Back to Journals » OncoTargets and Therapy » Volume 13
AMPK/mTOR/ULK1 Axis-Mediated Pathway Participates in Apoptosis and Autophagy Induction by Oridonin in Colon Cancer DLD-1 Cells
Authors Bu H, Liu D, Zhang G, Chen L, Song Z
Received 12 May 2020
Accepted for publication 5 August 2020
Published 25 August 2020 Volume 2020:13 Pages 8533—8545
DOI https://doi.org/10.2147/OTT.S262022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Heqi Bu,1,2 Dianlei Liu,3 Guolin Zhang,1 Li Chen,1 Zhangfa Song1
1Department of Colorectal Surgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, People’s Republic of China; 2Department of Coloproctology Surgery, Tongde Hospital of Zhejiang Province, Hangzhou 310012, People’s Republic of China; 3Department of Surgery, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou 310006, People’s Republic of China
Correspondence: Zhangfa Song Department of Colorectal Surgery
Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, 3 East Qingchun Road, Hangzhou 310016, People’s Republic of China
Email [email protected]
Background: Oridonin has been demonstrated to exert strong antitumor activities in various types of human cancers. Our previous study established that oridonin induced the apoptosis of and exerted an inhibitory effect on colon cancer cells in vitro and in vivo. However, the mechanisms behind the antitumor effects of oridonin on colorectal cancer are not clearly known. This study explored whether autophagy was involved in antitumorigenesis effects caused by the usage of oridonin in colon cancer and examined whether the AMPK/mTOR/ULK1 signaling pathway was involved in this process.
Methods: Cell viability was determined using CCK-8 assay. The distribution of cell apoptosis was evaluated using flow cytometry. RT-PCR and Western blotting analysis were conducted to identify the key target genes and proteins involved in the AMPK/mTOR cascade. AMPK siRNA was used to disturb AMPK expression. A DLD-1 cell orthotopic transplantation tumor model was established to explore the anti-cancer effects in vivo.
Results: Oridonin exhibited a suppressive effect on DLD-1 cells in a concentration- and time-dependent manner. Additionally, in a dose-dependent manner, oridonin induced cell apoptosis via inducing the protein expression levels of cleaved caspase-3, cleaved PARP and stimulated autophagy by increasing protein expression levels of Becin1, LC3-II, decreasing protein expression levels of LC3-I, p62, which were respectively attenuated and elevated by autophagy inhibitor 3-MA. Furthermore, oridonin upregulated the expression level of p-AMPK and downregulated the expression levels of p-mTOR, p-ULK1 in the DLD-1 cells in a dose-dependent manner. Moreover, knockdown of AMPK by a specific siRNA reversed the expression levels of proteins involved in the AMPK/mTOR pathway, autophagy and apoptosis. In addition, outcomes from the in vivo experiments also showed that oridonin treatment significantly repressed tumorigenic growth of DLD-1 cells without any side effects, which was accompanied by the upregulation of p-AMPK, LC3-II, active caspase-3 protein expression levels and the downregulation of p-mTOR and p-ULK1 protein expression levels.
Conclusion: This study demonstrated that oridonin induced apoptosis and autophagy of colon cancer DLD-1 cells via regulating the AMPK/mTOR/ULK1 pathway, which indicated that oridonin may be used as a novel therapeutic intervention for patients with colorectal cancer.
Keywords: oridonin, AMPK/mTOR/ULK1 pathway, autophagy, apoptosis, colon cancer
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths and has shown an increasing rate of morbidity.1 Although during recent times advancements have been made for its early diagnosis along with the development of more effectual treatment methods for CRC, some CRC patients have not positively responded to treatment due to severe clinical toxicity, side effects and the development of multidrug-resistance (MDR). Therefore, the development of new cytotoxic drugs is a huge challenge.2 The use of natural substances is known to be effective and less toxic for the treatment for a variety of disorders, including several types of human cancers.3–5 Therefore, at present, chemotherapeutic agents derived from natural sources have been studied and used for treatment of several types of cancers.
Oridonin (Figure 1A), is an ent-kaurene diterpenoid compound isolated from Chinese medicinal herb Rabdosia rubescens and has been proved to have multiple pharmacological and physiological effects including anti-inflammatory, anti-bacterial and anticancer effects, etc.6 Recently, in China, oridonin has been used to detect many kinds of cancer due to its low toxicity.7 Researchers have shown that the anticancer effects of oridonin in colorectal cancer may be mediated by the activation of the BMP7/p38MAPK/p53 signaling pathway, or by enhancing the function of PTEN through activating p38 MAPK.8,9 We have previously established that the inhibition caused by oridonin on colon cancer LoVo cells involved inactivation of the TGF-β1/Smads-PAI-1 signaling cascade in vitro and in vivo.10 Though oridonin could impede tumor cells growth in numerous cancer types by inhibiting propagation and apoptosis induction, the specific cellular targets of oridonin-induced cytotoxicity in colon cancer cells have not been probed enough and further exploration is required.
