Back to Journals » International Journal of Nanomedicine » Volume 17
Amphiphilic Dendritic Nanomicelle-Mediated Delivery of Gemcitabine for Enhancing the Specificity and Effectiveness
Authors Zhao W, Yang S, Li C, Li F , Pang H, Xu G, Wang Y, Cong M
Received 21 April 2022
Accepted for publication 15 July 2022
Published 26 July 2022 Volume 2022:17 Pages 3239—3249
DOI https://doi.org/10.2147/IJN.S371775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Weidong Zhao,1,2,* Shaoyou Yang,3,* Chunxiao Li,1,2 Feifei Li,1,2 Houjun Pang,3 Guangling Xu,1,2 Yuxin Wang,3 Mei Cong3
1Henan Key Laboratory of Immunology and Targeted Drugs, School of Laboratory Medicine, Xinxiang Medical University, Xinxiang, People’s Republic of China; 2Henan Collaborative Innovation Center of Molecular Diagnosis and Laboratory Medicine, Xinxiang Medical University, Xinxiang, People’s Republic of China; 3School of Pharmacy, Xinxiang Medical University, Xinxiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mei Cong, School of Pharmacy, Xinxiang Medical University, Xinxiang, 453003, People’s Republic of China, Tel +86 0373 3029879, Fax + 86 0373 3029879, Email [email protected]
Purpose: Gemcitabine is the first line and the gold standard drug for pancreatic cancer. However, the anticancer efficacy is severely limited by its instability and poor cellular uptake. To enhance the clinical efficacy of gemcitabine, we constructed a novel nanodrug delivery system based on amphiphilic dendrimers and aliphatic gemcitabine prodrug.
Methods: An aliphatic gemcitabine prodrug and a small amphiphilic dendrimer were synthesized and characterized by high resolution mass spectrometry (HRMS) as well as nuclear magnetic resonance (NMR). Then the aliphatic gemcitabine prodrug was encapsulated into the small amphiphilic dendrimer by film dispersion method, resulting in a novel nanodrug delivery system. Subsequently, the size, morphology, drug loading, stability, drug release profiles, cell uptake, toxicity, the anticancer activity and in vivo distribution of the new developed gemcitabine delivery system were systematically evaluated by different technical methods, including transmission electron microscopy (TEM), dynamic light-scattering (DLS), ultraviolet spectrophotometer, flow cytometry, in vivo imaging system etc.
Results: We developed a novel nanodrug delivery system of gemcitabine using amphiphilic dendrimer. This dendrimer-based gemcitabine nanoformulation reported here possess a high drug loading of 33%. With the features of small size, stable formulation and pH-responsive drug release, the obtained gemcitabine nanoformulation could effectively accumulate in tumor site and rapid uptake in cells. Finally, the gemcitabine nanoformulation displayed more potent anticancer activity compared to free gemcitabine both in vitro and in vivo. Moreover, the nanodrug displayed greatly reduced adverse effects and satisfactory biocompatibility.
Conclusion: Benefiting the advantageous features of both amphiphilic dendrimers and nanotechnology-based drug delivery, this gemcitabine nanosystem constitutes a promising therapeutic candidate for pancreatic cancer treatment. This study also underlines the potential use of self-assembling amphiphilic dendrimer-based nanotechnology for improving drug efficacy as well as reducing drug toxicity.
Keywords: gemcitabine, amphiphilic dendrimer, self-assembling, pancreatic cancer, anticancer candidate
Graphical Abstract:
Introduction
Pancreatic cancer (PC) is one of the most lethal and devastating human cancers. It has an overall survival rate less than 10% and a median survival less than 6 months.1 As only less than 20% of PC patients can benefit from surgery treatment,2 chemotherapy is therefore a mainstream treatment against this cancer. Gemcitabine (Gem, 20, 20-difluorodeoxycytidine, dFdC), a pyrimidine analogue, is the first line and the gold standard drug for all stages of advanced PC since 1997.3 However, its efficacy remains largely unsatisfactory, the 5-year survival rate is only 2%.4 The clinical efficacy of Gem is mainly limited by its metabolic instability and poor cellular uptake, which results in frequent administration of Gem with high dosage, hence ending with serious systemic toxicity.5,6 In addition, it has reported that combination of Gem with other agents only has limited improvements in survival rates.7,8 Due to these facts, there is a pressing need to explore more effective therapeutic strategies for improving treatment efficacy of Gem to combat pancreatic cancer.
