.4 pg/mL to 159.3 pg/mL. Compared with normal healthy controls, AIA rats showed higher levels of anti-CCP, RF and CRP. Whereas, RSV significantly reduced these RA diagnostic indicators in the treated rats (Figure 4B). At the same time, RSV treatment generally restored metabolic changes that occurred in AIA rats. Decrease in triglyceride and pyruvate as well as increase in lactate were significantly attenuated in the treated rats. But RSV did not restore blood glucose level but further decreased it (Figure 4C). Increase in PKM1 expression in the liver of AIA rats confirmed the accelerated glycolysis, which was subsequently suppressed by RSV treatment (Figure 4D). Although RSV slightly improved the body weight gain, its effects on fat storage loss in AIA rats were the opposite. RSV treatment barely affected AIA-caused spleen hyperplasia in rats. Nonetheless, RSV attenuated AIA severity a lot, and reduced paw swelling (Figure 4E). This conclusion was supported by morphological observation of the paws. Meanwhile, RSV greatly ameliorated cartilage degradation in AIA rats (Figure 4F).
Up-Regulation of SIRT1 in AIA Monocytes Reshaped Their Phenotype
Above evidences confirmed that activating SIRT1 with RSV has significant potentials in alleviating AIA. The therapeutic effects were probably related to altered monocytes polarization, because RSV treatment was obviously unfavorable for glycolysis but favored IL-10 production. To further validate this assumption, we activated SIRT1 in AIA monocytes in vitro. Compared to normal controls, AIA rats-derived monocytes released less IL-10 when cultured in vitro. RSV treatment dramatically increased the production of IL-10 (Figure 5A). Although RSV is usually recognized as a SIRT1 agonist, interestingly, its stimulus promoted SIRT1 expression. It could be as a result of the self-regulatory mechanism of this signaling. It would reinforce RSV-induced up-regulation of SIRT1 in these cells. Pyruvate kinase is a key glycolytic enzyme. Due to the negative effects of SIRT1 on glycolysis, RSV suppressed the abnormal increase in the expression of PKM1/2, the genes encoding different types of pyruvate kinase isozymes (Figure 5B). To further confirm the role of SIRT1 deficiency in changes of AIA monocytes, we overexpressed SIRT1 in the cells. It was revealed that SIRT1 overexpression decreased pyruvate and increased lactate production (Figure 5C). Besides, IL-10 secretion in AIA monocytes was significantly increased when IL-10 was overexpressed (Figure 5D). ATP production was synchronized with the above changes. Increased ATP production in AIA monocytes indicated the increased energy requirement and supply. SIRT1 overexpression impaired glycolysis-related extra energy supply and consequently reduced ATP production (Figure 5E). Metabolic reprogramming eventually reflected in expression changes of the relevant regulators, and immunoblotting assays further demonstrated that SIRT1 overexpression reshaped monocytes phenotype (Figure 5F). AMPK is a sensor of ATP/ADP.22 Impaired phosphorylation of AMPK in AIA monocytes could be caused by the accumulated ATP. SIRT1 overexpression greatly increased p-AMPK expression. Meanwhile, it significantly inhibited the expression of hypoxia-inducible factor 1-Alpha (HIF-1α) as well as its downstream TPI1 (Figure 5G).23,24 These results partially explained the declined glycolysis levels in those AIA monocytes overexpressing SIRT1. Besides, phosphorylation of STAT3 (a transcriptional factor controlling M2 polarization) was inhibited in AIA monocytes compared to normal control, which was restored by SIRT1 overexpression (Figure 5G). All the raw immunoblotting images related to Figure 5 were shown in Supplementary S2. PCR analysis found that overexpressed TPI1 in AIA monocytes was down-regulated by SIRT1 overexpression, while significantly higher expression of TGM2 and c-MAF (two indicators for M2 polarization) were detected in SIRT1 overexpressing cells (Figure 5H).
AMPK is Involved in SIRT1-Mediated Changes in AIA Monocytes
As a key upstream of SIRT1, AMPK could potentially affect SIRT1-controlled events.25 Activating AMPK signaling can mimic the effects brought by SIRT1 up-regulation. This claim was basically supported by immunoblotting experiments (Figure 6A). AMPK antagonist CC further exacerbated the abnormal changes in AIA monocytes, while treatment with AMPK agonist AICAR achieved the similar effects to SIRT1 overexpression. AICAR significantly promoted the phosphorylation of STAT3 and AMPK, and suppressed the expression of TPI1 and HIF-1α (Figure 6B). It revealed the similar role of AMPK with SIRT1 in AIA-related immune disruption. By taking their relationship into consideration, it is reasonable to assume that insufficient activation of AMPK in AIA rats is related to the abnormal down-regulation of SIRT1, because AMPK controls the production of NAD, a coenzyme indispensable for SIRT1 to function properly. Indeed, when treated with CC, normal monocytes showed certain features shared by AIA monocytes, namely up-regulation of glycolysis-related regulators TPI1 and HIF-1α (Figure 6C). Interestingly, SIRT1 overexpression could not totally offset the changes caused by CC-induced AMPK inhibition (Figure 6D). Raw images of above immunoblotting assays were included in Supplementary S3. These results demonstrated that AMPK signaling is required by SIRT1 to effectively inhibit inflammatory polarization of monocytes. Due to the up-regulated glycolytic regulators and glycolysis efficiency, the production of pyruvate and citric acid (two key intermediate metabolites related to aerobic oxidation of glucose) were decreased when the monocytes were treated with CC. Although SIRT1 overexpression did not affect their synthesis, it attenuated CC-induced aerobic oxidation deficiency (Figure 6E). As such, it can be inferred that SIRT1 could also regulate AMPK in a feedback mechanism.
