Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
Amoxicillin concentrations in relation to beta-lactamase activity in sputum during exacerbations of chronic obstructive pulmonary disease
Authors Brusse-Keizer M , VanderValk P, van der Zanden R, Nijdam L, van der Palen J , Hendrix R, Movig K
Received 1 July 2014
Accepted for publication 5 September 2014
Published 3 March 2015 Volume 2015:10(1) Pages 455—461
DOI https://doi.org/10.2147/COPD.S70355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Marjolein Brusse-Keizer,1 Paul VanderValk,2 Rogier W van der Zanden,3 Lars Nijdam,4 Job van der Palen,1,5 Ron Hendrix,6,7 Kris Movig4
1Medical School Twente, 2Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, 3Department of Clinical Pharmacy and Toxicology, Maastricht University Medical Centre, Maastricht, 4Department of Clinical Pharmacy, Medisch Spectrum Twente, 5Department of Research Methodology, Measurement and Data Analysis, University of Twente, 6Regional Laboratory of Public Health, Enschede, 7Department of Medical Microbiology, University Medical Centre Groningen, and University of Groningen, Groningen, the Netherlands
Background: Acute exacerbations of chronic obstructive pulmonary disease (COPD) are often treated with antibiotics. Theoretically, to be maximally effective, the antibiotic concentration at sites of infection should exceed the minimum inhibitory concentration at which 90% of the growth of potential pathogens is inhibited (MIC90). A previous study showed that most hospitalized COPD patients had sputum amoxicillin concentrations <MIC90 when treated with amoxicillin/clavulanic acid. Those with adequate sputum concentrations had better clinical outcomes. Low amoxicillin concentrations can be caused by beta-lactamase activity in the lungs. This study investigated whether patients with sputum amoxicillin concentrations <MIC90 had higher beta-lactamase activity in sputum than patients with a concentration ≥MIC90.
Methods: In total, 23 patients hospitalized for acute exacerbations of COPD and treated with amoxicillin/clavulanic acid were included. Sputum and serum samples were collected at day 3 of treatment to determine beta-lactamase activity in sputum and amoxicillin concentrations in both sputum and serum.
Results: We found no difference in beta-lactamase activity between patients with sputum amoxicillin concentrations <MIC90 and ≥MIC90 (P=0.79). Multivariate logistic regression analysis showed no significant relationship between beta-lactamase activity and sputum amoxicillin concentrations <MIC90 or ≥MIC90 (odds ratio 0.53; 95% confidence interval 0.23–1.2; P=0.13). Amoxicillin concentrations were <MIC90 in 78% of sputum samples and in 30% of serum samples.
Conclusion: In patients treated with amoxicillin/clavulanic acid for an acute exacerbation of COPD, sputum beta-lactamase activity did not differ between those with sputum amoxicillin concentrations <MIC90 or ≥MIC90. The finding that the majority of patients had sputum amoxicillin concentrations <MIC90 suggests that current treatment with antibiotics for acute exacerbations of COPD should be optimized.
Keywords: chronic obstructive pulmonary disease, exacerbation, amoxicillin, clavulanic acid, MIC90, concentration
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality throughout the world. The prevalence and burden of COPD are projected to increase in the coming decades due to continued exposure to COPD risk factors and the changing age structure of the world’s population, with COPD likely to become the third leading cause of death by 2020.1 Morbidity and mortality among patients with COPD are in large part related to acute exacerbations (AECOPD), which impair respiratory, physical, social, and emotional functioning both acutely and longitudinally.2–4
The management of AECOPD is empirical and includes oral corticosteroids, often combined with antibiotics, although the need to prescribe these antibiotics is still not convincingly demonstrated.5 Most placebo-controlled antibiotics trials that have been performed have important limitations and are difficult to compare because different definitions of COPD and AECOPD and different endpoints have been used.6,7 Further, these trials were conducted several decades ago, before systemic steroids were widely introduced for the treatment of AECOPD.8–11 In a more recent placebo-controlled study by Llor et al, treatment with amoxicillin/clavulanic acid did show a beneficial effect; however, only 17% of these patients received systemic steroids.12 In a study by Daniels et al, in which the add-on effect of antibiotics was investigated, no difference in clinical outcome after 30 days was observed.13
Theoretically, to be maximally effective, the antibiotic concentration at sites of infection should exceed the minimum inhibitory concentration at which 90% (MIC90) of the growth of potential COPD pathogens such as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis is inhibited. Levels of antimicrobial agents in sputum, where as a representation of the site of infection many potential pathogenic microorganisms are located, may be a more relevant predictor of efficacy of antibiotics in treatment of an AECOPD than concentrations in serum.14–16 An antibiotic widely used in the treatment of AECOPD is amoxicillin/clavulanic acid.