Nanotechnology has made remarkable advancement in cancer drug delivery. It is widely prospected to be a ground-breaking platform to improve the drug efficacy by delivering the drug to the tumor site and enhancing cellular internalization.9,10 As a result, there is an increasing interest to develop effective nanoformulations for improving treatment efficacy of Gem.6,11 Although various gemcitabine related nanoparticles have been evaluated in preclinical studies,12–14 the therapeutic benefit in terms of improved clinical outcomes has not been realized so far and the nanoformulation of gemcitabine is not currently clinically available. Therefore, more efficient and safety nanoformulations of PC chemotherapeutics are urgently needed.
Dendrimers are promising platform for the delivery of anti-cancer drugs and therapeutic oligonucleotides thanks to good biocompatibility, monodisperse, highly branched structure, tuneable size, presence of internal cavities, and easy functionalization.15,16 Especially, the spontaneous self-assembly of small amphiphilic dendrimers harness both the advantages offered by the self-assembling ability of lipid and the peculiar structure and stability of dendrimer for high drug loading capacity yet preserving small size and stable formulation.17,18
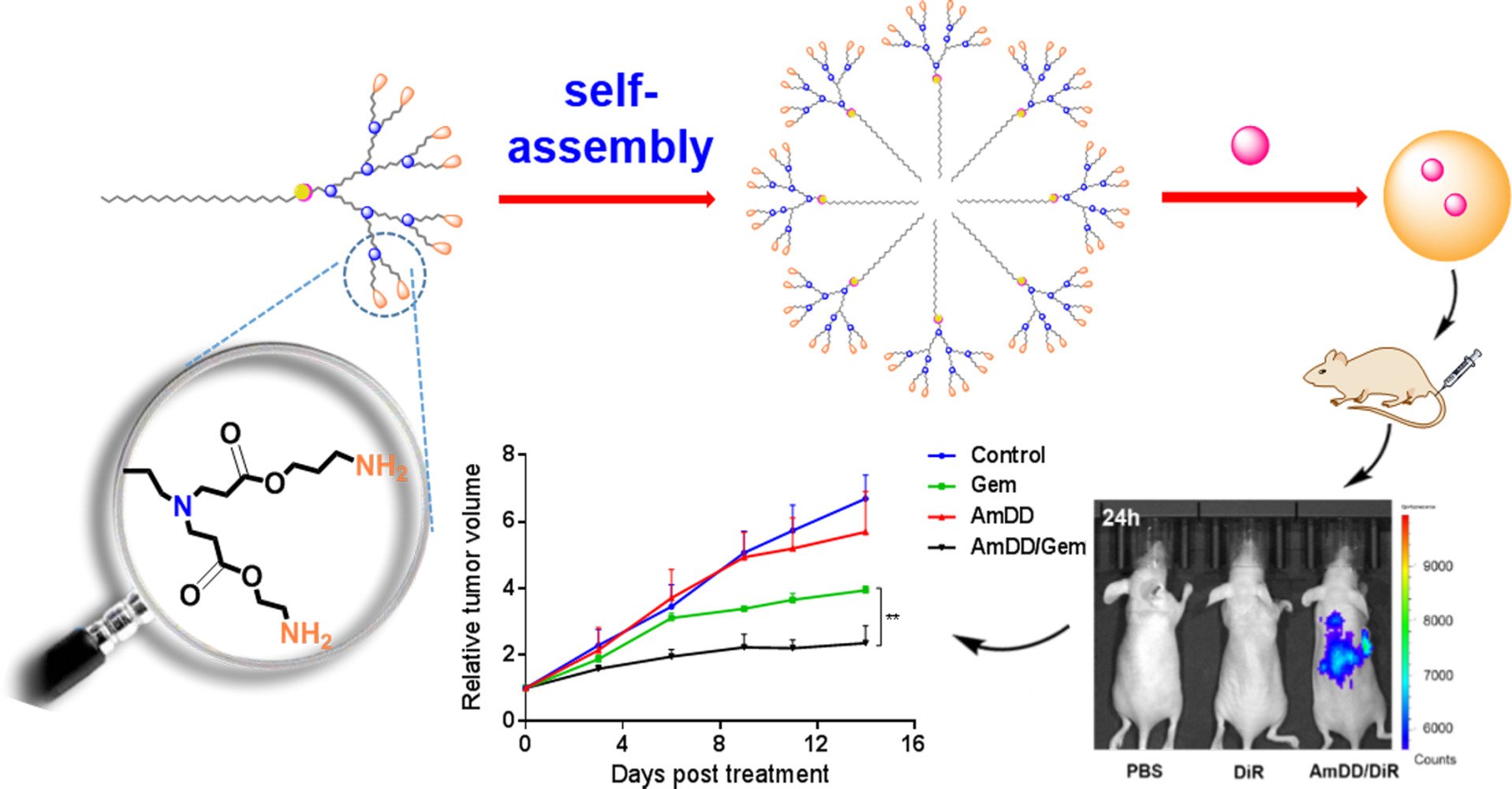
In this study, we firstly synthesized an aliphatic Gem prodrug, then encapsulated the gemcitabine prodrug into a small amphiphilic dendrimer which could self-assemble spontaneously into nanomicelles in water to improve the drug efficacy of Gem (Figure 1). Our resulting nanosystem based on small amphiphilic dendrimer exhibited remarkable features such as: (i) excellent stability to protect loaded drug from premature release, (ii) small size, resulting in effective accumulation, and (iii) efficient pH responsive drug release to boost tumor site drug concentration. Importantly, Gem-loaded nanomicelles (AmDD/Gem) possessed more potent anticancer activity and reduced adverse effects compared to standard drug Gem, which constitutes therefore an effective and promising candidate for pancreatic cancer treatment.
