Back to Journals » OncoTargets and Therapy » Volume 15
Amivantamab in the Treatment of Metastatic NSCLC: Patient Selection and Special Considerations
Authors Petrini I, Giaccone G
Received 29 July 2022
Accepted for publication 22 September 2022
Published 12 October 2022 Volume 2022:15 Pages 1197—1210
DOI https://doi.org/10.2147/OTT.S329095
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Iacopo Petrini,1 Giuseppe Giaccone2
1Medical Oncology, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; 2Weill-Cornell Medicine, Meyer Cancer Center, New York, NY, USA
Correspondence: Giuseppe Giaccone, Weill-Cornell Medicine, Meyer Cancer Center, 1300 York Ave. 6th floor, Rm A603C, New York, NY, 10021, USA, Tel +1 646 962-4969, Email [email protected]
Abstract: Amivantamab is a bispecific antibody that recognizes epidermal growth factor receptor (EGFR) and MET proto-oncogene (MET). In May 2021, the Food and Drug Administration gave an accelerated approval of amivantamab for the treatment of non-small cell lung cancer (NSCLC) patients with EGFR exon 20 insertions (Exon20ins) who progressed after platinum-based chemotherapy. Amivantamab prevents ligand binding to EGFR and MET and the dimerization of the receptors suppressing the downstream signal transduction. Moreover, amivantamab determines antibody dependent cellular cytotoxicity and down regulation of cell surface proteins through internalization of the receptor and trogocytosis. Preliminary results of the Phase I/IB CHRYSALIS trial demonstrated an objective response rate of 40% with a median duration of response of 11.1 months (95% CI 9.6-not reached) in 81 patients treated with amivantamab with pretreated NSCLC with Exon20ins EGFR mutations. In a different cohort of the CHRYSALIS trial, patients with Ex19del and L858R EGFR mutations were enrolled after progression on osimertinib. 121 and 45 patients received amivantamab or a combination with lazertinib, a third-generation tyrosine kinase inhibitor, respectively. The objective response rate was 19% and 36% in patients treated with amivantamab alone or in combination with lazertinib, with a median progression-free survival of 6.9 (95% CI: 3.2– 5.3) and 11.1 (95% CI: 3.7– 9.5) months, respectively. All 20 patients with Ex19del and L858R EGFR mutations who received amivantamab and lazertinib as their first line treatment achieved an objective response. Amivantamab is currently under evaluation in Phase III clinical trials for the first line treatment of NSCLCs with Exon20ins EGFR mutations in combination with chemotherapy (PAPILLON), for the first line therapy of Ex19del and L858R mutated NSCLCs in combination with lazertinib (MARIPOSA) and in combination with chemotherapy and lazertinib in NSCLCs who progressed on osimertinib (MARIPOSA-2).
Keywords: amivantamab, exon 20 insertion of EGFR, NSCLC
Antibody Bispecific Structure
Amivantamab (JNJ-61186372) is a fully human monoclonal antibody with bispecific binding: one portion of its antigen-binding fragment (FAB) recognizes the epidermal growth factor receptor (EGFR), while the other one recognizes the MET proto-oncogene (MET) (Figure 1). Amivantamab has been generated using Genmab’s DuoBody® technology: a novel FAB-arm exchange platform.1 JNJ-61186372 was selected through a screening of 40 possible bispecific molecules given by the combination of 8 different anti-EGFR and 5 anti-MET antibodies. Among them, amivantamab has been selected based on the binding affinity of the monovalent antibodies, capability to prevent MET and EGFR phosphorylation and ability to arrest proliferation in cell lines.2 The binding of amivantamab to the extracellular portion of MET was demonstrated by crystal structure and the ability of the antibody to prevent HGF activation of the receptor was also confirmed.2 Using insertional mutagenesis, the EGFR epitope recognized by amivantamab was found to be located in the third domain near the TGFα binding site in the proximity of the cetuximab epitope.2
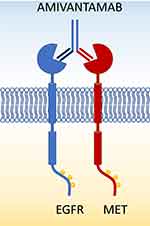 |
Figure 1 Bispecific structure of amivantamab with binding of EGFR and MET on the surface of the cells. |
Using surface plasmon resonance, the binding affinity of amivantamab to EGFR and MET were (KD) 1.43 and 0.04 nmol/L, respectively.3 Amivantamab simultaneously binds the extracellular domains of EGFR and MET and blocks the ligand binding of each receptor in a dose-dependent manner.3 Amivantamab blocks the binding of EGF to the extracellular domains of EGFR with an IC50 of 10 nmol/L (measured by ruthenium-labeled EGF) and the binding of HGF to the extracellular domains of MET with an IC50 of 30 nmol/L (measured by biotinylated-labeled HGF). The ability of amivantamab to block ligand-induced phosphorylation of EGFR and MET has been evaluated in a panel of cell lines with different levels of expression of the receptors. The degree of phosphorylation inhibition was proportional to the receptor density for both EGFR and MET.3 Amivantamab blocks the ligand-induced phosphorylation of the receptors more potently than the corresponding monovalent antibodies. This effect is stronger against the less expressed receptor on the surface of the cell between EGFR and MET: for example, amivantamab inhibits MET phosphorylation more potently in cell lines with higher EGFR and lower MET expression.3 Likely, amivantamab interacts first with the receptor with the higher density level on the surface of the target cells increasing the local cell-surface concentration of the antibody. Subsequently, it binds the less expressed receptor and strongly suppresses its phosphorylation. Moreover, this preferential binding to highly expressed receptors could be relevant for the selective recognition of cancer cells that overexpress the receptors and, consequently, to spare normal cells, and thereby increasing the therapeutic index of the drug.3
Amivantamab is an antibody derived from an IgG1 isotype and therefore it is able to bind to receptors of Fc fragment of IgG (FcγRs) on immune cells. Post transcriptional modifications influence the binding to FcγRs. A region within the CH2 domain of Fc was demonstrated to be critical for the binding to the cellular receptors and for the recognition of C1q complement fragment.4 Within the Fc, the asparagine (Asn) residue in position 297 is linked to a core structure containing n-acetyl-D-glucosamine and mannose. Modifications of this structure can incorporate fucose, galactose or sialic acid with a reduction of the binding affinity to FcγRs.5 Amivantamab is produced in a mammalian cell line (Chinese Hamster Ovary, CHO) using recombinant DNA technology that incorporates low levels of fucose within its Fc domain resulting in low levels (<10%) of antibodies containing a fucose attached to the glycan core linked to Asn297.6 The production of amivantamab in cell lines with low and high levels of fucosylation dramatically modifies the binding to FcγRIIIa on natural killer (NK) cells and macrophages.6
Mechanism of Action (Table 1)