Apoptosis and autophagy are two common types of cell death. Both apoptosis and autophagy are closely associated with cell death, growth, differentiation and survival of cancer cells.11,12 Various stimuli can trigger autophagy, apoptosis, or both. Moreover, studies have demonstrated that apoptosis may be delayed or promoted by autophagy in certain circumstances.13–17 For example, pemetrexed and simvastatin cotreatment was found to have increased apoptosis and autophagy in cancerous mesothelioma and NSCLC cells, whereas the prevention of pemetrexed and simvastatin-induced autophagy enhanced apoptosis.13 The inhibition of autophagy repressed cell apoptosis in oridonin phosphate-treated MDA-MB-436 cells.14 Flavonoid-induced suppression of autophagy increased their apoptotic impact on cancerous cells.15,16 It has also been documented that procyanidin B2 promoted the autophagy of CRC cells, and that induction of apoptosis was reversed in the presence of an autophagy inhibitor.17 Nevertheless, the exact mechanisms by which autophagy, apoptosis and their interactions are recognized are not clearly known. Up to now, it has never been verified whether antitumorigenesis effect exerted by oridonin on CRC is mediated through autophagy.
Numerous mechanisms and signaling pathways are involved in the management of autophagy and they are regulated by various cytoplasmic proteins, membrane-spanning proteins and numerous protein complexes.18 Previous studies have confirmed that tumor growth was hindered by the stimulation of autophagy and apoptosis in CRC cells.17,19-22 Furthermore, emerging evidence has suggested that mTOR suppression results in substantial activity against a variety of cancers in vitro and in tumor transplantation models.23 Furthermore, accumulating evidence has indicated that AMP‑activated protein kinase (AMPK) and mTOR play a decisive function in autophagy and apoptosis.24–27 More interestingly, it has been reported that oridonin augmented cisplatin sensitivity through its pro-apoptotic activity, which was regulated by AMPK/Akt/mTOR-dependent autophagosome stimulation in A549 cells.28 However, the detailed mechanisms by which oridonin acts against CRC cells need further research, specifically regarding the targeting mTOR-related modulators via the AMPK signaling pathway.
Hence, in the present study, we emphasized on autophagy and apoptosis to identify novel treatment options. We explored the effects of oridonin on autophagy and apoptosis in colon cancer DLD-1 cells and in a murine orthotopic tumor model. Meanwhile, we examined the effects of oridonin on the AMPK/mTOR-related modulator signaling cascade to detect underlying mechanisms. The results of this study offer new unique evidence for the usage of oridonin as a promising therapeutic option for colorectal cancer.
Methods
Materials and Reagents
Oridonin (purity>97%, P0290) was bought from Shanghai PureOne Biotechnology, dissolved in DMSO as stock solution (10 mM)) and stored in refrigerator at −20 °C. Fetal bovine serum (FBS), DSMO, RPMI-1640, penicillin/streptomycin, 0.25% trypsin containing EDTA were obtained from Abcam (Cambridge, UK). TRIzol reagent and RIPA lysis buffer were acquired from Thermo Fisher Scientific Inc; Cell Counting Kit-8 (CCK-8), Bicinchoninic Acid (BCA) Protein Assay kit and Annexin V-FITC/PI Apoptosis Detection Kit were gained from Sigma-Aldrich Inc. (USA). The antibodies used were as follows: rabbit monoclonal antibodies against GAPDH (#5174T), active caspase-3 (#2774S), cleaved caspase-3 (#9661S) and PARP (#5625S), LC3 (#4108S), Beclin 1 (#3495S), P62 (# 8025S) were bought from Cell Signaling Technology, Inc. (Boston, USA); p-mTOR (Ser2448)(ab84400), mTOR (ab25880), p-AMPK (ab23875), AMPK (ab80039), p-ULK1 (ab256537), ULK1 (ab167139) were purchased from Abcam (Cambridge, MA, USA). Goat anti-rabbit IgG-HRP (D110058) were obtained from Sangon Biotech Co., Ltd. (Shanghai, China).
Cell Culture
The colon cancer cell line DLD-1 (C0009007) was purchased from Morey Biosciences Inc. (Shanghai, China). The cells were cultured in a humidified environment of 5% CO2 and 95% O2 at 37 °C in a RPMI-1640 medium containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin.
Cell Viability Assay
A CCK-8 kit was used to assess the cytotoxicity of oridonin on DLD-1 cells, following the supplier’s instructions. Cells (5 × 103/well) at the logarithmic phase were cultured in 96-well culture plates overnight. Then, the cells were treated with indicated dosages of oridonin (0, 5, 10, 15, 20 and 25 μM) for 24, 48 and 72 h, respectively. Then, 10 µL of CCK working solution was added into each well. The plates were incubated for 1–3 h under standard conditions. Absorbance at 450 nm was determined using a Multi-Detection Microplate Reader (TECAN, Switzerland).
