Back to Journals » Lung Cancer: Targets and Therapy » Volume 9
Amifostine- and chemoradiotherapy-related esophagitis in small cell lung cancer: a single institutional series and literature update
Authors Pollock AE, Shinn L, Anderson R, Butler S , Pollock J
Received 27 October 2017
Accepted for publication 29 May 2018
Published 10 September 2018 Volume 2018:9 Pages 79—84
DOI https://doi.org/10.2147/LCTT.S155315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Ariel E Pollock,1 Lowell Shinn,2 Richard Anderson,3 Sarah Butler,3 Jondavid Pollock3
1Department of Graduate Medical Education, Icahn School of Medicine at Mt. Sinai, New York, NY, USA; 2Division of Medical Oncology, Schiffler Cancer Center, Wheeling Hospital, 3Division of Radiation Oncology, Schiffler Cancer Center, Wheeling Hospital, Wheeling, WV, USA
Objectives: Concurrent chemoradiotherapy is considered a standard option for patients with stage 3 small cell lung carcinoma. A 25% risk of acute esophagitis is experienced by patients as a result of the volume of esophagus encompassed within a conformal radiotherapy technique. We reviewed our institutional experience administering the radioprotectant amifostine prior to daily radiotherapy to determine its effects on the onset of esophagitis.
Materials and methods: From 2005 to 2016, 49 patients diagnosed with stage 3 small cell lung carcinoma received concurrent chemoradiotherapy. Chemotherapy (CT) consisted of cisplatin and etoposide with radiotherapy (RT) encompassing CT-identified gross tumor volume. In 32 patients (group 1), amifostine was delivered (500 mg subcutaneously divided in two injections) prior to the second daily RT fraction. The remaining 17 patients (group 2) did not receive amifostine due to choice or drug intolerance.
Results: Metrics of esophagitis included weight loss and opiate requirement during treatment. About 31% of group 1 required opiates at a median RT dose of 3300 cGy, and 41% of group 2 required opiates at a median dose of 2250 cGy. The dose of radiotherapy delivered to 50% of the esophageal volume for group 1 was significantly greater than that in group 2 (3000 cGy vs 576 cGy).
Conclusion: In this modern retrospective series of thoracic chemoradiotherapy in the treatment of stage 3 small cell lung cancer, amifostine that was delivered subcutaneously postponed the onset of esophagitis.
Keywords: small cell lung cancer, amifostine, chemoradiotherapy, esophagitis
Introduction
Concurrent chemoradiotherapy (CMT), in which chemotherapy is delivered every 3 weeks in conjunction with radiotherapy (RT), is recognized as a standard treatment option in patients with stage 3 small cell lung carcinoma.1 Acute toxicities associated with CMT include fatigue, skin erythema, dyspnea, myelosuppression, and esophagitis. Esophageal inflammation has been described as a dose-limiting effect2 and is observed in ~25% of patients undergoing CMT.1 Studies have reported a relationship between the volume of the esophagus irradiated and the likelihood of an acute injury. Series have reported volumes of esophagus irradiated as most reflective of acute symptoms, and efforts to sculpt the esophagus as an organ to avoid with RT have met with mixed results.3 Drug trials using the radioprotectant amifostine have been reported as unsuccessful in reducing acute esophagitis, but those trials used subjective measures of esophageal irritation such as patient self-reports.4,5 In this article, we report our retrospective series of amifostine as a cytoprotectant against acute esophagitis in patients receiving definitive chemoradiotherapy for small cell lung cancer (SCLC), using objective measures of weight loss and opiate use.