Amivantamab binds the extracellular domains of EGFR and MET, inhibits the interaction with respective ligands, and prevents the activation of both receptors.3 In EGF and HGF stimulated cells, amivantamab inhibits the downstream signaling pathways (phospho-ERK and phospho-AKT) more potently than the respective monovalent antibodies (with only one FAB able to bind EGFR or MET) used individually or in combination.7 Likely, the bispecific molecule has additional biological effects not present in the combination of monovalent antibodies. In cell lines harboring MET amplification (H820 and H1993 cells), amivantamab weakly inhibited phospho-ERK and phospho-AKT.7 In lung cancer cell lines harboring an EGFR mutation that causes a constitutive activation of the receptor (H1975 cells, which harbor L858R and T790M mutations of EGFR), the stimulation with EGF marginally increases the level of the phosphorylation of the C-terminal tail of EGFR. Nevertheless, amivantamab reduces EGFR phosphorylation below the level of EGF unstimulated cells also in lines harboring EGFR activating mutations. Even if amivantamab significantly reduces the phosphorylation of ERK and AKT, it is not able to inhibit cell proliferation or to induce apoptosis in H1975 cells.7,8 In vivo, amivantamab inhibits ERK and AKT phosphorylation and arrests tumor growth in H1975 mouse xenograft and in patient derived xenografts of tumors harboring Ex19del EGFR mutation.7 In HCC827 cells or patient-derived xenografts with Ex19del EGFR mutation, tumors shrink during treatment with amivantamab or erlotinib. However, the inhibition of tumor growth was maintained after the suspension of the treatment with amivantamab but not with erlotinib. The combination of amivantamab with osimertinib or lazertinib potentiates the antiproliferative effect in mouse xenografts.7,9
 |
Table 1 Summary of Mechanism of Action of Amivantamab |
Amivantamab induces a downmodulation of EGFR and MET expression on the surface of the tumor cells. This process is mediated by internalization and lysosomal degradation of EGFR and MET and contributes to cause inhibition of downstream signaling and induction of apoptosis.10 In mice with xenograft of H1975 cells, the levels of both EGFR and MET proteins were significantly decreased following treatment with amivantamab compared to control by 76% and 61%, respectively. Amivantamab but not erlotinib was able to inhibit tumor growth of HCC827 mouse xenografts; this lung adenocarcinoma cell line which harbors EGFR Ex19del mutation, was engineered to overexpress MET.7
Amivantamab induces antibody-dependent cytotoxicity (ADCC) both in vitro and in vivo, thereby increasing the anti-tumoral efficacy of the drug.6 The production of amivantamab in engineered CHO cell line that incorporates low levels of fucose within its Fc domain is essential for an efficient ADCC.6 Amivantamab binds to FcγR-IIIa more potently than its analogue antibody produced with normal fucose levels; differences were not observed in FcγR-I and FcγR-IIa binding.6 Once amivantamab has opsonized tumor cells expressing EGFR and/or MET, the Fc fragments can cluster and can be bound by the FcγR-IIIa expressed by NK cells with consequent ADCC. ADCC was tested in vitro using human peripheral blood mononucleate cells (PBMCs) as the source of NK cells and non-small cell lung cancer (NSCLC) cell lines with either wild type (WT) or mutant EGFR and normal copy number or amplified MET. Low fucose amivantamab was more potent and demonstrated increased levels of tumor cell lysis compared to its analogue produced with normal fucose levels.7 Moreover, when IgG1 antibodies bind to the cell surface antigens, their Fc domains can engage receptors on macrophages and activate antibody-dependent cellular phagocytosis (ADCP).8 As macrophages express FcγR-IIIa, FcγR-I and FcγR-IIa, ADCP was not dependent on fucosylation in vitro. Interestingly, amivantamab induced changes in monocyte/macrophage cytokine expression patterns, especially chemotactic cytokines, compared to modified variants of the bispecific antibody that are not able to bind the Fcγ receptors.8 In mice xenografts of H1975, the low fucosylated amivantamab inhibited tumor growth much more than its analogue produced with normal fucose levels. With an identical inhibition of EGFR and MET pathway, this observation supports the relevance of amivantamab induced ADCC in vivo.6 On the contrary, amivantamab does not induce complement dependent cytotoxicity in NSCLC cell lines even if it binds to the C1q complement fragment.8
Amivantamab can induce receptor degradation through trogocytosis.8 Trogocytosis is another important consequence of the interaction between the Fc fragment and its receptors on myeloid cells. It consists of an antibody-mediated transfer of membrane fragments and ligands from tumor cells to monocytes, macrophages, or neutrophils.11 It has been shown that amivantamab can induce EGFR and MET downregulation in vivo, but such activity was weak in cell culture.8 Interestingly, the addition of PBMCs to H1975 cells markedly potentiated amivantamab-mediated downregulation of EGFR and MET by about 50%. Predominantly, monocytes isolated from peripheral blood and macrophages determine downmodulation of EGFR and MET proteins through trogocytosis. Using time-lapse microscopy, fluorescently labeled amivantamab was transferred from tumor cells (H1975) to monocytes or macrophages. This transfer was observed with amivantamab but not with an isotype IgG1 control (not specific for EGFR or MET) or with an effector silent variant of amivantamab that is not able to bind FCγ receptors.8 In H1975 xenograft mice, the depletion of tumor-associated macrophage by anti-CSF1R antibody significantly reduced (from 72.2% to 35.8%) tumor growth inhibition of amivantamab treatment supporting the relevance of trogocytosis for the antitumor efficacy of amivantamab.8
Results of Clinical Trials
CHRYSALIS
CHRYSALIS (NCT02609776) was the first in human phase-I trial of amivantamab in patients with unresectable or metastatic NSCLC. The study was divided in the dose escalation and the dose expansion parts. The dose escalation had a typical 3+3 design with dose increments up to the maximum tolerated dose. Amivantamab monotherapy, or the combinations with lazertinib, or with carboplatin plus pemetrexed were evaluated. Data regarding amivantamab monotherapy have been reported.12 From the starting dose of 140 mg once a week in the first 28-day cycle and every other week for subsequent cycles, increments were made up to 1750 mg without reaching the maximum tolerated dose. The recommended dose declared for Phase 2 was 1050 mg (1400 mg for patients ≥ 80 kg), based on safety, pharmacokinetic, and pharmacodynamic data.12 The same dose was used for the combination with lazertinib, a novel third generation EGFR TKI, given orally at a daily dose of 240 mg. For the dose expansion, amivantamab was administered to six different cohorts assigned on the basis of EGFR and/or MET mutations or amplifications, and previous therapy (Figure 2).