Flow Cytometry Analysis
The cells (5×105/well) were cultured in 6-well plates. Following overnight incubation at 37°C, the cells were exposed to the indicated dosages (0, 10, 15 or 20 μM) of oridonin for 48 h. The cells were harvested at a density of 5×105 cells/mL and were washed with ice-cold PBS. Next, they were incubated with FITC conjugated Annexin V and propidium (PI) in a binding buffer at room temperature (RT) for 30 min in the dark. The cells were washed again with PBS and were evaluated using a flow cytometer (BD Bioscience, USA).
Western Blot Analysis
The cells collected or from frozen tissue samples were dissolved and homogenized in a RIPA lysis buffer. The BCA Protein Assay Kit was employed to quantify final protein concentrations. Forty micrograms of total protein from each sample was separated on 12% sodium dodecyl sulphate-polyacrylamide gels through electrophoresis and were transferred onto PVDF membranes. The blots were blocked at RT for 2 h using 5% skim milk in TBST, and then probed overnight with primary antibodies (1:1000) p-AMPK, AMPK, mTOR, p-mTOR, p-ULK1, ULK1, active caspase-3, cleaved caspase-3 and PARP, LC3, p62, Beclin 1 at 4°C. GAPDH (1:1000) served as the loading control. Following three washes with TBST, corresponding HRP-conjugated secondary antibodies (1:1000) were used for incubation for 1 h at RT. The immunoreactive bands were visualized using a Western Lightning Plus-ECL (PerkinElmer, USA). Densitometry was used for the evaluation conducted on Image J software.
Reverse Transcription PCR
The cultured cells were dissolved in TRIzol reagent for the extraction of RNA, following the supplier’s instructions. The cDNA was synthesized using a RevertAidTM First Strand cDNA Synthesis Kit (Thermo Scientific Corp, USA). The PCR reaction was run using a CFX96 TouchTM Real-Time PCR Detection System (Fermentas, USA) with an initial denaturation step at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 57°C for 30 s and 72°C for 2 min. Final extension was performed at 72°C for 10 min. The specific primers used were provided by GeneSail Biotech Co. Ltd. (Shanghai, China) (Table 1). The PCR products were identified through gel electrophoresis performed on 1.5% agarose. The images were captured using a gel imaging system and the data were analyzed using Image J software.
 |
Table 1 Primer Sequences Used for RT-PCR Analysis |
Small Interfering RNA (siRNA)
siRNA oligonucleotides (20 nM) specific for AMPK and non-silencing scrambled siRNA were bought from Santa Cruz Biotechnology (California, USA). siRNA transfection was conducted by using a Lipofectamine 2000 system (Invitrogen, USA), following the supplier’s instructions. Cells (~2×105/well) were seeded into 6-well and transiently transfected when the cell density grew to ~80% to 90% confluence. After additional incubation for 6 to 8 h, all samples were cultured with or without 20 μM oridonin in complete fresh medium for 48 h. Then, the samples were used in the following experiments.
Experimental Animals
A total of 15 male athymic nude mice (BALB/c, nu/nu, 4–6 weeks old, weight 18–20 g) were bought from Shanghai SLAC Laboratory Animal Co., Ltd. for tumor transplantation. All the animals were fed in an SPF environment (temperature: 20–26 °C; relative humidity: 40–70%; light: 12 h light/dark cycles) with a continuous supply of forage irradiated with Co60 and purified water sterilized using high-pressure steam. All protocols were sanctioned by the Institutional Animal Care and Use Committee. All of the procedures described in this study were taken into account welfare considerations, implementation of the 3Rs, and compliance with the guidelines, through the involvement of the NC3Rs and approved by institutional Ethics Committee from Zhejiang Academy of traditional Chinese Medicine under assurance number KTSC2020028.
In vivo Orthotopic Transplantation Model
In brief, a suspension of DLD-1 cells (1 ×107/mL) in 0.2 mL of PBS was subcutaneously injected into the right flanks of the donor nude mice. When the size of the tumor reached around 1 cm3, the tumor was excised under sterile conditions. The peripheral connective tissues were removed and normal peripheral tissue was cut into 1 mm3 for transplantation in sterilized saline containing penicillin (120 units/mL). All mice were anesthetized, fixed, and incised longitudinally on the right lower abdomen. After the colon near to the cecum was gently removed, the colonic serosa was cut and the 1 mm3 tumor mass was inserted into the serosa, which was then sutured transversely using a 7-0 silk thread. Thereafter, the colon was gently placed inside the abdominal cavity and the incision was closed using 4-0 silk thread.
After 1 week, the mice were arbitrarily separated into control group and oridonin-treatment groups (5 mice/group) using a randomized block design. Intraperitoneal injections of 0.9% sodium chloride were administered to animals in the control group, whereas mice in the treatment group were administered with an intraperitoneal injection of oridonin (5 and 10 mg/kg), once a day for consecutive 2 weeks at a volume of 50 µl. The mice were euthanized, and the tumor was resected and weighed. Tumor volume was calculated using the formula: (shorter diameter2 × longer diameter/2). A section of the tumor tissue was fixed using 10% formalin and embedded in paraffin for HE staining, and other tissues were stored in liquid nitrogen for subsequent experiments.