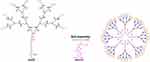 |
Figure 1 Cartoon illustration of self-assembling of amphiphilic dendrimer into nanomicelles for drug encapsulation. |
Materials and Methods
General Materials
The Gemcitabine prodrug Gem-C5 was synthesized according to the well-established protocol published by our group.19 The ester-terminating dendrimer C18-8E was synthesized according to a previously developed protocol.20,21 The chemical reagents used were purchased from Acros, Aldrich or Alfa Aesar. Methyl acrylate, N, N-dimethylethylenediamine, ethylenediamine, dichloromethane and methanol were dried using the described methods and distilled prior to use. The other chemicals were used without further purification. Analytical thin layer chromatography (TLC) was performed using silica gel 60 F254 plates 0.2 mm thick with UV light (254 and 364 nm) as revelator. Chromatography was prepared on silica gel (Merck 200–300 mesh). 1H NMR spectra were recorded at 400 MHz and 13C NMR spectra recorded at 100 MHz, on JEOL ECS 400 or VARIAN INOVA-400M. HRMS spectra were obtained on Bruker 7-Tesla FT-ICR MS equipped with an electrospray source or QStar Elite mass spectrometer (Applied Biosystems SCIEX, Concord, ON, Canada) equipped with an electrospray ionization source operated in the positive mode.
Synthesis, Preparation and Characterization of AmDD/Gem Nanomicelles
General Procedure for Synthesis of Compound AmDD
To a solution of C18-8E (112 mg) in methanol was added ethylenediamine. The reaction mixture was stirred under argon at 28°C until the IR analysis showed the complete consumption of the ester starting material. The reaction solution was evaporated, and the obtained residue was purified by a dialysis (MWCO 2000 Da), yielding the corresponding amine products. The obtained residue then purified by a dialysis to yield AmDD (108 mg, 85%) as a white solid. 1H NMR (400 MHz, CDCl3): δ 7.61 (s, 1H, CH), 4.25 (t, 2H, J = 8.0 Hz, CH2), 3.71 (s, 2H, CH2), 3.32–3.33 (m, 4H, CH2), 3.13–3.19 (m, 28H, CH2), 2.64–2.69 (m, 40H, CH2), 2.44–2.47 (m, 12H, CH2), 2.25–2.33 (m, 28H, CH2), 1.81 (br, 2H, CH2), 1.17 (br, 30H, CH2), 0.78–0.81 (m, 3H, CH3); 13C NMR (200 MHz, CD3OD): δ 175.3, 174.7, 144.7, 130.3, 128.2, 125.2, 124.0, 53.5, 51.4, 51.1, 50.4, 46.0, 41.9, 41.8, 38.6, 34.7, 33.1, 30.8, 30.6, 30.5, 27.6, 23.8, 22.8, 20.0, 14.5. HRMS: calcd for C91H184N32O14 2+ [M+2H]2+ 975.2343, found 975.2337. The data is in accordance to previous report.20
Preparation of Nanomicelles
AmDD/Gem loaded nanomicelles were prepared using amphiphilic dendrimer AmDD and Gem prodrug by the film dispersion method. Gem prodrug was dissolved in 1.0 mL mixed solvent (chloroform: methanol = 3:2, vol/vol) and mixed with amphiphilic dendrimer in 3.0 mL mixed solvent (chloroform: methanol = 3:2, vol/vol). The solvent was removed by vacuum rotary evaporation to form a dry drug-containing film. And then, this film was hydrated with phosphate buffered saline (PBS) for 30 min. Non-encapsulated Gem prodrug was separated by filtration of the micelle suspension through a polycarbonate membrane. The empty nanomicelles were prepared in an identical procedure except that no drug was present in the mix solvent. For fluorescent in vivo imaging, the near-infrared fluorescent probe, DiR, was encapsulated into nanomicelles. The DiR-loaded nanomicelles were prepared using the same procedures as Gem-C5 loading.