Exon20ins Cohort
During the course of the trial, amivantamab received Breakthrough Therapy Designation on the basis of the preliminary results of the patients harboring EGFR Exon20ins mutation, who had been previously treated with platinum-based chemotherapy. Of the 362 patients enrolled in the trial, 258 had been treated at the recommended dose of 1050 mg (1400 mg for patients ≥80 kg) and 81 had a tumor with Exon20ins EGFR mutation previously treated with chemotherapy, defining the efficacy population. There have been 3 (4%) complete and 29 (36%) partial responses, 39 (48%) stable disease, 8 (10%) progressive disease and 2 unevaluable patients in the efficacy population with a clinical benefit of 88% (stable disease plus complete and partial response), according to the blinded independent central review. The median duration of response was 11.1 months (95% CI: 6.9, not reached). The median progression-free survival and overall survival were 8.3 (95% CI: 6.5, 10.9) and 22.8 months (95% CI: 14.6, not reached), respectively. In-frame insertions of exon 20 of EGFR include more than 60 different mutations with different predictive value of response to tyrosine kinase inhibitors (TKI).10 The therapeutic challenge with selective Exon20ins TKIs is overcoming steric hindrance at the active site, while sparing activity against the WT receptor to minimize toxicity. Interestingly, patients achieving objective responses with amivantamab had tumors with EGFR mutations located in each region of exon 20 including the helical region (AA 762–766) and the proximal (AA 767–772) and distal region of the loop (AA 773-775).
A possible limitation of the drug is the predicted lack of penetration through the blood-brain barrier, because of the large size of the antibody. Interestingly, preliminary data in a limited number of patients treated with amivantamab suggest comparable outcomes in patients with brain metastases (n=18) and those without (n = 63).10 However, patients with active or untreated brain metastases were excluded from the CHRYSALIS trial and the activity of amivantamab in central nervous system (CNS) disease will need to be explored in future studies.10 A specific trial accruing NSCLC patients with EGFR mutations and brain metastases is evaluating the efficacy of amivantamab in combination with lazertinib (NCT04965090).
Upon the promising objective response rate (ORR) and duration of response, the Food and Drug Administration (FDA) gave an accelerated approval for the use of amivantamab on the 21st of May 2021 for the treatment of locally advanced or metastatic NSCLC patients harboring Exon20ins EGFR mutations whose disease has progressed on or after platinum-based chemotherapy.
The Context of Exon20ins Inhibitors
In NSCLC, the most common EGFR mutations are the inframe deletions of the exon 19 and the L858R missense mutation in exon 21 where leucine in position 858 is replaced by arginine. These two types of mutations account for approximately 90% of the EGFR mutations and are predictive factors of response to first, second and third generation EGFR TKIs. With the implementation of advanced diagnostic tools, including next generation sequencing, additional mutations are identified more frequently. In frame insertions of nucleotides within exon 20 account (Exon20ins) account for 4–12% of EGFR mutations in NSCLCs.13 Similarly to other EGFR mutations, Exon20ins occur more frequently in female patients, never-smokers with East Asian ancestry.14–16 Unlike Ex19del and L858R mutations, Exon20ins can be of variable length (3 to 21 base pairs) and involve different portions of exon 20, with more than 60 different variants described so far.17 Exon 20 of EGFR (amino acid 762–823) consist of 2 regions: the C-helix (residues 762–766) and the loop following the C-helix (residues 767–774). The C-helix of the tyrosine kinase domain is a regulator element that determines the activation status of EGFR by rotating from an outward to an inward position: the inactive and active conformation, respectively.18 When ligand binds to the WT receptor, the C-lobe of one kinase domain binds the N-lobe of the other partner of the dimer and pushes the C-helix into the active position. In silico prediction of the effect of the mutations on the structure of the tyrosine kinase domain suggests that insertions from amino acids 764 to 770 poorly interact with the TKI-binding pocket. On the contrary, insertions between amino acid 763 and 764 determine significant modification of the C-helix with consequent reduction of the affinity of first generation EGFR TKI. Insertions between amino acid 773 and 775 sterically obstruct the access of first generation TKIs to their binding pocket.14 90% of Exon20ins mutations occurs after the C-helix between codons 768–774.19 Exon20ins, located at the C-terminal end of the C-helix or in the following loop, push the C-helix into a permanent active conformation without affecting the ATP-binding pocket and, therefore, do not increase the affinity for EGFR TKIs.20 For example, D770_N771insNPG activates EGFR without increasing its affinity for EGFR TKIs.19 In contrast, EGFR-A763_Y764insFQEA, occurring within the C-helix, is highly sensitive to EGFR TKIs in vitro, and patients with tumors harboring this mutation respond to the first generation EGFR TKI erlotinib.19 As first generation TKIs are ineffective in most Exon20ins, carriers have a shorter median overall survival of 16.2 months (95% CI: 11.0, 19.4) compared to patients with exon 19 and 21 mutations with a median overall survival of 25.5 months (95% CI: 24.5, 27.0) benefiting from targeted therapies.21 Although second generation TKIs may be effective in Exon20ins mutations in vitro, but effective plasma concentrations cannot be reached in vivo, due to dose-limiting toxicities. In a pooled analysis of the LUX-Lung trials (2, 3 and 6), first line treatment with afatinib achieved a median overall survival of 9.2 months and a median progression free survival of 2.7 months in patients with Exon20ins.22 Case reports have described sensitivity to afatinib of H773dup, H773_V774insNPH, and N771delinsKG mutations.23–25