Statistical Analysis
All experiments were performed in triplicate. Data analysis was performed using SPSS 18.0 software. The representative outcomes are expressed as mean ± SD. Student’s t-test and one-way analysis of variance were used for the analyses. A P value of <0.05 was considered to indicate statistical significance.
Results
Effects of Oridonin on the Viability of Colon Cancer DLD-1 Cells
Cells were treated with or without oridonin at serial concentrations for 24, 48 and 72 h. CCK-8 assay was used to determine the impact of oridonin on the viability of DLD-1 cells. Our findings, which are shown in Figure 1B, indicated that oridonin effectively hindered cell viability in a time and dosage-dependent manner. Furthermore, the half-maximal cytotoxicity of oridonin on DLD-1 cells was ~15 μM at 48 h. The concentration of oridonin used in subsequent experiments to examine the probable molecular mechanisms was in the range of 10–20 μM concentration.
Oridonin Induced the Autophagy and Apoptosis of DLD-1 Cells
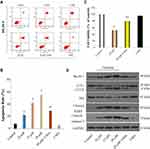
It has been previously shown that oridonin induced colon cancer cell apoptosis.8,9 In our study, flow cytometry with Annexin V/PI double staining was used to determine the rate of apoptosis of DLD-1 cells following oridonin treatment. As presented in Figure 2A and B, oridonin treatment substantially promoted the apoptosis of DLD-1 cells in a dosage-dependent manner. Furthermore, Western blotting analysis was used to establish that the expression levels of cleaved caspase-3 and cleaved PARP were clearly augmented in a concentration-dependent manner (Figure 2C).
To investigate if oridonin prompted autophagy in DLD-1 cells, using Western blotting analysis we established that the expression levels of Beclin 1 and LC3-II were upregulated, whereas the expression levels of LC3-I and p62 were downregulated in a dosage-dependent manner (Figure 2D). Together these results suggested that oridonin significantly induced autophagy of DLD-1 cells.
To further elucidate the interaction between apoptosis and autophagy of DLD-1 cells induced by oridonin, an autophagy inhibitor, 3-MA, was used in our experiment. The results of the CCK-8 assay and flow cytometry suggested that oridonin induced an anti-proliferation and apoptosis effect in DLD-1 cells, which was obviously reversed by 3-MA. Meanwhile, the viability and apoptosis of DLD-1 cells were not affected by 3-MA alone (Figure 2AC). Furthermore, the upregulation of Beclin 1 and LC3-II protein expression levels induced by oridonin was alleviated, whereas the downregulation of LC3-I and p62 protein expression levels induced by oridonin was reversed in the presence of 3-MA (Figure 2D). These results confirmed that prevention of autophagy diminished the anti-proliferation effects of oridonin on DLD-1 cells.
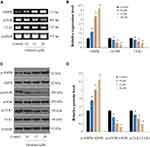
Oridonin Induced Apoptosis and Autophagy of DLD-1 Cells via Regulation of the AMPK/mTOR/ULK1 Signaling Pathway
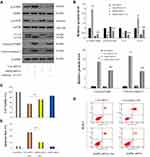
AMPK can stimulate the ULK1 protein kinase to promote cell death by hindering mTORC1 activity.29,30 Next, we investigated whether the AMPK/mTOR/ULK1 signaling pathway was involved in the induction of autophagy and apoptosis of DLD-1 cells caused by oridonin. As shown in Figure 3AD, we found that oridonin treatment increased the mRNA level of AMPK while decreased those of mTOR, ULK1; as well as upregulated expression level of p-AMPK, and downregulated expression levels of p-ULK1 and p-mTOR in DLD-1 cells, in a concentration-dependent manner. Interestingly, total protein levels of AMPK, mTOR, ULK1 were almost unchanged after oridonin treatment. Furthermore, to confirm the function of the AMPK/mTOR cascade, levels of autophagy‑related signals were analyzed in DLD-1 cells treated with AMPK siRNA. AMPK siRNA efficiently restored levels of p‑mTOR and LC3-I, which had been downregulated by oridonin, prevented the elevation of LC3-II, caspase-3 and PARP cleavage in DLD-1 cells, while total protein levels of AMPK, mTOR, ULK1 remained unchanged (Figure 4A and B). Besides, CCK-8 results indicated that AMPK knockdown rescued cellular viability to some extent compared with control group (Figure 4C). Moreover, flow cytometry confirmed that oridonin-mediated apoptosis was notably attenuated in DLD-1 cells (Figure 4D and E). Together, these outcomes suggested that the AMPK/mTOR signaling cascade participated in oridonin-induced autophagy and apoptosis of DLD-1 cells.