Drug Encapsulation Efficiency and Loading Content
Gem-C5 concentration in micelles was determined using a UV spectrometer with λmax = 249 nm. The drug encapsulation efficiency and drug-loading content were calculated as reported below: encapsulation efficiency (%) = Wt/Wi × 100%, drug loading content (%) = Wt/Ws × 100%, in which Wt represents the amount of Gem loaded into nanomicelles, Wi is the initial amount of Gem fed, and Ws represents the amount of drug loaded nanomicelles after lyophilization.
Micelle Morphology Characterization
The morphology of nanomicelles was determined using transmission electron microscope (TEM). Briefly, 5.0 μL of a solution of AmDD/Gem or AmDD were dropped onto the copper grids and air-dried at 42°C. The grids were stained with 1.0% (wt/vol) uranyl acetate solution for 30s before taking images. Then imaging was performed using a JEM-1200EX analytical electron microscope.
Micelle Size Characterization
Hydrodynamic sizes of the nanomicelles were measured in aqueous solution by dynamic light scattering (DLS) (Zetasizer Nano-ZS90, Malvern).
In vitro Drug Release
Release of Gem prodrug from AMDD/Gem was determined by the dialysis method. 200 μL of AMDD/Gem (1.0 mg/mL) were dialyzed at 37°C in PBS buffer of pH 7.4 and pH 5.0. Gem prodrug concentration was measured using a UV spectrometer (AS-Nano100a). Accumulative release of Gem produrg from AMDD/Gem nanomicelles was expressed as percentage of released Gem and plotted as a function of time.
In vitro Antiproliferation Activity Assay
The antiproliferation activity of free Gem, the Gem prodrug Gem-C5, and the AmDD/Gem nanomicelles against human PC cancer cells was evaluated using the CCK8 assays. The human pancreatic cancer cell lines SW1990, Mia-PaCa2 used in this study were generously provided by Dr. Zhong Genshen (Xinxiang Medical University) and approved by the Ethics Committee of Xinxiang Medical University (2019/11/20, XYLL-2019S017). The cells were cultured with DMEM medium with 10% FBS at 37°C with 5% CO2 humidified atmosphere. Mia-PaCa2 and SW1990 cells were seeded into a 96-well plate at 5000 and 4000 cells per well, respectively, and allowed to adhere overnight. Then the culture medium was removed and replaced with fresh media alone as control or containing different Gem formulation. After 72 h treatment, the number of viable cells remained was determined by Cell Counting Kit-8 (CCK8) assay. All experiments were done in triplicate and repeated three independent times.
Internalization of Free DiI and AmDD/DiI Nanomicelles
Internalization of free DiI and AmDD/DiI nanomicelles in SW1990 cells was examined using flow cytometry. 1.5×105 cells per well were seeded into six-well plates and incubated at 37°C overnight. The medium was then removed and replaced with free DiI and AmDD/DiI nanomicelles at a final concentration of 1.25 μg/mL for 10, 20, 30, 60, 120 and 240 min at 37°C. Finally, the cells were harvested and washed with 1× PBS solution three times and then analyzed by flow cytometry (FACS Calibur, BD). Each assay was performed in triplicate.
In vivo Anticancer Activity Assay
The in vivo behavior of free Gem, AmDD/Gem and Gem prodrug in pancreatic cancers was investigated in a Balb/c nude mice xenograft model. All animal procedures were performed according to guidelines provided by the Institutional Animal Care and Use Committee (IACUC) of the Xinxiang Medical University and approved by the Ethics Committee of Xinxiang Medical University (2019/11/20, XYLL-2019S017). Five-week-old female Balb/c nude mice were purchased from Vital River Laboratories (Beijing, China) and bred in the authorized SPF (specific pathogen free) animal facility at Xinxiang Medical University. Pancreatic cancer cell SW1990 (1×106) in 0.1 mL of cold PBS were inoculated subcutaneously to the nude mice. When tumors reached around 50 mm3, xenograft mice were randomly assigned to either treatment group or control group. 5.0 mg/kg body weight of compound was administrated by intravenous injections (i.v) to the mice (treatment group) once 3d for 15d. Tumor progression in the mice was measured by Vernier calipers and tumor volume was calculated as described previously using the following formula: L × W2/2,22 in which L and W are the longest and shortest tumor diameters, respectively. Mice weight was also assessed twice a week. After 14 days of treatment, the experiment was halted and animals in each group were sacrificed and different organs were harvested and half of which were fixed in 4.0% paraformaldehyde solution for histopathological analysis.