In-frame insertions of exon 20 occur also in HER2 in approximately 1–2% of NSCLC. Exon20ins mutations of HER2 are less heterogenous than those of EGFR, with Y772dupYVMA and A775_G776insYVMA accounting for more than 80% of the cases.26 HER2 mutations are potent oncogenic drivers, both in vitro and in vivo.26 Similarly to EGFR, Exon20ins of HER2 modify the conformation of the αC-helix, at residues 770–774, and the loop region comprising residues 775–783, blocking the receptor in an active conformation with a steric hindrance for the access of TKIs to their binding pocket. The tyrosine kinase domains of EGFR and HER2 have a similar structure and inhibitors have activity on both receptors. Currently, several TKIs with a molecular structure able to bypass the steric hindrance of EGFR and HER2 mutations are under evaluation including poziotinib, mobocertinib and tarloxotinib.26
Poziotinib has been the first TKI specifically evaluated in patients with NSCLC harboring Exon20ins mutations. In vitro, poziotinib has shown a promising activity in Ba/F3 cells expressing Exon20ins mutation with a potency of growth inhibition 40 and 100 times greater than afatinib and osimertinib, respectively.27 Despite an encouraging ORR of 58% in the first report on 40 treated patients from MD Anderson, poziotinib attained an ORR of only 14.8%, with a median progression free survival of 4.2 months in 115 patients enrolled in the multicenter Phase II ZENITH20 trial. Grade ≥3 rash and diarrhea were observed in 28% and 26% of patients, respectively, and this excessive toxicity caused frequent treatment interruptions and delays which precluded continuous treatment at active doses for a sufficiently long time. Trials in which changes in schedule are used (BID instead of daily) appear to have better tolerability.
Mobocertinib is the first irreversible TKI designed to selectively target Exon20ins mutations of EGFR in NSCLC. In the Phase 1/2 trial, 160 mg orally once a day was selected as the recommended phase II dose and based on its activity mobocertinib has recently been approved in the US for patients with locally advanced or metastatic NSCLC with EGFR Exon20ins mutations whose disease had progressed on prior platinum-based chemotherapy.28 Results have been published including 114 patients pretreated with platinum-based chemotherapy. An ORR of 28%, a median progression free survival of 7.3 months, and a median overall survival of 24 months have been reported. The drug penetrance into the CNS is crucial given the brain tropism of metastases in patients with EGFR exon 20 insertions. In patients with and without brain metastases the ORR of mobocertinib was 18% and 34%, respectively. These data suggest some activity in patients with brain metastases even if mobocertinib penetrates poorly into the central nervous system. Despite the low affinity for WT EGFR, mobocertinib has a typical EGFR TKI toxicity profile. Treatment-related adverse events of grade ≥3 occur in 47% of patients: diarrhea was the most frequently observed side effect (21% of patients). Also, rash and paronychia were frequent albeit of milder intensity: grade ≤2 in 45% and 38% of patients, respectively.28
Osimertinib, a third generation EGFR TKI, with activity on T790M mutation (in exon 20), at a daily dose of 80 mg has limited activity in Exon20ins mutated NSCLC. However high-dose osimertinib (160 mg daily) has shown an ORR of 25%, a stable disease rate of 60% and a median progression free survival of 9.7 months among the 20 patients enrolled in a phase II trial.27 Higher dose osimertinib was more toxic than 80 mg, with diarrhea (76% any grade), fatigue (67% any grade), and acneiform rash (38% any grade), but none of these adverse events were grade ≥3. Combination therapies of necitumumab and osimertinib are under evaluation in NSCLC patients with Exon20ins EGFR mutations.
CLN-081 (TAS6417) is a novel TKI with a specific structure designed to interact with the EGFR binding pocket with an Exon20ins mutation. CLN-081 has shown a potent activity in vitro and a phase I/II trial is ongoing with promising results on the first 17 patients treated (ORR 35%).10
Luminespib is an HSP90 inhibitor that has been tested in a phase II trial with 29 pretreated patients with Exon20ins. EGFR is a client of HSP90 for the conformational maturation of the protein that is necessary for the function of the receptor and its signal transduction. Also, the maturation and function of mutated EGFR depends on HSP90. The inhibition of HSP90 has shown anti proliferative activity in models that harbor EGFR mutations including those resistant to TKIs such as T790M and Exon20ins.29,30 In a phase II trial, 29 patients with advanced NSCLC with Exon20ins mutations of EGFR received luminespib after at least one previous line of treatment. Luminespib induced an ORR of 17% with a median progression free survival of 2.8 months and a median overall survival of 9.9 months.10
Of all the drugs tested in patients harboring Exon20ins only amivantamab and mobocertinib have been approved by FDA for treatment of platinum pretreated NSCLC patients to date. Since these drugs present different mechanisms of action, possibly different subgroups of patients could benefit from these treatments. However, there was no clear association between depth of response and response rate with specific EGFR ins20 variants in early trials of mobocertinib and amivantamab. On the contrary, responses to poziotinib were enriched in tumors with an insertion in the region proximal to the end of the C-helix between amino acids M766-D770.10 Brain metastases are common in patients with Exon20ins EGFR mutations. Patients with brain metastases experienced lower response rates than those without CNS disease if treated either with poziotinib or mobocertinib. Despite the large molecular size of amivantamab that hampers the penetration through the blood-brain barrier, patients with and without brain metastases experienced a similar outcome in a limited number of patients; confirmatory studies in larger populations are needed in order to draw definitive conclusions. High dose osimertinib might be an interesting alterative for patients with CNS disease, because of the high penetrance of this drug through the blood brain barrier. Both amivantamab and mobocertinib are under evaluation in two Phase 3 clinical trials (MARIPOSA and EXLAIM-2) in comparison to chemotherapy for the first line treatment of NSCLC patients harboring Exon20ins EGFR mutations.