In a former study by Brusse-Keizer et al, in which 33 COPD patients were treated with amoxicillin/clavulanic acid for AECOPD, sputum amoxicillin concentrations proved to be an important determinant of clinical outcome. Patients with sputum amoxicillin concentrations <MIC90 were hospitalized for 4 days longer than patients with an amoxicillin concentration in sputum ≥MIC90 (7 versus 11 days). Moreover, 67% of patients in this study had a sputum amoxicillin concentration <MIC90.17
The sputum amoxicillin concentration may differ markedly from the concentrations in serum due to various factors, such as the diffusion of amoxicillin into the airways,18 which could be both a host-related as well as a drug-related factor.
A well-known drug-related factor associated with amoxicillin is the susceptibility of amoxicillin to breakdown by beta-lactamase enzymes. In COPD, there are several potential pathogens (eg, H. influenza and M. catarrhalis) that produce these beta-lactamase enzymes.19 The use of beta-lactamase inhibitors, such as clavulanic acid, allows inactivation of certain beta-lactamases.20 An excessive beta-lactamase activity in sputum of COPD patients compared with the dosage of clavulanic acid could possibly be an explanation for the earlier observed low numbers of COPD patients in which an amoxicillin concentration above the MIC90 was reached.
We therefore conducted a study to investigate whether there was any difference in beta-lactamase activity between COPD patients with a sputum amoxicillin concentration <MIC90 and those with a concentration ≥MIC90.
Materials and methods
Patients
This study was part of the Cohort of Mortality and Inflammation in COPD (COMIC) study, a single-center prospective cohort study on the immune status of COPD patients as a determinant of survival. From December 2005 until April 2010, 795 patients were included in the cohort, with a follow-up period of 3 years. To be eligible for the cohort, patients had to meet the following criteria: a clinical diagnosis of COPD, as defined by the Global Initiative for Chronic Obstructive Lung Disease criteria;21 current or former smoker; age 40 years or over; no medical condition compromising survival within the follow-up period or serious psychiatric morbidity; absence of any other active lung disease (eg, sarcoïdosis); no maintenance therapy with antibiotics; and ability to speak Dutch.
In this exploratory study, we had no data available on which we could base our power calculations. We therefore included all patients from the COMIC study when they were hospitalized for an AECOPD that was treated with amoxicillin/clavulanic acid between November 2009 and March 2010.
An AECOPD was defined as an acute event characterized by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication.21,22 Patients with pneumonia were not excluded, and pneumonia was defined as an acute respiratory infection combined with an infiltrate of the lungs, which was visible on an X-ray of the chest. The infiltrate had to be non-pre-existent, nor reasonably caused by another cause than pneumonia. Further, patients had to be able to produce a sputum sample.
The medical ethics committee of Medisch Specrtum Twente, Enschede, the Netherlands approved the COMIC study and the amendment for the current study, and all patients provided written informed consent for both the COMIC study itself and the amendment. All patients received usual care, which included oral corticosteroids and amoxicillin/clavulanic acid started on the day of admission. Amoxicillin/clavulanic acid was administered according to regular care, which was either orally (500/125 mg three or four times a day) or intravenously (1,000/200 mg four times a day). Some patients received oral and intravenous amoxicillin/clavulanic acid sequentially.
Outcome measures
On the first day of admission, C-reactive protein was measured in blood using the NyoCard® CRP Single Test (Clindia Diagnostics, Leusden, the Netherlands). On the third day of treatment with amoxicillin/clavulanic acid, sputum and serum samples were collected. Sample collection was performed on the third day of treatment because, theoretically, steady-state amoxicillin concentrations in both serum and sputum should be reached by then.23 Serum samples were stored at –80°C. Sputum samples were stored at –80°C after a culture was performed.
Before measuring amoxicillin concentrations and beta-lactamase activity in sputum, sputum samples were thawed and homogenized using a MagNA lyser (3×60 seconds at 6,500 rpm; Roche Diagnostics, Indianapolis, IN, USA). Lysing of cells during the homogenization process was confirmed by microscopy. After homogenization, vials were centrifuged at 10,000 g for 5 minutes to separate cell debris. Amoxicillin concentrations in serum and homogenized sputum samples were determined using a high-performance liquid chromatography/tandem mass spectrometry method.