Materials and methods
From 2005 to 2016, 53 consecutive patients with stage 3 SCLC were evaluated at our large community hospital. This study was reviewed by the Wheeling Hospital IRB and deemed exempt as the data collection was retrospective, from chart review only, and did not include any patient identifiers. All patients had previously provided written informed consent for the use of their medical data in future research. A diagnostic procedure was performed (by bronchoscopy or mediastinal node biopsy), followed by a standard staging evaluation to include blood work to assess marrow, renal, and liver function along with a radiographic survey including a contrast-enhanced chemotherapy (CT) scan of the chest, abdomen, and pelvis. All patients underwent a pretreatment MRI of the brain and, with symptoms of skeletal pain, a bone scan. Our institutional approach is to deliver concurrent chemoradiotherapy with the RT targeting the thoracic tumor volume from the first cycle of chemotherapy. Four patients had bulky enough disease requiring induction chemotherapy prior to the initiation of the RT course, and they were excluded from this series; the remaining 49 patients received concurrent chemoradiotherapy (CMT) from the first chemotherapy cycle and were the subject of this analysis. Chemotherapy consisted of a 21-day cycle of cisplatin (120 mg/m2 on day 1) and etoposide (60 mg/m2 on days 1–3). The RT course consisted of 4500 cGy to the gross tumor volume in 150 cGy fractions delivered twice daily with a 6-hour interfraction interval. Elective nodal irradiation was not performed. The tumor volumes were identified at a diagnostic contrast-enhanced CT simulation. Three-dimensional conformal RT techniques were used. Intensity-modulated radiation therapy was not performed. The standard RT approach was to deliver 30 Gy in an opposed anterior-posterior beam arrangement and 15 Gy delivered in a spinal cord and esophagus-sparing oblique beam arrangement. In 32 patients (group 1), amifostine was delivered subcutaneously (500 mg) 30 minutes prior to the afternoon fraction of RT. Amifostine was not delivered on days when chemotherapy was given. Patients were encouraged to prehydrate and take compazine 10 mg per oral route 90 minutes prior to the amifostine injection. Patients were questioned by the nursing staff prior to the amifostine injection to ensure compliance with the hydration and anti-emetic regimen. Failure to comply resulted in holding the amifostine dose for that day. All patients were observed in the department following the amifostine injection for signs and symptoms of hypotension and nausea. The remaining 17 patients (group 2) did not receive pre-RT amifostine due to choice or drug intolerance.
The entire volume of the thoracic esophagus was contoured with an RT treatment planning system tool. This provided for a volumetric estimate of the esophagus that received the RT intended for the tumor volume; the esophagus, while adjacent to the tumor volume, received substantial dose. The dose of RT that was delivered to 20%, 30%, or 50% of the esophageal volume was classified as D20, D30, or D50, respectively.
Upon completion of the CMT course, patients completed a total of four cycles of cisplatin/etoposide and were then restaged and referred for consideration of consolidative prophylactic cranial irradiation based on at least a clinical partial tumor response. Following the completion of all therapies, patients were seen in a multidisciplinary clinic every 3 months. Follow-up consisted of physical examination, blood work, and a surveillance CT of the chest and abdomen twice a year. Targeted studies were performed in response to clinical concerns.
Statistical analysis
Representative results were shown in the present study. Numerical data were presented as the mean ± SD. The p-values between the two experimental groups were tested by 2-sample t-test, and p-values <0.05 were considered significant.
Results
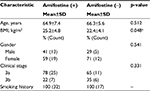
Table 1 depicts the patient characteristics. Patients in groups 1 and 2 presented with similar stages of disease with a nonsignificantly higher mediastinal nodal stage in the group not receiving amifostine (35% vs 22%). The median age of the two groups was similar (64 in group 1 and 67 in group 2), and there were more women in our series overall (59% in group 1 and 71% in group 2). The pretreatment body mass index was significantly higher in the group receiving pre-RT amifostine though, because each patient served as his/her own control in terms of weight loss during therapy, this was not considered to be a clinically significant factor. Table 2 illustrates the median absolute and relative weight changes as well as the median dose of RT at which point the opiate was prescribed. As is our clinical practice, all patients were prescribed a liquid hydrocodone/acetaminophen elixir (7.5 mg/500 mg per 15 mL) and could self-titrate to effect with no more than 60 mL delivered in a 24-hour period. In group 1, 10 patients (31%) initiated opiates at a median RT dose of 3300 cGy, whereas in the non-amifostine group, 7 patients (41%) initiated opiates at a median RT dose of 2250 cGy. The median absolute and relative weight loss experienced by group 1 was 4 pounds and 2% of the pretreatment body weight; 3 patients (9%) gained a median 3 pounds or 1.8%. The patients in group 2 not receiving amifostine lost a median 2.5 pounds and 1.4% of their pretreatment weight. Patients in group 1 could receive at most 13 injections of amifostine (1 dose/day minus the 2 days of chemotherapy). The average number of injections of amifostine received was 10. The reason for fewer than 13 injections was that the patient did not follow preinjection protocol with hydration and anti-emetic (number of patients [n]=8) or preinjection hypotension (n=6). Neither opiate initiation nor weight change was significantly affected by amifostine use. The patients in group 2 who were not receiving the amifostine either declined the drug (n=3) or experienced drug-related toxicity after one injection (n=14). The drug toxicities included hypotension and emesis (n=8) and allergy (n=6). The allergy consisted of a drug rash in 4 patients and asthenia in 2 others. The allergic reaction resolved after an average 2 days with appropriate antihistamine administration.