Amivantamab has shown a potent antiproliferative activity in models of NSCLC with EGFR exon 20 insertions.10 Utilizing Ba/F3 cells engineered to express EGFR V769_ D770insASV, D770delinsGY, H773_V774insH, and D770_ N771insSVD, Yun and colleagues showed that amivantamab reduces cell viability in a dose-dependent manner and that the inhibition is significantly greater than that observed with osimertinib. Amivantamab reduced the protein level of EGFR with inhibition of phosphorylation of downstream signaling pathways (phospho-ERK and phospho-AKT), and activates apoptosis in a BIM- and caspase-dependent manner.10 In patient-derived xenografts from NSCLC with Exon20ins, amivantamab was more effective in inhibiting tumor growth than poziotinib or cetuximab, and induced less skin toxicity.10 After amivantamab treatment, both internalization of the receptor with lysosomal degradation and FC-mediated trogocytosis contribute to down regulate EGFR protein levels in Ba/F3 cells transfected with Exon20ins and patient-derived xenografts from tumors with Exon20ins.10 Amivantamab-induced ADCC was confirmed in vitro in Ba/F3 cells transfected with Exon20ins EGFR mutation when PBMCs were added and in patient-derived xenograft of NSCLC harboring Exon20ins EGFR mutations. These data confirm the multiple known mechanisms of action of amivantamab in the context of Exon20ins mutations of EGFR and suggest a synergistic effect that could explain the positive results observed in clinical trials.
CHRYSALIS Post Osimertinib Cohorts
NSCLC patients with Ex19del and L858R EGFR mutations previously treated with osimertinib were included in the CHRYSALIS trial in multiple cohorts depending on the presence or absence of C797S mutation and MET amplification. One hundred and twenty-one patients received amivantamab in monotherapy and 45 in combination with lazertinib.9 Patients receiving amivantamab alone or in combination with lazertinib were unbalanced for the presence of C797S EGFR mutation (57% vs 16%) and for MET amplification (33% vs 11%). Moreover, patients treated with amivantamab had received a median of 3 (range 1–14) lines of therapies compared to 2 (range 1–4) of those receiving the combination. Patients with untreated or active CNS metastases were excluded from the trial; 26% and 29% of patients treated with single agent amivantamab or in combination with lazertinib had brain metastases, respectively. The ORR was 19% and 36% in patients treated with amivantamab alone and in combination with lazertinib, with a median duration of response of 5.9 (95% CI: 4.2–12.6) and 9.6 (95% CI: 5.3–not reached) months, respectively. The median progression-free survival was 6.9 (95% CI: 3.2–5.3) and 11.1 (95% CI: 3.7–9.5) months for patients treated with amivantamab alone or the combination with lazertinib. The median treatment duration with amivantamab was 3.7 months (range 0.03–32.2,) and 8.3 months (range 2.8–32.2) in those who achieved an objective response. The median treatment duration with amivantamab in combination with lazertinib was 5.6 months (range 0.5–14.8), and 12 months (range 4.1–14.6) in those who achieved an objective response. 17% and 7% of patients treated with amivantamab alone or in combination with lazertinib experienced CNS progression with the appearance of new brain lesions in 13% and 7% of cases, respectively.9
In the CRISALYS trial, 20 patients with Ex19del and L858R EGFR mutations received amivantamab and lazertinib as first line therapy. All patients achieved an objective response without any evidence of disease progression with a median follow-up of 7 months (range 4–7). Tumors rapidly shrunk with a median time to response of 1.5 months (range 1.2–2.6).9
CHRYSALIS-2
CHRYSALIS-2 is a phase I/Ib trial of lazertinib alone or in combination with amivantamab in patients with advanced NSCLC and EGFR mutations. Phase 1 of the trial is designed to confirmed the safety and tolerability of lazertinib. In the phase 1b the efficacy of the combination of lazertinib 240 mg/day and amivantamab 1050 mg (1400 mg, ≥80 kg) IV dosed weekly in cycle 1 (28-day cycle), every other week thereafter was evaluated. Moreover, a cohort of the phase 1b will evaluate the tolerability of the combination of lazertinib, amivantamab, pemetrexed and carboplatin. Four expansion cohorts were planned: “A” for patients with Ex19del and L858R EGFR mutations who had progressed on 1st or 2nd-line osimertinib followed by platinum chemotherapy; “B” for patients with EGFR Exon20ins mutations who had progressed on prior therapy; and “C” for patients with uncommon non-Exon20ins EGFR mutations (ie, S768I, L861Q, G719X) who were treatment-naïve or received 1st or 2nd-generation EGFR TKI as last therapy. At ESMO 2021, results of the expansion cohort “A” were presented.31 Two populations of NSCLC patients with Ex19del and L858R EGFR mutations were enrolled, designated by the authors as the “target” and the “heavily-pretreated” populations. In the “target” population, lazertinib and amivantamab were given as 3rd/4th line of treatment after progression to standard of care osimertinib (first line or after a 1st or 2nd generation TKI) and platinum chemotherapy. In the “heavily-pretreated” population, patients received lazertinib and amivantamab as 5th or subsequent line of treatment.
In the “target” population, ORR was 41% with a rate of clinical benefit of 69% among the 29 evaluable patients. The median treatment duration was 4.2 months (range 0.03–8.4 months) with 13 patients still on treatment (8 with a partial response and 5 with a disease stabilization).