Discussion
SIRT1 has already been identified as a potential target for RA treatment because it possesses unquestionable anti-inflammatory properties and several reports have revealed that its expression is generally insufficient in RA patients.26,27 However, the results from some other researches questions this conclusion. SIRT1 expression in the joint tissues of RA patients is obviously higher than that of general healthy population, and this abnormality positively correlates to RA severity.28,29 As such, whether SIRT1 deficiency is common under RA conditions needs to be further validated. Successfully answering this question is important for the development of SIRT1-targeting anti-rheumatic therapies. SIRT1 was initially identified as a metabolism regulator, which suppresses glycolysis and promotes aerobic oxidation of glucose and fatty acids. Based on accumulating acknowledge regarding metabolism reprogramming during the polarization of immune cells, it is revealed that SIRT1 serves as a key switch deciding their phenotypes by coordinating with immune regulators.9,30 Because monocytes and macrophages are the main cells utilizing glucose by means of glycolysis in RA patients, it is very plausible that SIRT1 deficiency-exacerbated RA inflammation is due to their excessive inflammatory polarization.31 Compared with tissues-resident macrophages, circulating monocytes are more accessible. In this study, we monitored the dynamic changes in SIRT1 expression in WBC, which contains monocytes. It was observed that during most time, SIRT1 expression in AIA rats was lower than normal healthy controls (Figure 2A). However, it was tricky to observe that before the onset of secondary inflammation on day 15, SIRT1 expression became higher compared to the normal controls, howbeit the difference was not statistically significant due to huge individual variations. In fact, similar fluctuation pattern in the expression of SIRT1 was observed in CIA rats.8 Nonetheless, it can be concluded that SIRT1 expression is generally insufficient in rheumatic subjects. According to the current understanding about the role of SIRT1 in monocyte polarization, its overexpression at certain stages could be a self-protective reaction to hamper the rapid progression of inflammation. This theory was supported by the differed functions of SIRT1 and its direct upstream NAMPT. Contrary to SIRT1, NAMPT generally facilitates glycolysis and it is thus taken as a M1 polarization indicator.32,33 In fact, NAMPT is not always overexpressed in CIA and AIA rats, but fluctuated in accordance with SIRT1. When NAMPT is overexpressed, up-regulation of SIRT1 is necessary to quench the flame.
Under these circumstances, it is not surprising to observe that RSV showed satisfying therapeutic effects on AIA rats. Actually, the anti-rheumatic and anti-inflammatory effects of RSV have been well documented.17,18 It is widely believed that its antioxidant properties greatly contribute to these therapeutic effects.34 But not all polyphenols can alleviate inflammatory arthritis like RSV. Despite the fact that oxidative stress would aggravate inflammation, the difference between AIA models and healthy rats was not always significant (Figure 3C). In this study, we highlighted the role of RSV as a SIRT1 agonist in treating AIA. Consistent to the immune functions of SIRT1, RSV promoted IL-10 production, and suppressed IL-1β and IL-6 secretion. This serves as a piece of useful evidence for the restored M1/M2 monocyte balance. However, these cytokines are not exclusively specific to certain monocyte subsets. Changes in metabolites provide more clues to support the aforementioned claim. Increase in lactate and decrease in pyruvate production indicated the existence of accelerated glycolysis, which eventually led to depletion in blood glucose level (Figure 4C). Because only inflammatory monocytes in blood can generate energy via glycolysis, RSV-induced inhibition on glycolysis indicated its potent negative effects on M1 polarization of these cells. These results were further confirmed by in vitro experiments. Interestingly, RSV stimulus increased SIRT1 expression (Figure 5B). It thus suggests that activating SIRT1 would up-regulate its own expression. But there is yet another possibility. The up-regulation of SIRT1 was resulted from the promoted M2 polarization of monocytes. That is, RSV treatment favored M2 polarization, and M2 monocytes highly express SIRT1.