  | Table 2 Weight change and opiate use Notes: (+) denotes the absolute and relative weight gain. (−) denotes the absolute and relative weight loss. |
Because of the apparent difference between groups in terms of the dose of RT at which opiates were initiated, we evaluated the RT dose delivered to 20% (D20), 30% (D30), and 50% of the esophagus (D50) in both groups. The average D50 for group 1 was 2569 cGy (CI 1949–3189 cGy) and for group 2 was 1420 cGy (CI 523–2316 cGy), which met statistical significance (p<0.03) by 2-sample t-test. The average D30 for group 1 was 4032 cGy and for group 2 was 3392 cGy (p<0.08), and the average D20 for group 1 was 4231 cGy and for group 2 was 3712 cGy (p<0.11; Table 3). Because 5 of the patients (3 in group 1 and 2 in group 2) gained weight and did not experience any evidence of esophagitis during the course of treatment, they were not included in the statistical analysis evaluating the dose of RT that could be delivered before esophagitis was experienced. Based on the clinical records of the remaining 44 patients, all experienced varying degrees of esophagitis, but because it was not our approach, none were formally scored by the RTOG (Radiation Therapy Oncology Group) or CTCAE (Common Terminology Criteria for Adverse Events) parameters. Again, our approach was to measure weight loss and opiate introduction as an objective indicator of esophagitis.
Discussion
The importance of minimizing treatment-related toxicities has become a focus of clinical research over the past several years. Evidence exists demonstrating that treatment breaks or dose reductions impact negatively on outcomes.6 In the management of lung cancer, and particularly SCLC that often presents with bulky hilar and mediastinal nodal disease adjacent to the esophagus, efforts have been undertaken to minimize RT dose to the esophagus, with either advanced conformal avoidance RT techniques7 or reduction in the volume of the mediastinum treated.8
Amifostine was introduced into clinical cancer practice in the early 1990s, and reproducibly has shown benefit in reducing certain toxicities while preserving local tumor control and tumor-related survival; its earliest beneficial effects were demonstrated in cancers of the head and neck9 and rectum.10 The American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines currently support the use of amifostine prior to RT delivery in patients with head and neck cancers as a way of reducing xerostomia and prior to chemotherapy for ovarian cancer as a means to reduce platinum-based nephrotoxicity. However, because the number of patients potentially receiving chemoradiotherapy for lung cancer is far greater than that diagnosed with head and neck and ovarian cancers combined, several investigators have evaluated amifostine as a means of reducing esophagitis, reducing treatment breaks and dose reductions, and improving outcomes.