In the “heavily pretreated” population, 47 of the 56 enrolled patients were evaluable for efficacy with 21% of them obtaining a partial response and 30% a stabilization of the disease. The median time on treatment was 3.7 months (range 0.03–9.7 months) with 20 patients still on treatment: 10 with a partial response and 10 with a disease stabilization.31
The Context of Acquired Resistance to Osimertinib
Osimertinib is the standard of care for the first line treatment of patients with advanced or metastatic NSCLC with Ex19del or L858R EGFR mutations. However, after a median progression free survival of 18.9 months tumors progress and platinum-based chemotherapy is the standard of care.32 Several acquired resistance mechanisms have been described: on target secondary mutations of EGFR in 6–10% of cases, bypass signaling mutations including amplification of MET in 7–15% of cases or HER2 in 1–2% of cases, fusion genes of ALK, RET and BRAF (1–8%), mutations of molecules in downstream signaling pathways (BRAF V600E 3%, PIK3CA 7%, KRAS 3–4%, HER2 1%), amplification of CDK and cyclins including CCND1, CCDN2, CCNE1, CDK4, CDK6 (10%) and transformation into SCLC or squamous histologies.33 Anecdotal responses and promising early clinical experience with specific inhibitors have been reported for some mechanisms of acquired resistance.34–36 Phase 3 clinical trials are evaluating MET inhibitors such as savolitinib (NCT03778229), tepotinib (NCT03940703) or capmatinib (NCT04816214) in combination with osimertinib versus chemotherapy for the treatment of patients with EGFR mutated NSCLC who progressed after osimertinib with acquired MET amplification. Given the heterogeneity of the acquired resistance mechanisms, the development of specific strategies of treatment for each type of mutation remains difficult as demonstrated by the slow accrual of ongoing trials. The identification of the acquired resistance mechanism and its inhibition, when feasible, remains the best opportunity for targeted therapies. The re-biopsy of tumors can be challenging in some cases and an extended panel of molecular tests is requested preferentially using next generation sequencing. For not biopsiable tumors the analysis of circulating tumor DNA is a valuable option37 with the limitation that 20% of tumors do not show any DNA shedding in plasma, at least with the current technologies available. Even with the best technologies, the acquired resistance mechanisms remain undetermined in about 50% of the patients.
Amivantamab can interfere with multiple mechanisms of acquired resistance to osimertinib. Amplification and overexpression of MET and an increased local concentration of HGF are mechanisms of acquired resistance to EGFR inhibitors.6 Moreover, EGFR and MET are expressed in the same tumor cells making particularly appealing the use of the bispecific binding affinity of this antibody that is able to inhibit both downstream signaling pathways in vitro and in vivo.7 Amivantamab binding to the extracellular domains is not affected by secondary mutations of the tyrosine kinase domain of EGFR, such as C797S, L718Q, G742S and S769I.7 Therefore, to date amivantamab is possibly the drug that is able to target simultaneously the highest number of acquired resistance mechanisms to osimertinib. The promising results of amivantamab alone and of its combination with lazertinib in EGFR mutated NSCLC who acquired resistance to osimertinib are in line with these preclinical findings. Currently, amivantamab and its combination with lazertinib are being evaluated in combination with platinum-based chemotherapy in NSCLC patients with Ex19del and L859R EGFR mutations after progression to osimertinib.
Ongoing Clinical Trials (Table 2)
PAPILLON is a phase 3 clinical trial enrolling treatment-naïve patients with advanced or metastatic NSCLC with Exon20ins mutations. Patients are randomly assigned to receive carboplatin plus pemetrexed with or without amivantamab. A platinum doublet schedule is the standard of care for NSCLC and the combination of carboplatin AUC (area under the curve) 5 and pemetrexed 500 mg/m2 every 21 days is the preferred option for non-squamous tumors.38 Recently, the combination of platinum pemetrexed and immunotherapy has become the new standard for treatment of NSCLC but EGFR mutated and ALK rearranged tumors were mostly excluded from the clinical trials because less responsive to immune checkpoint inhibitors.39,40 Therefore, the combination of carboplatin and pemetrexed is considered the standard treatment for NSCLC with Exon20ins mutations. Chemotherapy is given for 4 cycles followed by maintenance with pemetrexed in monotherapy or in combination with amivantamab in the experimental arm. In order to synchronize the administration with chemotherapy schedule, amivantamab is given as an intravenous infusion at a dose of 1400 mg (1750 mg if body weight is ≥80 kg) once a week up to Cycle 2 Day 1, then 1750 mg (2100 mg if body weight is ≥80 kg) on Day 1 of each 21-day cycle, starting with Cycle 3. The primary endpoint of the trial is progression-free survival assessed by blinded independent central review according to RECIST v1.1 with a target accrual of about 300 patients in 200 sites in 25 countries. Crossover from the chemotherapy arm to monotherapy amivantamab is allowed.41
 |
Table 2 Amivantamab Ongoing Clinical Trials in NSCLC |
MARIPOSA is a phase 3 clinical trial enrolling treatment naïve patients with advanced or metastatic NSCLC with Ex19del or L858R mutations of EGFR. Patients are randomly assigned 2:2:1 in tree arms to receive amivantamab and lazertinib, lazertinib alone, or the standard of care osimertinib until disease progression. Osimertinib is given at the dose of 80 mg daily according to the Flaura trial32 and lazertinib at the dose of 240 mg daily according to the dose identified in the Phase I/II trial.42 In the combination, lazertinib is given at the dose of 240 mg daily and amivantamab at the dose of 1050 mg intravenously for body weight less than 80 kg and 1400 mg for ≥80 kg, in 28-day cycles: once weekly in Cycle 1 (with a split dose on Days 1–2), and then every 2 weeks in subsequent cycles, according to the data of CHRYSALIS trial. The primary endpoint of MARIPOSA trial is progression-free survival based on blinded independent central review according to RECIST v1.1 with a target accrual of about 1000 patients across 262 sites in 27 countries.43
MARIPOSA 2 (NCT04988295) is a phase 3 clinical trial enrolling patients with advanced or metastatic NSCLC with Ex19del or L858R EGFR mutations after progression on osimertinib. Patients are randomly assigned to one of three arms: pemetrexed and carboplatin, amivantamab plus pemetrexed and carboplatin, or the combination of amivantamab and lazertinib plus pemetrexed and carboplatin. The primary endpoint is progression-free survival based on blinded independent central review according to RECIST v1.1.
A phase II trial (NCT04965090) is evaluating the efficacy of amivantamab and lazertinib in EGFR mutant NSCLC patients with CNS metastases, including brain and leptomeningeal metastases. Finally, the Phase I PALOMA trial is evaluating the subcutaneous administration of amivantamab in solid tumors, whereas 3 phase II trials are evaluating its efficacy in gastric (NCT04945733), esophageal (NCT05117931) and adenoid cystic carcinoma of salivary and other glands (NCT05074940).