For amoxicillin, an a priori cut-off level of 2 mg/L was defined as an adequate concentration in both serum and sputum. This value corresponds to the MIC90 for amoxicillin/clavulanic acid. The MIC90 used in this study is derived from local susceptibility tests from the Regional Laboratory of Public Health and is comparable with data published by the European Committee On Susceptibility Testing.24
Beta-lactamase activity was measured directly in homogenized sputum samples by measuring the turnover rate of nitrocefin (Calbiochem, San Diego, CA, USA). Nitrocefin is a beta-lactam with chromogenic properties; it changes color from yellow to red under the influence of beta-lactamase activity. Nitrocefin turnover was measured with spectrophotometry at λ=490 nm in a mixture of 50 μL of homogenized sputum and 50 μL of a 0.025% nitrocefin solution. Beta-lactamase activity was quantified by comparing the measured activity with the activity of a beta-lactamase positive lysate from a H. influenzae culture. Beta-lactamase activity was calculated as a percentage of the activity of the H. influenzae lysate.
Statistical analysis
Continuous variables are expressed as the mean (standard deviation [SD]) or as the median (interquartile range [IQR]). Categorical variables are displayed as numbers (percentages). The crude relationship between beta-lactamase activity and sputum amoxicillin concentrations (<MIC90 or ≥MIC90) was analyzed using the Mann–Whitney U test. To identify confounders in this relationship, first the association of a priori selected possible confounders with beta-lactamase activity was analyzed by Pearson correlation tests, independent samples t-tests, and analysis of variance for normally distributed (continuous/dichotomous/categorical) variables. For non-normally distributed variables, this was performed with, respectively, Spearman correlation tests, Mann–Whitney U tests, and Kruskal–Wallis tests. Variables associated with beta-lactamase activity with a significance of P<0.15 were tested for an association with sputum amoxicillin concentration (<MIC90 or ≥MIC90). For categorical variables, these associations were tested by chi-square tests or by Fisher’s Exact tests, and for continuous variables by independent samples t-tests or Mann–Whitney U tests. Variables that were also associated with sputum amoxicillin concentration with a significance of P<0.15 were considered as potential confounders in the relationship between beta-lactamase activity and sputum amoxicillin concentration and were entered in a multivariate logistic regression model. Subsequently, variables with the highest P-values were eliminated step by step, until the fit of the model decreased significantly, based on the –2 log likelihood. The statistical analyses were performed using Statistical Package for the Social Sciences version 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
Between November 2009 and March 2010, 147 patients were screened for eligibility (Figure 1). Of the 30 patients included, 23 provided a sufficient amount of sputum. Table 1 shows the demographic and clinical characteristics of these 23 patients. The organisms isolated in all patients were confirmed to be susceptible to amoxicillin.
  | Figure 1 Flowchart of study inclusion. |
The univariate analysis showed no difference in beta-lactamase activity between patients with a sputum amoxicillin concentration <MIC90 and patients with a concentration ≥MIC90 with, respectively, a median beta-lactamase activity of 0.35 (IQR 0.26–0.59) and 0.32 (IQR 0.18–0.62; P=0.79). Also when individual data of beta-lactamase activity and sputum amoxicillin concentrations were plotted in a scatter diagram (Figure 2) no correlation could be observed (r=−0.06, P=0.80). In 18 of 23 sputum samples (78%), amoxicillin concentrations were below the MIC90. In six of those samples (26%), the amoxicillin concentration was undetectable. Seven of 23 serum samples (30%) had an amoxicillin concentration below the MIC90. All serum samples had detectable levels of amoxicillin.
  | Figure 2 Scatter diagram of amoxicillin sputum concentration and beta-lactamase activity. |
Only route of administration, median daily clavulanic acid dose, and C-reactive protein concentration at admission were univariately associated with beta-lactamase activity (P<0.15; Table 2) and were tested for an association with sputum amoxicillin concentration. All three were also significantly associated with sputum amoxicillin concentration <MIC90 or ≥MIC90 (Table 3). Multivariate logistic regression analysis showed no significant relationship between beta-lactamase activity and sputum amoxicillin concentrations <MIC90 versus ≥MIC90 (odds ratio 0.53 for a 0.1% increase in beta-lactamase activity; 95% confidence interval 0.23–1.2; P=0.13). The likelihood of reaching a sputum concentration ≥MIC90 was 80 times greater when amoxicillin/clavulanic acid was administered intravenously versus via the oral route (odds ratio 80.6; 95% confidence interval 1.6–4100; P=0.03).
  | Table 2 Potential confounders: association with beta-lactamase activity |
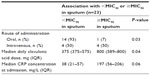  | Table 3 Potential confounders: association with <MIC90 or ≥MIC90 |
Discussion
No difference in beta-lactamase activity was found between patients with an amoxicillin concentration in sputum <MIC90 and patients with a concentration ≥MIC90 when treated with amoxicillin/clavulanic acid. Although we found that 78% of the patients had a low sputum concentration of amoxicillin (<MIC90), beta-lactamase activity does not seem to be the reason for this observation.