Antitumor Effect of Oridonin on Orthotopically Transplanted Colon Cancer in Nude Mice
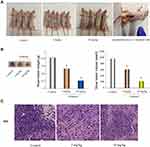
Based on the findings of our in vitro experiments, DLD-1 orthotopic nude mouse models were used to explore the therapeutic effects of oridonin in vivo. As presented in Figures 5A and B, different doses (5 mg/kg, 10 mg/kg) of oridonin significantly suppressed tumor growth, compared with the control group. Mean tumor weight as well as volume in oridonin-treated groups were decreased and the inhibition ratio reached 35.7% and 73.2%, respectively, for each of the two different dosages. Nevertheless, the body weight of mice in oridonin-treated group was not significantly affected. Moreover, HE staining, as shown in Figure 5C, demonstrated that there were no substantial changes in tumor morphology, including size, arrangement, nucleus and the cytoplasm of the tumor cells in the oridonin-treated groups. However, uneven necrotic areas were observed in the parenchyma of the tumor tissue.
Effects of Oridonin on AMPK/mTOR/ULK1 Pathway, Apoptosis and Autophagy-Related Protein Expressions in Tumor Tissues
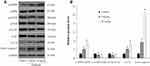
To further evaluate the mechanisms for anticancer capacity of oridonin in vivo, next the related protein expression levels were determined in tumor tissues. Data revealed that the protein expression levels of p-AMPK, LC3-II and active caspase-3 were much higher in the oridonin-treated groups compared to that in the untreated control group, while the protein expression levels of p-mTOR, p-ULK1 were downregulated in oridonin-treated tumor tissues, and while total protein levels of AMPK, mTOR, ULK1 were the same as in vitro experimental results (Figure 6A and B). These data suggested oridonin could inhibit tumorigenicity through AMPK/mTOR/ULK1 signaling pathway in vivo, which was in agreement with in vitro data.
Discussion
The prevalence of CRC is increasing worldwide. Even though medical technology has been advancing rapidly, CRC is responsible for a large number of cancer-related deaths. Induction of cell death is comprised of four major morphological processes: necrosis, apoptosis, autophagy and pyroptosis, of which apoptosis and autophagy are closely associated with cell growth, differentiation, death, invasion, metastasis and the development of tumors. Hence, it is essential to explore the relationship between autophagy and apoptosis in elucidating the mechanism of action of antitumor drugs. In this study, we explored the anti-cancer effect of oridonin on colon cancer DLD-1 cells in vitro and in vivo. We found that apoptosis and autophagy of DLD-1 cells induced by oridonin were mediated via the AMPK/mTOR/ULK1 signaling pathway.
Apoptosis is a key process for tumor development and treatment response.31,32 Caspase-3 is closely associated with apoptosis and it is usually present as an inactive proenzyme. However, once activated, it can stimulate other procaspases, initiating a cascade of protease reactions.33 To evaluate the role of oridonin in inducing apoptosis, manifestation levels of apoptotic proteins, cleavage status of caspase-3 and poly ADP ribosomal polymerase (PARP, an apoptosis marker) after oridonin treatment were detected. We found that oridonin increased the expression levels of cleaved caspase-3 and PARP in DLD-1 cells. These results were in accordance with that of recent findings.34,35 Autophagy plays a vital function in maintaining intracellular environmental homeostasis and participates in basic biological activities.36 Autophagy plays dual roles in promoting and inhibiting cancer.37 To date, many anticancer drugs have been found to exert autophagy-inducing functions, which can lead to cell survival or cell death.38,39 The development of autophagosomes comprising autophagy-related genes and the conversion of LC3-I to LC3-II via photolytic cleavage and lipidation are regarded as hallmarks of autophagy.40 The protein Beclin 1 is a vital regulator of autophagy.41 Key cargo adaptor protein, p62, directly binds to Bcl-2 to inhibit the interaction between Bcl-2 and Beclin 1, and then directly binds to the two autophagy effectors, LC3-I and LC3-II, during the process of autophagy.42 Autophagy-dependent apoptosis with the recognized participation of p62 has also been reported and the loss of the p62 ZZ domain has been documented to restore the sensitivity of cells to apoptotic death.43 Studies have shown that DLD-1 cell death can be induced via the upregulation of autophagy markers.44 We confirmed here that oridonin aroused DLD-1 cell autophagy via upregulating the expression levels of LC3-II, Beclin 1 and downregulating the expression levels of LC3-I, p62.