Although RSV is always used as an agonist of SIRT1, in fact, it mainly activates the upstream AMPK. The reason why RSV is extensively used to up-regulate SIRT1 signaling is that most functions of AMPK rely on SIRT1.35 Therefore, AMPK agonist treatment can achieve similar effects to SIRT1 overexpression (Figure 6A). However, SIRT1 overexpression cannot totally compensate AMPK deficiency (Figure 6C). These facts indicate that as the upstream of SIRT1 signaling, the role of AMPK is decisive. AMPK is required by SIRT1 to fulfill its functions. From this perspective, AMPK down-regulation could be the fundamental reason for SIRT1 deficiency observed in rheumatic subjects. It was observed that ATP production in AIA monocytes was significantly increased (Figure 5E). It would substantially suppress the activation of AMPK, the most important metabolism sensor and regulator.
Conclusion
SIRT1 expression in WBC of AIA rats was insufficient, which consequently contributed to the imbalanced polarization of monocytes. By suppressing M1 polarization, SIRT1 agonist RSV restored cytokine network balance and metabolism homeostasis, which eventually attenuated AIA severity in rats. Overexpressing SIRT1 and activating its upstream AMPK affected AIA monocytes in the similar way. These clues hint that AMPK/SIRT1 deficiency is implicated in monocytes-mediated inflammation in AIA rats, and activating this pathway is a feasible anti-rheumatic strategy.
Abbreviations
RA, rheumatoid arthritis; AIA, adjuvant-induced arthritis; CIA, collagen-induced arthritis; AMPK, AMP-activated protein kinase; RF, rheumatoid factor; ARG-1, arginase-1; CRP, C-reactive protein; ELISA, enzyme linked immunosorbent assay; GSH, superoxide dismutase; HIF-1α, hypoxia-inducible factor 1-alpha; BCG, Bacillus Calmette-Guerin; IFA, incomplete Freund’s adjuvant; CFA, complete Freund’s adjuvant; IL-10, interleukin 10; MDA, malonaldehyde; NAMPT, nicotinamide phosphoribosyltransferase; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; PKM1/2, pyruvate kinase isozyme type M1/2; RSV, resveratrol; SIRT1, silent information regulator 1; SOD, superoxide dismutase; SPF, specific pathogen free; TPI1, triosephosphate isomerase 1; WBC, white blood cells; AICAR, 5-Aminoimidazole-4-carboxamide1-β-D-ribofuranoside; CC, compound C; PVDF, poly(vinylidene fluoride).
Author Contributions
All authors made a significant contribution to conception and design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (81973828), The Open Fund of Key Laboratory of Anti-Inflammatory and Immune Medicine, Ministry of Education, P.R. China (Anhui Medical University) (KFJJ-2020-08), Anhui Natural Science Foundation Project (2108085QH386) and The Scientific Research Project of Health Commission of Anhui Province (AHWJ2021b061).
Disclosure
The authors report no declarations of interest.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi:10.1016/S0140-6736(16)30173-8
2. Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400. doi:10.1016/j.jaut.2019.102400
3. Okano T, Saegusa J, Takahashi S, Ueda Y, Morinobu A. Immunometabolism in rheumatoid arthritis. Immunol Med. 2018;41:89–97. doi:10.1080/25785826.2018.1531186
4. Hua S, Dias TH. Hypoxia-inducible factor (HIF) as a target for novel therapies in rheumatoid arthritis. Front Pharmacol. 2016;7:184. doi:10.3389/fphar.2016.00184
5. O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. doi:10.1084/jem.20151570
6. Harmon GS, Lam MT, Glass CK. PPARs and lipid ligands in inflammation and metabolism. Chem Rev. 2011;111:6321–6340. doi:10.1021/cr2001355
7. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi:10.1172/JCI29881
8. Lei M, Tao MQ, Wu YJ, et al. Metabolic enzyme triosephosphate isomerase 1 and nicotinamide phosphoribosyltransferase, two independent inflammatory indicators in rheumatoid arthritis: evidences from collagen-induced arthritis and clinical samples. Front Immunol. 2022;12:795626. doi:10.3389/fimmu.2021.795626
9. Wu YJ, Fang WJ, Pan S, et al. Regulation of Sirt1 on energy metabolism and immune response in rheumatoid arthritis. Int Immunopharmacol. 2021;101:108175. doi:10.1016/j.intimp.2021.108175
10. Chen WG, Zhang SS, Pan S, et al. α-Mangostin treats early-stage adjuvant-induced arthritis of rat by regulating the CAP-SIRT1 pathway in macrophages. Drug Des Devel Ther. 2022;16:509–520. doi:10.2147/DDDT.S348836