Beta-lactamase activity could not explain the low numbers of patients with an adequate amoxicillin concentration in sputum, so it seems that there should be other host-related or drug-related factors that influence the penetration across the blood-bronchus and alveolar-capillary barriers.
The most important host-related factor is the integrity of the anatomical barriers which may be damaged by inflammation and mechanical injury. In the presence of inflammation, the distribution of amoxicillin may be altered because of increased membrane permeability.25,26 As observed in this and our previous study a high level of C-reactive protein, a marker of systemic inflammation, was related to higher amoxicillin levels.17 C-reactive protein levels could therefore possibly be used as a marker to determine COPD patients in whom adequate concentrations could be reached. However, this does not solve the problem of low concentrations in the majority of patients; this is a worrying feature, especially because our previous study showed that this is also associated with significantly worse clinical outcomes.17 This warrants further investigation into more optimal treatment in these patients.
Since beta-lactam antibiotics such as amoxicillin do not cross membranes readily,27 it might be interesting to look at other antibiotics for the treatment of AECOPD that have a better penetration in sputum.28 Also, the possibility of individually tailored amoxicillin dosing could be investigated, since it has been shown that increased systemic dosing of amoxicillin is associated with increased levels in sputum. We observed that intravenous administration was associated with a higher probability of reaching a sputum amoxicillin concentration ≥MIC90 and led to higher serum concentrations (data not shown), but still 50% of patients had a concentration <MIC90. Further, due to the wide confidence interval that was observed with this probability and since we did not observe any differences in the numbers of patients who reached the MIC90 between intravenous and oral administration in our earlier study,17 we cannot recommend a preference for use of either intravenous or oral administration to reach adequate amoxicillin sputum levels.
Alternative routes of administration such as inhalation of antibiotics could be considered. Aerosolized antibiotics have been proven to deliver high concentrations of antibiotics into the airways with low systemic bioavailability. Stockley et al treated patients with bronchiectasis with nebulized amoxicillin and observed significant reductions in sputum purulence and volume after failure with the same drug given orally.29 It would therefore be interesting to administer amoxicillin/clavulanic acid via inhalation to reach the MIC90, and perhaps to improve the efficacy of antibiotic treatment, as seen in cystic fibrosis.29,30
The observed concentration of amoxicillin in serum is in the range of concentrations found in earlier published studies, which showed concentrations between 3.87 and 45.2 mg/L. Observed concentrations of amoxicillin in sputum ranged between 0.23 and 4.4 mg/L.31–36 However, these concentrations were measured in non-COPD patients and in healthy subjects.
Our study could have been influenced by selection bias, since we only included patients who were able to produce a sufficient amount of sputum on day 3. There might have been a difference in beta-lactamase activity and/or amoxicillin concentration between patients who were able to produce sputum and those who were not. Patients who were able to produce sputum may have had other characteristics in terms of presence/absence of bacteria or cause of exacerbation (eg, viral/bacterial infection). To overcome this issue, induction of sputum with hypertonic saline and the effects on possible dilution of amoxicillin concentration could be explored. Sputum induction has proven to be safe even during exacerbations in COPD patients.37
Since the majority of patients had an amoxicillin concentration <MIC90, the beta-lactamaseactivity of these patients could be compared to the beta-lactamaseactivity of only a small group of patiets with a concentration ≥MIC90. Although there seems to be no difference in median beta-lactamase activity, this study cannot definitely conclude that no relationship exists, and further studies are still necessary.
Conclusion
We observed no relationship between beta-lactamase activity and sputum amoxicillin concentration (<MIC90 or ≥MIC90) in patients treated with amoxicillin/clavulanic acid for an acute exacerbation in COPD. More studies are necessary to confirm this finding. Further, we repeatedly observed that the majority of patients had low sputum concentrations of amoxicillin (<MIC90), suggesting that current treatment with antibiotics should be optimized.
Disclosure
The COMIC study was partly funded by GlaxoSmithKline, the Netherlands, through an unrestricted research grant. Otherwise, the authors report no conflicts of interest in this work.