Both autophagy and apoptosis are pivotal mechanisms for mediating the survival or death of cancer cells. The intersection between the two has been demonstrated in the tumorigenesis and development of cancer,45 whereas the interplay between the two cascades in CRC has not been well understood. Even though autophagy and apoptosis show significant differences between the metabolic pathways involved, their signaling cascades are closely associated.46 Based on distinct regulation modes, their interface can be roughly divided into three types: cooperative, antagonistic and promotion relationships.12 Considering that different drugs cause the activation of different signaling pathways, which act on different cells, their autophagic and apoptotic consequences may be different.47 Therefore, the role of autophagy was further investigated. The outcomes indicated that oridonin-induced apoptosis of DLD-1 cells was reversed by 3-MA. These findings recommended that the pro-apoptotic impacts of oridonin in DLD-1 cells decreased due to the inhibition of autophagy, which was consistent with the findings of recent research.17
It is well known that mTOR interacts with other proteins to form mTORC1 and mTORC2, and that among them, mTORC1 plays a vital function in cell growth and propagation.48,49 In the absence of energy and nutrients, the formation of mTORC1 is hindered and autophagy is stimulated.50 In addition to autophagy, mTOR also exerts a robust effect on the regulation of apoptosis. AMPK signaling is one of the main signals that regulates the mTOR cascade and is regarded as a central negative modulator of autophagy.51 In this cascade, AMPK functions as a stimulator of autophagy, and its stimulation triggers the dephosphorylation of mTOR, which causes its separation from the ULK1 complex, which then results in the successful dephosphorylation of ULK1 that leads to autophagy.52 It has been documented that the activation of AMPK prompts an autophagy response in tumor cells,53,54 while AMPK is a main modulator of autophagy in various types of cells.55 Although previous studies have shown that oridonin can induce autophagy via inhibition of glucose metabolism induced by deactivation of AMPK in p53-mutated colorectal cancer cells.56 What we find here was that oridonin augmented level of AMPK phosphorylation and decreased the levels of mTOR and ULK1 phosphorylation of DLD-1 cells in a concentration-dependent manner. Based on this, we consider that induction of autophagy varies with tumor cell types and signal transduction. To further validate the pathway, AMPK knockdown was applied using an AMPK-specific siRNA. We established that oridonin-induced DLD-1 cell cytotoxicity could be reversed through AMPK silencing. In addition, AMPK knockdown decreased levels of apoptosis in DLD-1 cells induced by oridonin. Further, silencing of AMPK decreased p-AMPK, LC3-II, cleaved PARP and caspase-3 expression levels, whereas it ameliorated p-mTOR and LC3-I expression levels. These findings indicated that the AMPK/mTOR cascade performed a vital function in oridonin‑induced DLD-1 cell autophagy and apoptosis. Additionally, orthotopic tumor growth significantly decreased in oridonin-treated mice and the same expression level changes of p-AMPK, p-mTOR, p-ULK1 were observed in tumor tissue, further confirming our results in vitro.
Conclusion
The results presented here demonstrate that that oridonin promoted the autophagy and apoptosis of colon cancer DLD-1 cells through regulating AMPK/mTOR/ULK1 signaling pathway. This finding may provide novel insights into the therapeutic action of oridonin, which may be used as a promising chemotherapeutic agent against colorectal cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Bach DH, Luu TT, Kim D, et al. BMP4 upregulation is associated with acquired drug resistance and fatty acid metabolism in EGFR‑mutant non‑small‑cell lung cancer cells. Mol Ther Nucleic Acids. 2018;12:817–828. doi:10.1016/j.omtn.2018.07.016
3. Shahabipour F, Caraglia M, Majeed M, Derosa G, Maffioli P, Sahebkar A. Naturally occurring anti‑cancer agents targeting EZH2. Cancer Lett. 2017;400:325–335. doi:10.1016/j.canlet.2017.03.020
4. Wali AF, Majid S, Rasool S, et al. Natural products against cancer: review on phytochemicals from marine sources in preventing cancer. Saudi Pharm J. 2019;27(6):767–777. doi:10.1016/j.jsps.2019.04.013
5. Wei G, Sun J, Luan W, et al. Natural product albiziabioside a conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis-ferroptosis m2-tams polarization for combined cancer therapy. J Med Chem. 2019;62(19):8760–8772. doi:10.1021/acs.jmedchem.9b00644
6. Xu J, Wold EA, Ding Y, Shen Q, Zhou J. Therapeutic potential of oridonin and its analogs: from anticancer and antiinflammation to neuroprotection. Molecules. 2018;23(2):