In vivo Biodistribution
AmDD/DiR nanomicelles were prepared using the same method described above. To determine their in vivo biodistribution, female Balb/c nude mice bearing SW1990 tumors were injected intravenous (i.v.) with free DiR and AmDD/DiR nanomicelles at a dose corresponding to 60 μg/Kg of DiR via tail vein. The mice injected with PBS were used as control. The real-time distribution and tumor accumulation of free DiR, AmDD/DiR nanomicelles and PBS were recorded using an in vivo imaging system (PerkinElmer IVIS Lumina III).
Statistics
Statistical analysis was performed with Prism software v6 (GraphPad Software). Data were expressed as the means ± standard error of the mean (SEM). Student’s t test (two-tailed) was applied to determine differences between two means. For the comparison of multiple groups, one-way ANOVA followed by a Tukey’s multiple comparisons test was used. A P-value of < 0.05 was considered statistically significant.
Results and Discussion
The Amphiphilic Dendrimer AmDD Forms Stable Nanomicelles with Lipophilic Gemcitabine Prodrug
Gemcitabine is used as clinical drug either alone or in combination, to treat certain types of advanced or metastatic cancers including pancreatic, non-small cell lung, ovarian cancers.23–25 However, its clinical application is severely limited by its rapid inactivation and serious side effects.5,26 Nanotechnology-based drug delivery is expected to overcome these problems and improve drug efficacy.27 Various nanosystems of gemcitabine have been reported for improving its therapeutic efficacy.6,28–31 Most of those studies are mainly focused on lipid- or polymer-based nanosystems. However, lipid-based nanosystems usually have the drawback of limited stability,32 whereas polymers are plagued with poor drug loading and dispersed molecular weight distribution.33,34 An ideal nanosystems-based delivery system is expected to harbor the advantageous features of both lipid and polymer vectors while overcoming their pitfalls. It has been reported that nanosystems formed by amphiphilic dendrimers are able to combine the self-assembling feature of lipids and the stability of dendrimeric polymers.18 Here, we proposed to construct a novel gemcitabine drug delivery system based on amphiphilic dendrimer.
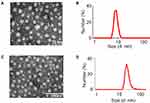
The preparation of the amphiphilic dendrimer-based gemcitabine nanosystem (AmDD/Gem) is facile and can be performed in three steps. First, an aliphatic chain-functionalized Gem prodrug (Gem-C5) was synthesized by the amination of Gem with 4-pentynoic acid to protect against deamination (Supplementary Figure S1), which was verified by nuclear magnetic resonance (NMR) analysis and high resolution mass spectrometry (HRMS) (Supplementary Figures S2 and S3). Moreover, this modification delivered a more lipophilic drug (Gem-C5) for its loading into small amphiphilic dendrimer (AmDD). Second, the AmDD which contains one hydrophilic poly (amido) amine dendron part and one hydrophobic a C18 alkyl chain was synthesized according to previous reported method (Supplementary Figures S4),20 which was verified by NMR analysis and HRMS (Supplementary Figures S5 and S6). AmDD could easily form nanomicelles by using the film dispersion method, as revealed by transmission electron microscopy (TEM) imaging (Figure 2A) and dynamic light-scattering (DLS) analysis (Figure 2B). Finally, the fabrication of the AmDD/Gem nanosystems were prepared by using the same film dispersion method. According to TEM observations, the obtaining AmDD/Gem systems consisted in small size, spherical morphology and uniformly dispersed (Figure 2C). The particle size distribution measurement results further confirmed this conclusion. The average size distribution of AmDD/Gem was ∼20 nm (Figure 2D).
AmDD/Gem Nanomicelles Showed High Drug Loading and Stability, and pH-Sensitive Release
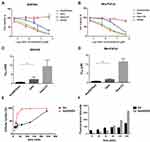
Next, the drug encapsulation efficiencies at different weight ratios of AmDD/Gem were measured and a weight ratio of 3/2 (Supplementary Table S1, entry 2) was chosen for subsequent experiments for its high drug loading (33%) and encapsulation efficiency (69%). Additionally, AmDD/Gem nanomicelles did not exhibit any notable change in size even after storage at 4°C for 4 weeks (Figure 3A). All these evidences indicate that AmDD/Gem nanomicelles have high drug loading efficiency and stability.
The controlled release of drugs at the tumor site is an important parameter of a drug delivery system in cancer therapy, which could reduce the toxicity to the normal tissues. It has been reported that tumor lesions are more acidic than normal tissues.35 Therefore, acid promoted/triggered drug release is a favorable advantage of drug delivery systems to achieve specific release at tumor sites. We hence evaluated Gem prodrug release from AmDD/Gem micelles under the mimic acidic environment of tumor site (pH 5.0) in contrast to the neutral physiological pH 7.4. As shown in Figure 3B, Gem prodrug release from AmDD/Gem nanomicelles at pH 5.0 was more rapid and efficient when compared to that at neutral pH 7.4. Specifically, the drug release attained more than 80% of the total Gem prodrug at pH 5.0 after 24 h, while only around 20% at pH 7.4. This stability of AmDD/Gem nanomicelles under neutral environment was conducive to the efficient delivery of loaded drugs to tumor sites, while reducing systemic toxicity associated with drug leakage in the circulation during transport. These results highlight an acid-promoted release of Gem from the AmDD/Gem nanomicelles, which is beneficial for prolonging the plasma half-life of Gem and enhancing the delivery of the drug to tumor sites.