References
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176: 532–555. | |
Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29:1224–1238. | |
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. | |
Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. | |
Wilson R. Bacterial infection and chronic obstructive pulmonary disease. Eur Respir J. 1999;13:233–235. | |
Hirschmann JV. Do bacteria cause exacerbations of COPD? Chest. 2000;118:193–203. | |
Hirschmann JV. Bacteria and COPD exacerbations redux. Chest. 2001;119:663–667. | |
Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756–766. | |
Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941–1947. | |
Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354:456–460. | |
Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165: 698–703. | |
Llor C, Moragas A, Hernandez S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186: 716–723. | |
Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:150–157. | |
Baldwin DR, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother. 1992;36:1171–1175. | |
Gould IM, Harvey G, Golder D, et al. Penetration of amoxycillin/clavulanic acid into bronchial mucosa with different dosing regimens. Thorax. 1994;49:999–1001. | |
Davies B, Maesen F. Serum and sputum antibiotic levels after ampicillin, amoxycillin and bacampicillin chronic bronchitis patients. Infection. 1979;7 Suppl 5:S465–S468. | |
Brusse-Keizer M, ten Bokum L, Movig K, et al. Relation between amoxicillin concentration in sputum of COPD patients and length of hospitalization. COPD. 2011;8:66–70. | |
Pennington JE. Penetration of antibiotics into respiratory secretions. Rev Infect Dis. 1981;3:67–73. | |
Sportel JH, Koëter GH, van Altena R, Löwenberg A, Boersma WG. Relation between beta-lactamase producing bacteria and patient characteristics in chronic obstructive pulmonary disease (COPD). Thorax. 1995;50:249–253. | |
Barcelona L, Marin M, Stamboulian D. [Betalactam antibiotics combined with bectalactamases inhibitors. Amoxicillin-sulbactam]. Medicina (B Aires). 2008;68:65–74. Spanish. | |
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD, 2011. Available from: http://www.goldcopd.org/. Accessed October 9, 2014. | |
Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. | |
Lovering AM, Pycock CJ, Harvey JE, Reeves DS. The pharmacokinetics and sputum penetration of ampicillin and amoxycillin following simultaneous iv administration. J Antimicrob Chemother. 1990;25: 385–392. | |
European Committee for Antimicrobial Susceptibility Testing. MIC determination. 2014. Available from: http://www.eucast.org/ antimicrobial_susceptibility_testing/qc_tables. Accessed December 1, 2014. | |
Braude AC, Cohen RD, Penner JL, Preston MA, Rebuck AS. Pulmonary disposition of moxalactam. Chest. 1984;86:881–883. | |
Bergogne-Berezin E. Penetration of antibiotics into the respiratory tree. J Antimicrob Chemother. 1981;8:171–174. | |
Honeybourne D. Antibiotic penetration into lung tissues. Thorax. 1994;49:104–106. | |
Baldwin DR, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother. 2011;36:1176–1180. | |
Stockley RA, Hill SL, Burnett D. Nebulized amoxicillin in chronic purulent bronchiectasis. Clin Ther. 2011;7:593–599. | |
Hill SL, Morrison HM, Burnett D, Stockley RA. Short term response of patients with bronchiectasis to treatment with amoxycillin given in standard or high doses orally or by inhalation. Thorax. 1986;41:559–565. | |
Fraschini F, Scaglione F, Falchi M, et al. Pharmacokinetics and tissue distribution of amoxicillin plus clavulanic acid after oral administration in man. J Chemother. 1990;2:171–177. | |
Maesen FP, Davies BI, Baur C. Amoxycillin/clavulanate in acute purulent exacerbations of chronic bronchitis. J Antimicrob Chemother. 1987;19:373–383. | |
Havard CW, Fernando A, Brumfitt W, Hamilton-Miller JM. A pilot study of [#x2018;]Augmentin’ in lower respiratory tract infections: pharmacokinetic and clinical results. Br J Dis Chest. 1982;76:255–260. | |
Ingold A. Sputum and serum levels of amoxycillin in chronic bronchial infections. Br J Dis Chest. 1975;69:211–216. | |
Lovering AM, Pycock CJ, Harvey JE, Reeves DS. The pharmacokinetics and sputum penetration of ampicillin and amoxycillin following simultaneous iv administration. J Antimicrob Chemother. 1990;25: 385–392. | |
Hill SL, Burnett D, Lovering AL, Stockley RA. Use of an enzyme-linked immunosorbent assay to assess penetration of amoxicillin into lung secretions. Antimicrob Agents Chemother. 1992;36:1545–1552. | |
Bathoorn E, Liesker J, Postma D, Koëter G, van Oosterhout AJ, Kerstjens HA. Safety of sputum induction during exacerbations of COPD. Chest. 2007;131:432–438. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