Several investigators have published work evaluating amifostine when patients with non-small cell lung cancer (NSCLC) received definitive RT or chemoradiotherapy (CMT). In a Greek randomized trial of definitive RT alone in 146 patients with stage 2b–4 NSCLC receiving 60 Gy, 50% of the patients received amifostine 340 mg/m2 intravenous (IV) 15 minutes prior to the RT course. Endpoints included toxicity and tumor control. The authors observed a significant reduction in esophagitis beginning in the third week of RT, which continued until 2 weeks following all treatment delivery in those patients receiving amifostine. A reduction was also seen in acute pulmonary toxicity starting in the fifth week of treatment. There was no worsening in terms of overall or progression-free survival in the amifostine arm.11
In a second randomized trial from MD Anderson, 60 patients with stage 2a–3b NSCLC were randomized to 500 mg IV amifostine twice weekly or placebo prior to CMT. The chemotherapy consisted of oral VP-16 and infusional cisplatin. The RT was delivered twice daily 1.2 Gy to a total dose of 69.6 Gy. With a median follow-up of 6 months, there was a reduction in opiate requirement (7% vs 31%) and less pneumonitis (4% vs 27%) in the amifostine arm. No significant difference in tumor response rates was seen. A significant number of patients receiving amifostine (70%) experienced hypotension, as defined as a 20 mm Hg decrease from baseline.12
In the largest study to date evaluating amifostine as a potential protective agent, the RTOG enrolled 243 patients with stage 2–3b NSCLC receiving concurrent chemoradiotherapy. The randomization was between placebo and IV amifostine 500 mg 4 times weekly. After a median follow-up of nearly 8 years, there was no difference in overall survival between groups nor was there a benefit in terms of esophagitis or pneumonitis. The amifostine group experienced greater acute toxicities including nausea, emesis, and hypotension. Criticisms of the study were that there was less weight loss and a lower incidence of swallowing symptoms reported by patients in the amifostine arm, but the health care personnel did not report a lower incidence of esophagitis.13
In total, the amifostine experience with chemoradiotherapy and NSCLC has been compelling; that is, several of these studies have clearly demonstrated a protective benefit though the benefit may be underestimated by the competing acute toxicity of nausea, emesis, and hypotension often seen with the infusional delivery of the drug. More recently, it has been suggested that esophagitis is dependent upon the volume of the esophagus irradiated.
In an excellent review of the literature addressing risk factors for the development of esophagitis, concluding that 25% of patients receiving concurrent chemoradiotherapy develop severe acute esophageal toxicity, the authors indicated that there is inconsistency regarding the definition of esophageal toxicity, defining the actual volume of the esophagus irradiated, the actual cause of esophageal injury, and whether it can be minimized with drug intervention or modification of RT dose delivery.14
In our series, we elected to collect objective data regarding esophageal injury (weight loss and opiate use) rather than scoring the degree of subjective patient complaints, particularly given the discrepancy between patients reported swallowing function and that reported by clinicians as seen in the RTOG trial.13 We used a standard volume measurement of the esophagus by contouring the entire circumference of the structure from the thoracic inlet to the gastroesophageal junction.
We then calculated the total RT dose delivered to 20%, 30%, and 50% of the contoured esophagus by a volume approach (0.20× esophagus volume in cm3 irradiated for the D20 value).
Our definition of what makes amifostine a potential esophageal cytoprotectant was less weight loss, less use of opiates during treatment, and/or allowing for a higher dose of RT to be delivered before objective measurements of esophagitis could be observed. In our series, we did not observe less weight loss or less opiate use overall between the two groups; however, we did observe that a significantly higher RT dose could be delivered to 50% of the esophagus, before opiates were prescribed, in the group receiving amifostine.
The meta-analysis14 evaluating the esophageal dose–volume histograms suggested that the D50 was the most predictive metric of acute esophageal toxicity; our series was corroborative. Importantly, it is notable that the published series included in the meta-analysis investigated NSCLC. None of the series investigated esophagitis in SCLC.
Given the volume of esophagus typically encompassed in the high-dose RT penumbra in SCLC, several studies have also evaluated the role of amifostine. In a Phase II trial of 34 patients with limited stage SCLC, patients received amifostine 340 mg/m2 IV daily before the morning fraction of RT. The RT course was 1.5 Gy twice daily to a total dose of 45 Gy. Chemotherapy consisted of cisplatin 60 mg/m2 on day 1 and etoposide 120 mg/m2 on days 1–3 of each cycle. About 30% of the patients experienced hypotension, 32% developed nausea and emesis, and 3% a rash. Response rates and rates of esophagitis scored as grade 0–4 were not different than historic controls. The authors concluded that there was no benefit to amifostine administered IV prior to the morning RT fraction in patients with SCLC; they characterized this as inconsistent with the effect seen with NSCLC.5 It is possible that the acute toxicity seen with IV amifostine in this study precluded analysis of esophagitis as nearly a third of patients had emesis from the drug.