Pharmacokinetics
Amivantamab exposure increases proportionally in the dose range 350–1750 mg with steady state achieved by the 9th infusion; the accumulation ratio is 2.4.44 The mean volume of amivantamab distribution is 5.13 L. The mean clearance is 360 mL/day with a terminal half-life of 11.3 days.44 The volume of distribution and clearance of amivantamab increased with bodyweight.44 The exposure in patients weighting≥ 80 kg is 30–40% lower than in those <80 kg. A 1050 mg dose in patients weighting <80 kg and of 1400 mg in patients ≥80 kg resulted in a similar exposure. Even if the drug exposure is dependent on patients’ weight, the variation does not affect the efficacy or safety profile when patients are divided in those weighting < and ≥80 kg and amivantamab given at the dose of 1050 mg and 1400 mg, respectively. Therefore, a fixed dose instead of a schedule based on patient body weight has been chosen. Age, sex, creatinine clearance (≥30 mL/min) or mild hepatic impairment had no clinically relevant effect on the pharmacokinetics of amivantamab. The effect of severe kidney failure or moderate (total bilirubin 1.5–3 × ULN) to severe (total bilirubin >3 × ULN) hepatic impairment have not been evaluated.44
Adverse Events
Infusion-related reactions are common with the first administration of amivantamab, with 69% of adverse reactions observed; however, only 2% are grade ≥3 and tend not to recur with subsequent cycles of therapy.9 The first dose of amivantamab is split in two subsequent days and administered in a peripheral line after steroid and antihistamine premedication. Steroids are usually not required in subsequent administrations of amivantamab but pre-treatment with antihistamines and antipyretics is required. With the first administration of amivantamab at a reduced rate of 25 mL/h in the first 2 hours and increased to 50 mL/h thereafter, the majority of infusion related reactions were grade ≤2. The safety profile has been reported for 258 and 114 patients treated in the CHRYSALIS trials with amivantamab at the dose recommended for the phase II and for those included in the Exon20ins cohort, respectively.12 In NSCLC patients with Exon20ins EGFR mutations treated with amivantamab, grade ≥3 adverse events were observed. Treatment related adverse event of grade ≥3 were 16%, with rash (4%) infusion related reaction (3%) and neutropenia (3%) being the most common. Diarrhea was a serious adverse event in 2 patients (2%). Dose was reduced because of toxicity in 13% of the patients mainly because of rash, and treatment was discontinued in 5 patients because of rash (2 patients), infusion related reaction (2 patients) and paronychia (1 patient). Common toxicities grade ≤2 include rash (83%), paronychia (44%), hypoalbuminemia (24%), constipation (24%), stomatitis (21%), nausea (19%), dyspnea (18%), peripheral edema (19%) and pruritus (22%).12 Pneumonitis/interstitial lung disease are uncommon: 2% with amivantamab alone and 4% in combination with lazertinib.9
In the CHRYSALIS trial, the combination of amivantamab and lazertinib was well tolerated with a low rate of treatment discontinuation (6%) and 11% of treatment related adverse events of grade ≥3.45 65% of patients treated with the combination experienced an infusion-related reaction at the first infusion but all cases were not severe (grade ≤2).45 The most common adverse event related to EGFR inhibition was skin rash (65%) but only 4% was grade 3 leading to treatment discontinuation in 1 patient only. Other common treatment related adverse events (grade ≥1) included paronychia (57%), stomatitis (33%), pruritus (28%), diarrhea (18%), hypoalbuminemia (37%), and peripheral edema (18%) but grade ≥3 toxicities were uncommon with hypoalbuminemia (2%), paronychia, diarrhea, nausea, transaminase increase (1% each).45
In the CHRYSALIS-2 trial, the safety profile was consistent with previous reports of amivantamab and lazertinib combination, with 37% of patients developing a grade ≥3 treatment-related adverse event. Grade ≥3 treatment related adverse events were infusion related reactions (9%), rash (including acneiform dermatitis, 6%), stomatitis (2%), paronychia (3%), hypoalbuminemia (4%) and increased alanine aminotransferase (3%). Four patients (3%) developed interstitial lung disease. Adverse events led to treatment discontinuation, dose reduction and dose interruption in 11%, 18% and 46% of patients, respectively. The safety profile suggests a cumulative toxicity with the two EGFR inhibitors but the combination treatment was still manageable.31
Conclusions
Amivantamab is the first bifunctional antibody effective for the treatment of NSCLC. It has shown promising activity in phase IB/II clinical trials for a subset of patients with tumor harboring Exon20ins mutations of EGFR. First and second generation TKIs targeting EGFR are not effective in these tumors since they cannot access their binding pocket because of the sterical hindrance of the kinase domain determined by exon 20 mutations. Therefore, targeting the extracellular domain of EGFR with an antibody is an interesting therapeutic approach. The drug has an acceptable toxicity profile and has been recently approved by the FDA for the treatment of advanced NSCLCs with Exon20ins mutations of EGFR after progression to a first line treatment with platinum doublets. Amivantamab is currently under evaluation in a phase III trial in combination with chemotherapy for the first line treatment of NSCLC with Exon20ins. Moreover, amivantamab is under investigation also in other types of EGFR and MET mutations as single agent and in combination with lazertinib.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Labrijn AF, Meesters JI, de Goeij BE., et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110(13):5145–5150. doi:10.1073/pnas.1220145110
2. Neijssen J, Cardoso RMF, Chevalier KM, et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem. 2021;296:100641. doi:10.1016/j.jbc.2021.100641
3. Jarantow SW, Bushey BS, Pardinas JR, et al. Impact of Cell-surface Antigen Expression on Target Engagement and Function of an Epidermal Growth Factor Receptor × c-MET Bispecific Antibody. J Biol Chem. 2015;290(41):24689–24704. doi:10.1074/jbc.M115.651653
4. Vafa O, Gilliland GL, Brezski RJ, et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. 2014;65(1):114–126. doi:10.1016/j.ymeth.2013.06.035
5. Chung S, Quarmby V, Gao X, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs. 2012;4(3):326–340. doi:10.4161/mabs.19941
6. Grugan KD, Dorn K, Jarantow SW, et al. Fc-mediated activity of EGFR x c-Met bispecific antibody JNJ-61186372 enhanced killing of lung cancer cells. MAbs. 2017;9(1):114–126. doi:10.1080/19420862.2016.1249079
7. Moores SL, Chiu ML, Bushey BS, et al. A Novel Bispecific Antibody Targeting EGFR and cMet Is Effective against EGFR Inhibitor-Resistant Lung Tumors. Cancer Res. 2016;76(13):3942–3953. doi:10.1158/0008-5472.CAN-15-2833
8. Vijayaraghavan S, Lipfert L, Chevalier K, et al. Amivantamab (JNJ-61186372), an Fc Enhanced EGFR/cMet Bispecific Antibody, Induces Receptor Downmodulation and Antitumor Activity by Monocyte/Macrophage Trogocytosis. Mol Cancer Ther. 2020;19(10):2044–2056. doi:10.1158/1535-7163.MCT-20-0071
9. Leighl N Amivantamab monotherapy and in combination with lazertinib in post-osimertinib EGFR-mutant NSCLC: analysis from the CHRYSALIS study.