AmDD/Gem Nanomicelles Improved the Antiproliferative Activity of Gem on Pancreatic Cancer Cells Through an Effectively and Consistently Enhanced Cellular Uptake
Motivated by the high drug loading and acid-promoted release offered by AmDD/Gem nanomicelles, we further assessed the antiproliferative activity of AmDD/Gem by using CCK8 assays in human PC cell lines (Mia-PaCa2 and SW1990). Free Gem, Gem-C5 and AmDD/Gem nanomicelles all showed concentration-dependent ability to inhibit the cancer cell proliferation. AmDD had no impact on cell viability in the two tested PC cell lines. AmDD/Gem displayed an enhanced antiproliferative efficacy than free Gem on either Mia-PaCa2 or SW1990 cells (Figure 4A and B). The half-maximal inhibitory concentration (IC50) values for AmDD/Gem and Gem were observed at 0.69 ± 0.18 and 1.78 ± 0.22 (p ˂ 0.01) in Mia-PaCa2 cells, 0.72 ± 0.15 and 2.35 ± 0.59 (p ˂ 0.05) in SW1990 cells, respectively (Figure 4C and D, Supplementary Table S2).
Gem-C5 showed slightly weaker antiproliferation activity on tumor cells than Gem, possibly as a consequence of the prodrug effect. A prodrug is normally a biologically inactive form that needs a chemical transformation to release the drug in active form within the body. Therefore, it is acceptable that the activity of a prodrug is sometimes lower or equivalent compared to the parent drug in an in vitro assay. In addition, much lower IC50 values were obtained for Gem-C5 (11.48 ± 0.85 and 9.46 ± 1.70 in Mia-PaCa2 and SW1990 cells, respectively) than AmDD/Gem (Figure 4C and D), showing AmDD/Gem nanomicelles exhibited more effective antiproliferation activity than the prodrug Gem-C5, which might be attributed to the enhanced cellular uptake of the nanomicelles. In order to confirm this speculation, a fluorescently labeled AmDD was developed via loading a lipophilic fluorescent dye DiI. Then the DiI-loaded nanoparticles (hereby referred to as AmDD/DiI) were incubated with SW1990 cells. The cellular uptake of AmDD/DiI was observed via flow cytometry. As represented in Figure 4E and F, the cellular uptake of AmDD/DiI was markedly rapid and efficient compared with that of free DiI (Figures 4E and F, S7). After 4 h treatment, the mean fluorescence intensity detected in AmDD/DiI-treated cells was about 2 times higher than that of DiI-treated cells (Figure 4F). These results show that the cellular uptake of AmDD/Dil nanomicelles was obviously faster and more efficient than free DiI. As a consequence, the intracellular drug concentration was higher in cells treated with the nanomicelles than in cells treated with the free drug, confirming that the enhanced antiproliferative effects of AmDD/Gem was attribute to the increased cellular uptake of the drugs.
AmDD/Gem Nanomicelles Behaved Superior Antitumor Activity and Lower Toxic Side Effects Compared to Gem in PC Cancer Bearing Mice
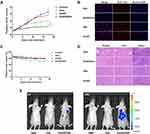
We next evaluated the anticancer activity of AmDD/Gem nanomicelles using pancreatic cancer SW1990 xenograft mice. We set the dosage of Gem of 5.0 mg/kg on the basis of our in vitro evaluation data and numerous reported studies1,23,36 in the view of inspecting the efficacy and the safety profile of AmDD/Gem nanomicelles. When the tumor reached to a certain volume of approximately 50 mm3, the mice were separated randomly into 4 groups and treated intravenously with PBS (Control), AmDD, Gem and AmDD/Gem nanomicelles respectively. During the course of the experiment, AmDD/Gem nanomicelles treatment resulted in significant attenuation on tumor growth in mice compared with Gem treatment (Figures 5A and S8), underlining the superior anticancer activity of AmDD/Gem nanomicelles. This was further confirmed using immunohistochemical (IHC) analysis with Ki-67 that is used as a proliferation marker for tumor cells. The expression of Ki-67 in tumor cells from AmDD/Gem-treated PC tumor bearing mice was decreased than that from Gem group. As a control, Ki-67 maintained a high level of expression in tumor cells from PBS group (Figures 5B and S9). In addition, the body weight changes of mice were also recorded every 2 days during the experiment. As presented in Figure 5C, a significant body weight loss was observed in the free Gem group, while no obvious body weight loss was found in the other groups, suggesting the less body harm of AmDD/Gem treatment than that of Gem treatment. Additionally, Gem has been shown to cause many side effects such as nephrotoxicity and hepatotoxicity.37 Therefore, we further assessed the pathological changes caused by AmDD/Gem in mice organs of kidney and liver using hematoxylin and eosin (H&E) staining (Figure 5D). There were no visible effects of toxicity in either kidney or liver in both AmDD/Gem and AmDD groups as compared with those in the PBS group. However, the obvious tissue injuries were obtained in both liver and kidney in the free Gem group. These results highlight the superior safety of AmDD/Gem nanomicelles compared with free Gem.