7. Li D, Han T, Liao J, et al. Oridonin, a promising ent-Kaurane diterpenoid lead compound. Int J Mol Sci. 2016;17:17.
8. Liu RX, Ma Y, Hu XL, et al. Anticancer effects of oridonin on colon cancer are mediated via BMP7/p38 MAPK/p53 signaling. Int J Oncol. 2018;53(5):2091–2101. doi:10.3892/ijo.2018.4527
9. Wu QX, Yuan SX, Ren CM, et al. Oridonin upregulates PTEN through activating p38 MAPK and inhibits proliferation in human colon cancer cells. Oncol Rep. 2016;35(6):3341–3348. doi:10.3892/or.2016.4735
10. He-Qi B, Shen F, Cui J. The inhibitory effect of oridonin on colon cancer was mediated by deactivation of TGF-β1/Smads-PAI-1 signaling pathway in vitro and vivo. Onco Targets Ther. 2019;12:7467–7476. doi:10.2147/OTT.S220401
11. Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi:10.1111/j.1365-2184.2012.00845.x
12. Qian HR, Shi ZQ, Zhu HP, et al. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget. 2017;8(37):62759–62768. doi:10.18632/oncotarget.18663
13. Hwang KE, Kim YS, Jung JW, et al. Inhibition of autophagy potentiates pemetrexed and simvastatin-induced apoptotic cell death in malignant mesothelioma and non-small cell lung cancer cells. Oncotarget. 2015;6(30):29482–29496. doi:10.18632/oncotarget.5022
14. Li Y, Wang Y, Wang S, Gao Y, Zhang X, Lu C. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med Oncol. 2015;32(1):365. doi:10.1007/s12032-014-0365-1
15. Fukuda T, Oda K, Wada‑ Hiraike O, et al. Autophagy inhibition augments resveratrol‑induced apoptosis in Ishikawa endometrial cancer cells. Oncology Lett. 2016;12(4):2560–2566. doi:10.3892/ol.2016.4978
16. Huang YH, Sun Y, Huang FY, et al. Toxicarioside O induces protective autophagy in a sirtuin-1-dependent manner in colorectal cancer cells. Oncotarget. 2017;8(32):52783–52791. doi:10.18632/oncotarget.17189
17. Zhang R, Yu Q, Lu W, et al. Grape seed procyanidin B2 promotes the autophagy and apoptosis in colorectal cancer cells via regulating PI3K/Akt signaling pathway. Onco Targets Ther. 2019;12:4109–4118. doi:10.2147/OTT.S195615
18. Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi:10.1016/j.molcel.2010.09.023
19. Fan X-J, Wang Y, Wang L, Zhu M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36(6):3559–3567. doi:10.3892/or.2016.5138
20. Chang TC, Wei PL, Makondi PT, et al. Bromelain inhibits the ability of colorectal cancer cells to proliferate via activation of ROS production and autophagy. PLoS One. 2019;14(1):e0210274. doi:10.1371/journal.pone.0210274
21. Kaluzki I, Hailemariam-Jahn T, Doll M, et al. Dimethylfumarate inhibits colorectal carcinoma cell proliferation: evidence for cell cycle arrest, apoptosis and autophagy. Cells. 2019;8(11):
22. El-Kott AF, Al-Kahtani MA, Shati AA. Calycosin induces apoptosis in adenocarcinoma HT29 cells by inducing cytotoxic autophagy mediated by SIRT1/AMPK-induced inhibition of Akt/mTOR. Clin Exp Pharmacol Physiol. 2019;46(10):944–954. doi:10.1111/1440-1681.13133
23. Duarte A, André-Grégoire G, Trillet K, et al. Inhibition of mTOR in head and neck cancer cells alters endothelial cell morphology in a paracrine fashion. Mol Carcinog. 2019;58(1):161–168. doi:10.1002/mc.22911
24. Cheng Y, Huang L, Wang Y, et al. Strontium promotes osteogenic differentiation by activating autophagy via the the AMPK/mTOR signaling pathway in MC3T3‑E1 cells. Int J Mol Med. 2019;44(2):652–660. doi:10.3892/ijmm.2019.4216
25. Xing JJ, Hou JG, Ma ZN, et al. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019;52(4):e12627. doi:10.1111/cpr.12627
26. Teng JF, Qin DL, Mei QB, et al. Polyphyllin VI, a saponin from Trillium tschonoskii Maxim, induces apoptotic and autophagic cell death via the ROS triggered mTOR signaling pathway in non-small cell lung cancer. Pharmacol Res. 2019;147:104396. doi:10.1016/j.phrs.2019.104396
27. Sun J, Feng Y, Wang Y, et al. α-hederin induces autophagic cell death in colorectal cancer cells through reactive oxygen species dependent AMPK/mTOR signaling pathway activation. Int J Oncol. 2019;54(5):1601–1612. doi:10.3892/ijo.2019.4757
28. Yang H, Gao Y, Fan X, Liu X, Peng L, Ci X. Oridonin sensitizes cisplatin-induced apoptosis via AMPK/Akt/mTOR-dependent autophagosome accumulation in A549 cells. Front Oncol. 2019;9:769. doi:10.3389/fonc.2019.00769
29. Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32(1):2–11. doi:10.1128/MCB.06159-11
30. Mao K, Klionsky DJ. AMPK activates autophagy by phosphorylating ULK1. Circ Res. 2011;108(7):787–788. doi:10.1161/RES.0b013e3182194c29
31. Shu YJ, Bao RF, Wu XS, et al. Baicalin induces apoptosis of gallbladder carcinoma cells in vitro via a mitochondrial-mediated pathway and suppresses tumor growth in vivo. Anticancer Agents Med Chem. 2014;14:1136–1145. doi:10.2174/1871520614666140223191626