10. Meador CB, Sequist LV, Piotrowska Z. Targeting. Cancer Discov. 2021;11(9):2145–2157. doi:10.1158/2159-8290.CD-21-0226
11. Taylor RP, Lindorfer MA. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood. 2015;125(5):762–766. doi:10.1182/blood-2014-10-569244
12. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: initial Results From the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391–3402. doi:10.1200/JCO.21.00662
13. Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J Thorac Oncol. 2018;13(10):1560–1568. doi:10.1016/j.jtho.2018.06.019
14. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220–229. doi:10.1158/1535-7163.MCT-12-0620
15. Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23–31. doi:10.1016/S1470-2045(11)70129-2
16. Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179–184. doi:10.1097/JTO.0b013e3182779d18
17. Bauml JMV, Minchom S, Bazhenova A. Underdiagnosis of EGFR Exon 20 Insertion Mutation Variants: estimates from NGS-based Real-World Datasets. J Thoracic Oncol. 2021;16:S208–S209. doi:10.1016/j.jtho.2021.01.112
18. Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125(6):1137–1149. doi:10.1016/j.cell.2006.05.013
19. Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5(216):216ra177. doi:10.1126/scitranslmed.3007205
20. Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta. 2010;1804(3):559–566. doi:10.1016/j.bbapap.2009.12.010
21. Bazhenova L, Minchom A, Viteri S, et al. Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer. 2021;162:154–161. doi:10.1016/j.lungcan.2021.10.020
22. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of Afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi:10.1016/S1470-2045(15)00026-1
23. Zöchbauer-Müller S, Kaserer B, Prosch H, et al. Case Report: afatinib Treatment in a Patient With NSCLC Harboring a Rare. Front Oncol. 2020;10:593852. doi:10.3389/fonc.2020.593852
24. Urbán L, Dóczi R, Vodicska B, et al. Major Clinical Response to Afatinib Monotherapy in Lung Adenocarcinoma Harboring EGFR Exon 20 Insertion Mutation. Clin Lung Cancer. 2021;22(1):e112–e115. doi:10.1016/j.cllc.2020.09.005
25. Lin L, Wu X, Yan S, et al. Response to Afatinib in a Patient with NSCLC Harboring Novel. Onco Targets Ther. 2020;13:9753–9757. doi:10.2147/OTT.S268694
26. Friedlaender A, Subbiah V, Russo A, et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol. 2022;19(1):51–69. doi:10.1038/s41571-021-00558-1
27. Friedlaender A, Subbiah V, Russo A, et al. Author Correction: EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol. 2022;19(1):70. doi:10.1038/s41571-021-00571-4
28. Zhou C, Ramalingam SS, Kim TM, et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: a Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol. 2021;7(12):e214761. doi:10.1001/jamaoncol.2021.4761
29. Shimamura T, Shapiro GI. Heat shock protein 90 inhibition in lung cancer. J Thorac Oncol. 2008;3(6Suppl 2):S152–9. doi:10.1097/JTO.0b013e318174ea3a
30. Jorge SE, Lucena-Araujo AR, Yasuda H, et al. EGFR Exon 20 Insertion Mutations Display Sensitivity to Hsp90 Inhibition in Preclinical Models and Lung Adenocarcinomas. Clin Cancer Res. 2018;24(24):6548–6555. doi:10.1158/1078-0432.CCR-18-1541
31. Shu C Amivantamab plus lazertinib in post-osimertinib, post-platinum chemotherapy EGFR-mutant non-small cell lung cancer (NSCLC): preliminary results from CHRYSALIS-2.
32. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
33. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–737. doi:10.1038/s41416-019-0573-8
34. Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. 2020;8(11):1132–1143. doi:10.1016/S2213-2600(20)30154-5
35. Gautschi O, Menon R, Bertrand M, Murer C, Diebold J. Capmatinib and Osimertinib Combination Therapy for EGFR-Mutant Lung Adenocarcinoma. J Thorac Oncol. 2020;15(1):e13–e15. doi:10.1016/j.jtho.2019.07.027
36. Kim L, Chae YK, Jung CM, Lee AD, Yu E Addition of selpercatinib to overcome osimertinib resistance in non-small cell lung cancer (NSCLC) with acquired RET fusion detected in ctDNA at very low allele frequency.
37. Ramalingam S, Cheng Y, Zhou C, et al. LBA50 - Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study.
38. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi:10.1200/JCO.2007.15.0375
39. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
40. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi:10.1016/S1470-2045(20)30641-0
41. Agrawal T, Artis E, Xie J, et al. P76.74 PAPILLON: randomized Phase 3 Study of Amivantamab Plus Chemotherapy vs Chemotherapy Alone in EGFR Exon20ins NSCLC.
42. Ahn MJ, Han JY, Lee KH, et al. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol. 2019;20(12):1681–1690. doi:10.1016/S1470-2045(19)30504-2
43. Shreeve M, Martinez M, Verheijen R, et al. P76.73 MARIPOSA: randomized Phase 3 Study of First-line Amivantamab + Lazertinib vs Osimertinib vs Lazertinib in EGFR-mutant NSCLC.
44. Syed YY. Amivantamab: first Approval. Drugs. 2021;81(11):1349–1353. doi:10.1007/s40265-021-01561-7
45. Cho B. Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