To shed light on the mechanism by which AmDD/Gem enhances the anticancer activities and reduces the side effects, we inspected the distribution of free drug and nanodrug in mice via an in vivo imaging system. We first loaded a near-infrared fluorescent dye DiR into AmDD (AmDD/DiR). The fluorescence of DiR can effectively penetrate muscle and skin tissues and is sensitively detected by live imaging. Then SW1990 tumor bearing mice were separated into 3 groups and treated by intravenous injection with AmDD/DiR nanomicelles, PBS or free DiR respectively. The fluorescence signals in the mice were detected 4 h and 24 h post-injection by small animal live imaging system. As shown in Figure 5E, the fluorescence intensity of the tumor site was markedly enhanced at 24 h than that at 4h in the AmDD/DiR group, and the fluorescence intensities were much stronger in AmDD/DiR group than those in the free DiR group at each corresponding time points. These results reveal that AmDD/Gem nanomicelles are capable of inducing the drug accumulation at tumor site.
Conclusion
Pancreatic cancer is one of the most lethal malignancies that is usually detected at an advanced stage. Due to the most current treatment regimens are ineffective and contributes to the side effects and poor prognosis, more effective agents are urgently needed. In this present work, we have elaborated an innovative Gem nanodrug based on amphiphilic dendrimer. The nanosystem is particularly promising because of its high drug loading, small size, stable formulation and acid-promoted drug release. By virtue of these remarkable features, this dendrimer nanosystem can be effectively accumulated at tumor and displays rapid drug uptake profile in the cancer cells, leading to potent anticancer activity and reduced adverse effects in mice model. Collectively, these results demonstrate that this simple and convenient dendrimer nanodrug system constitutes a promising therapeutic candidate for further PC translational investigations. This study has also demonstrated the potential use of self-assembling amphiphilic dendrimer-based nanotechnology for improving drug efficacy and reducing drug toxicity.
Acknowledgments
Financial support was from the National Natural Science Foundation of China (81903567, 31600109), Henan Programs for Science and Technology Development (182102310221), the PhD startup fund of Xinxiang Medical University (505158). We thank Jixia Zhang and Tangqiang Sun from Xinxiang Medical University for their help in the NMR experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85. doi:10.1016/S0140-6736(16)00141-0
2. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi:10.1056/NEJMra1404198
3. Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi:10.1200/JCO.1997.15.6.2403
4. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi:10.1056/NEJMoa1304369
5. Moysan E, Bastiat G, Benoit JP. Gemcitabine versus modified gemcitabine: a review of several promising chemical modifications. Mol Pharm. 2013;10(2):430–444. doi:10.1021/mp300370t
6. Paroha S, Verma J, Dubey RD, et al. Recent advances and prospects in gemcitabine drug delivery systems. Int J Pharm. 2021;592:120043. doi:10.1016/j.ijpharm.2020.120043
7. Reni M, Cereda S, Galli L. PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) for patients with advanced pancreatic cancer: the ghost regimen. Cancer Lett. 2007;256(1):25–28. doi:10.1016/j.canlet.2007.04.009
8. Tahara J, Shimizu K, Otsuka N, et al. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2018;82(2):245–250. doi:10.1007/s00280-018-3611-y
9. Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
10. Adiseshaiah PP, Crist RM, Hook SS, et al. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol. 2016;13(12):750–765. doi:10.1038/nrclinonc.2016.119
11. Tucci ST, Kheirolomoom A, Ingham ES, et al. Tumor-specific delivery of gemcitabine with activatable liposomes. J Control Release. 2019;309:277–288. doi:10.1016/j.jconrel.2019.07.014
12. Sun J, Kim D-H, Guo Y, et al. A c (RGDfE) conjugated multi-functional nanomedicine delivery system for targeted pancreatic cancer therapy. J Mater Chem B. 2015;3(6):1049–1058. doi:10.1039/C4TB01402B