32. Lu J, Qin Q, Zhan LL, et al. AT-406, an IAP inhibitor, activates apoptosis and induces radiosensitization of normoxic and hypoxic cervical cancer cells. Pharmacol Sci. 2014;126:56–65. doi:10.1254/jphs.14079FP
33. Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2015;1219:1–9. doi:10.1007/978-1-4939-1661-0_11
34. Heqi B, Liu D, Cui J, Cai K, Shen F. Wnt/β-catenin signaling pathway is involved in induction of apoptosis by oridonin in colon cancer COLO205 cells. Transl Cancer Res. 2019;8:1782–1794. doi:10.21037/tcr.2019.08.25
35. Zhang D, Zhou Q, Huang D, et al. ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem Biophys Res Commun. 2019;513(3):594–601. doi:10.1016/j.bbrc.2019.04.011
36. Zhang D, Pan J, Xiang X, et al. Protein kinase Cδ suppresses autophagy to induce kidney cell apoptosis in cisplatin nephrotoxicity. J Am Soc Nephrol. 2017;28(4):1131–1144. doi:10.1681/ASN.2016030337
37. Duffy A, Le J, Sausville E, Emadi A. Autophagy modulation: a target for cancer treatment development. Cancer Chemother Pharmacol. 2015;75(3):439–447. doi:10.1007/s00280-014-2637-z
38. Cheng X, Feng H, Wu H, et al. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018;431:105–114. doi:10.1016/j.canlet.2018.05.046
39. Ni Y, Wu S, Wang X, et al. Cucurbitacin I induces pro-death autophagy in A549 cells via the ERK-Mtor-STAT3 signaling pathway. J Cell Biochem. 2018;119(7):6104–6112. doi:10.1002/jcb.26808
40. Xie K, Tian L, Guo X, et al. BmATG5 and BmATG6 mediate apoptosis following autophagy induced by 20-hydroxyecdysone or starvation. Autophagy. 2016;12(2):381–396. doi:10.1080/15548627.2015.1134079
41. ÁF F, Sebti S, Wei Y, et al. Disruption of the beclin 1–BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136–140. doi:10.1038/s41586-018-0162-7
42. Zhou L, Wang HF, Ren HG, et al. Bcl-2-dependent upregulation of autophagy by sequestosome 1/p62 in vitro. Acta Pharmacol. 2013;34(5):651–656.
43. Islam MA, Sooro MA, Zhang P. Autophagic regulation of p62 is critical for cancer therapy. Int J Mol Sci. 2018;19(5):1405. doi:10.3390/ijms19051405
44. Lin LT, Uen WC, Choong CY, et al. Paris polyphylla inhibits colorectal cancer cells via inducing autophagy and enhancing the efficacy of chemotherapeutic drug doxorubicin. Molecules. 2019;24(11):2102. doi:10.3390/molecules24112102
45. Booth LA, Roberts JL, Dent P. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin Cancer Biol. 2019;
46. Ou L, Lin S, Song B, Liu J, Lai R, Shao L. The mechanisms of graphene‑based materials‑induced programmed cell death: a review of apoptosis, autophagy, and programmed necrosis. Int J Nanomed. 2017;12:
47. Zhai B, Hu F, Jiang X, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13:
48. Yamate-Morgan H, Lauderdale K, Horeczko J, et al. Functional effects of cuprizone-induced demyelination in the presence of the mTOR-inhibitor rapamycin. Neuroscience. 2019;406:667–683. doi:10.1016/j.neuroscience.2019.01.038
49. Zhou JY, Huang DG, Qin YC, et al. mTORC1 signaling activation increases intestinal stem cell activity and promotes epithelial cell proliferation. J Cell Physiol. 2019;234(10):19028–19038. doi:10.1002/jcp.28542
50. Chen Y, Shiqiang X, Wang N, et al. Dynasore suppresses mTORC1 activity and induces autophagy to regulate the clearance of protein aggregates in neurodegenerative diseases. Neurotox Res. 2019;36(1):108–116. doi:10.1007/s12640-019-00027-9
51. Mokarram P, Albokashy M, Zarghooni M, et al. New frontiers in the treatment of colorectal cancer: autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13(5):781–819. doi:10.1080/15548627.2017.1290751
52. Zha QB, Zhang XY, Lin QR, et al. Cucurbitacin E induces autophagy via downregulating mTORC1 signaling and upregulating AMPK activity. PLoS One. 2015;10:e0124355. doi:10.1371/journal.pone.0124355
53. Xu Z, Huang CM, Shao Z, et al. Autophagy induced by areca nut extract contributes to decreasing cisplatin toxicity in oral squamous cell carcinoma cells: roles of reactive oxygen species/AMPK signaling. Int J Mol Sci. 2017;18(3):524. doi:10.3390/ijms18030524
54. Wang H, Zhang G. Activation of CaMKKβ-AMPK-mTOR pathway is required for autophagy induction by β,β-dimethylacrylshikonin against lung adenocarcinoma cells. Biochem Biophys Res Commun. 2019;517(3):477–483. doi:10.1016/j.bbrc.2019.07.100
55. Huang Q, Wang T, Yang L, Wang HY. Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. Int J Mol Sci. 2017;18(5):1063. doi:10.3390/ijms18051063
56. Yao Z, Xie F, Li M, et al. Oridonin induces autophagy via inhibition of glucose metabolism in p53-mutated colorectal cancer cells. Cell Death Dis. 2017;8(2):e2633. doi:10.1038/cddis.2017.35
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.