11. Kong S, Yeung P, Fang D. The class III histone deacetylase sirtuin 1 in immune suppression and its therapeutic potential in rheumatoid arthritis. J Genet Genom. 2013;40:347–354. doi:10.1016/j.jgg.2013.04.001
12. Conforti A, Lussignoli S, Bertani S, et al. Specific and long-lasting suppression of rat adjuvant arthritis by low-dose Mycobacterium butyricum. Eur J Pharmacol. 1997;324:241–247. doi:10.1016/S0014-2999(97)00068-X
13. Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68:744–750. doi:10.1136/ard.2007.086066
14. Rhoads JP, Major AS, Rathmell JC. Fine tuning of immunometabolism for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:313–320. doi:10.1038/nrrheum.2017.54
15. Zuo J, Yin Q, Wang YW, et al. Inhibition of NF-κB pathway in fibroblast-like synoviocytes by α-mangostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int Immunopharmacol. 2018;56:78–89. doi:10.1016/j.intimp.2018.01.016
16. Nie Q, Zhang J, He B, et al. A novel mechanism of protection against isoproterenol-induced cardiac inflammation via regulation of the SIRT1/NRF2 signaling pathway with a natural SIRT1 agonist. Eur J Pharmacol. 2020;885:173398. doi:10.1016/j.ejphar.2020.173398
17. Wahba MG, Messiha BA, Abo-Saif AA. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm Biol. 2016;54:1705–1715. doi:10.3109/13880209.2015.1125931
18. Chen X, Lu J, An M, Ma Z, Zong H, Yang J. Anti-inflammatory effect of resveratrol on adjuvant arthritis rats with abnormal immunological function via the reduction of cyclooxygenase-2 and prostaglandin E2. Mol Med Rep. 2014;9:2592–2598. doi:10.3892/mmr.2014.2070
19. Hu YH, Han J, Wang L, et al. α-Mangostin alleviated inflammation in rats with adjuvant-induced arthritis by disrupting adipocytes-mediated metabolism-immune feedback. Front Pharmacol. 2021;12:692806. doi:10.3389/fphar.2021.692806
20. Yang G, Chang CC, Yang Y, et al. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways, and suppressing angiogenesis. J Agric Food Chem. 2018;66:12953–12960. doi:10.1021/acs.jafc.8b05047
21. Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin Rheumatol. 2018;37:2035–2042. doi:10.1007/s10067-018-4080-8
22. Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi:10.1242/jcs.01540
23. Wang ZH, Bao XG, Hu JJ, Shen SB, Xu GH, Wu YL. Nicotinamide riboside enhances endothelial precursor cell function to promote refractory wound healing through mediating the Sirt1/AMPK pathway. Front Pharmacol. 2021;12:671563. doi:10.3389/fphar.2021.671563
24. Jiang TT, Ji CF, Cheng XP, et al. α-Mangostin alleviated HIF-1α-mediated angiogenesis in rats with adjuvant-induced arthritis by suppressing aerobic glycolysis. Front Pharmacol. 2021;12:785586. doi:10.3389/fphar.2021.785586
25. Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–760. doi:10.1152/ajpendo.00745.2009
26. Li G, Xia Z, Liu Y, et al. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-κB pathway. Biosci Rep. 2018;38:BSR20180541. doi:10.1042/BSR20180541
27. Hao L, Wan Y, Xiao J, Tang Q, Deng H, Chen L. A study of Sirt1 regulation and the effect of resveratrol on synoviocyte invasion and associated joint destruction in rheumatoid arthritis. Mol Med Rep. 2017;16:5099–5106. doi:10.3892/mmr.2017.7299
28. Zhou J, He YW, Fu L, et al. Gene polymorphisms of SIRT1 in patients with rheumatoid arthritis. Int J Rheum Dis. 2022;25:210–217. doi:10.1111/1756-185X.14257
29. Niederer F, Ospelt C, Brentano F, et al. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann Rheum Dis. 2011;70:1866–1873. doi:10.1136/ard.2010.148957
30. Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet. 2014;30:271–286. doi:10.1016/j.tig.2014.04.007
31. Hah YS, Cheon YH, Lim HS, et al. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS One. 2004;9:e87733. doi:10.1371/journal.pone.0087733
32. Halvorsen B, Espeland MZ, Andersen GØ, et al. Increased expression of NAMPT in PBMC from patients with acute coronary syndrome and in inflammatory M1 macrophages. Atherosclerosis. 2015;243:204–210. doi:10.1016/j.atherosclerosis.2015.09.010
33. Wang QH, Li Y, Dou DY, et al. Nicotinamide mononucleotide-elicited NAMPT signaling activation aggravated adjuvant-induced arthritis in rats by affecting peripheral immune cells differentiation. Int Immunopharmacol. 2021;98:107856. doi:10.1016/j.intimp.2021.107856
34. Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules. 2021;26:229. doi:10.3390/molecules26010229
35. Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–1634. doi:10.1016/j.bbapap.2009.10.025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.