13. Zhao X, Wang X, Sun W, et al. Precision design of nanomedicines to restore gemcitabine chemosensitivity for personalized pancreatic ductal adenocarcinoma treatment. Biomaterials. 2018;158:44–55. doi:10.1016/j.biomaterials.2017.12.015
14. Sobot D, Mura S, Yesylevskyy SO, et al. Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat Commun. 2017;8(1):1–9. doi:10.1038/ncomms15678
15. Mintzer MA, Grinstaff MW. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40(1):173–190. doi:10.1039/B901839P
16. Mignani S, Shi X, Rodrigues J, et al. First-in-class and best-in-class dendrimer nanoplatforms from concept to clinic: lessons learned moving forward. Eur J Med Chem. 2021;219:113456. doi:10.1016/j.ejmech.2021.113456
17. Percec V, Wilson DA, Leowanawat P, et al. Self-assembly of janus dendrimers into uniform dendrimersomes and other complex architectures. Science. 2010;328(5981):1009–1014. doi:10.1126/science.1185547
18. Lyu Z, Ding L, Tintaru A, et al. Self-assembling supramolecular dendrimers for biomedical applications: lessons learned from poly (amido) amine dendrimers. Acc Chem Res. 2020;53(12):2936–2949. doi:10.1021/acs.accounts.0c00589
19. Cong M, Xu G, Yang S, et al. A self-assembling prodrug nanosystem to enhance metabolic stability and anticancer activity of gemcitabine. Chinese Chem Lett. 2022;33(5):2481–2485. doi:10.1016/j.cclet.2021.11.083
20. Yu T, Liu X, Bolcato-Bellemin A, et al. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew Chem Int Ed. 2012;51(34):8478–8484. doi:10.1002/anie.201203920
21. Zhou Z, Cong M, Li M, et al. Negative dendritic effect on enzymatic hydrolysis of dendrimer conjugates. Chem Commun. 2018;54(47):5956–5959. doi:10.1039/C8CC01221K
22. Wei T, Chen C, Liu J, et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci USA. 2015;112(10):2978–2983. doi:10.1073/pnas.1418494112
23. Zhang Y, Chen L, Hu G-Q, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–1135. doi:10.1056/NEJMoa1905287
24. Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi:10.1016/S0140-6736(20)30974-0
25. Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled Phase 3 trial. Lancet Oncol. 2015;16(7):763–774. doi:10.1016/S1470-2045(15)00021-2
26. Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. 2007;97(2):145–151. doi:10.1038/sj.bjc.6603860
27. van der Meel R, Sulheim E, Shi Y, et al. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–1017. doi:10.1038/s41565-019-0567-y
28. Birhanu G, Javar HA, Seyedjafari E, et al. Nanotechnology for delivery of gemcitabine to treat pancreatic cancer. Biomed Pharmacother. 2017;88:635–643. doi:10.1016/j.biopha.2017.01.071
29. Chen Z, Zheng Y, Shi Y, et al. Overcoming tumor cell chemoresistance using nanoparticles: lysosomes are beneficial for (stearoyl) gemcitabine-incorporated solid lipid nanoparticles. Int J Nanomedicine. 2018;13:319–336. doi:10.2147/IJN.S149196
30. Wang Y, Fan W, Dai X, et al. Enhanced tumor delivery of gemcitabine via peg-dspe/tpgs mixed micelles. Mol Pharmaceut. 2014;11(4):1140–1150. doi:10.1021/mp4005904
31. Daman Z, Ostad S, Amini M, et al. Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: a comparison with its self-assembled nanoparticles. Int J Pharm. 2014;468(1):142–151. doi:10.1016/j.ijpharm.2014.04.021
32. Grimaldi N, Andrade F, Segovia N, et al. Lipid-based nanovesicles for nanomedicine. Chem Soc Rev. 2016;45(23):6520–6545. doi:10.1039/C6CS00409A
33. Ferrari R, Sponchioni M, Morbidelli M, et al. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale. 2018;10(48):22701–22719. doi:10.1039/C8NR05933K
34. Shah S, Famta P, Raghuvanshi RS, et al. Lipid polymer hybrid nanocarriers: insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interfac Sci. 2022;46:100570. doi:10.1016/j.colcom.2021.100570
35. Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335(6070):813–817. doi:10.1126/science.1205962
36. Shroff RT, Javle MM, Xiao LC, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824–830. doi:10.1001/jamaoncol.2019.0270
37. Khare V, Kour S, Alam N, et al. Synthesis, characterization and mechanistic-insight into the antiproliferative potential of PLGA-gemcitabine conjugate. Int J Pharm. 2014;470(470):51–62. doi:10.1016/j.ijpharm.2014.05.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.