In a Phase I trial investigating sequential and concurrent CMT, 15 patients with limited stage SCLC received topotecan and paclitaxel for 2 cycles followed by cisplatin and etoposide concurrent with 1.2 Gy twice daily starting at 48 Gy and escalating to 66 Gy. Pre-RT amifostine was delivered subcutaneously at 500 mg. This study demonstrated that amifostine provided for dose escalation to 60 Gy before dose-limiting esophagitis was observed.15
In a Phase II Korean study, 76 patients with limited stage SCLC were treated with 2 cycles of induction cisplatin and irinotecan followed by concurrent cisplatin, etoposide, and RT 1.5 Gy twice daily to 45 Gy. Patients were randomized to either pre-RT subcutaneously amifostine 500 mg or epoetin-alpha, both delivered 3 times each week. Of the 36 patients randomized to receive amifostine, only 21 received the drug due to supply shortages. Response rates were similar between the 2 arms, but there was no benefit in terms of reduction of esophagitis in the amifostine arm. The authors concluded that in this intensive chemotherapy regimen, epoetin-alpha reduced hematologic toxicity.16
A British trial of 84 patients with limited or extensive stage SCLC was randomized to amifostine 740 mg/m2 IV prior to chemotherapy alone or placebo. RT was not studied in this group. The authors did not demonstrate a protective benefit from amifostine, with particular attention paid to hematologic effects.17
Based on these reports investigating patients with SCLC who received intensive induction chemotherapy followed by concurrent chemoradiotherapy, there does not appear to be a protective benefit to pre-RT amifostine. However, sequential and concurrent CMT has not been an accepted method of treating non-metastatic SCLC. Indeed, concurrent CMT with the RT initiated along with the first cycle of chemotherapy has been considered the optimal therapy.18 It is questionable to conclude that amifostine does not reduce esophagitis in a treatment regimen that is not considered standard. Furthermore, these series of SCLC did not report the volumes of the esophagus irradiated and whether amifostine impacted on the D50 RT dose.
Conclusion
Our series represents a contemporary concurrent chemoradiotherapy approach in patients with stage 3 SCLC. Based on level 1 evidence demonstrating an overall survival advantage to hyperfractionated RT,19 omitting elective nodal irradiation, and using modern doublet chemotherapy, our series represents a compelling argument that pre-RT amifostine delivered subcutaneously can postpone the development of acute esophagitis. These findings represent first to our knowledge that evaluating the esophageal D50 in SCLC provides evidence that subcutaneous amifostine postpones the onset of acute esophagitis. These findings should be replicated in a prospective controlled study and also to determine whether subjective scoring of patient-reported esophagitis supplements data on weight change and opiate introduction.
Acknowledgments
A portion of the data associated with this manuscript was presented at the International Association for the Study of Lung Cancer, Chicago, 2017, in the form of a poster. The poster’s abstract was published in the Poster Session of J Thorac Oncol 12(11), Suppl 1:S1575 (www.jto.org/article/S1556-0864(17)30801-8/pdf).
Disclosure
The authors report no conflicts of interest in this work.
References
Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage 3 non-small cell lung cancer: randomized phase 3 trial RTPG 9410. J Natl Cancer Inst. 2011;103(19):1452–1460. | ||
Bradley J, Graham M, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase 1-2 dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;61(20):318–328. | ||
Hu X, He W, Wen, S, et al. Is IMRT superior or inferior to 3DCRT in radiotherapy for NSCLC? A meta-analysis. PLoS One. 2016;11(4): e0151988. | ||
Han HS, Han JY, Yu SY, et al. Randomized phase 2 study of subcutaneous amifostine versus epoetin-alpha given 3 times weekly during concurrent chemotherapy and hyperfractionated radiotherapy for limited-disease small cell lung cancer. Cancer. 2008;113(7):1623–1631. | ||
Arquette M, Wasserman T, Govindan R, et al. Phase II evaluation of amifostine as an esophageal mucosal protectant in the treatment of limited-stage small cell lung cancer with chemotherapy and twice-daily radiation. Semin Radiat Oncol. 2002;12(Suppl 1):59–61. | ||
Cox JD, Pajak TF, Asbell S, et al. Interruptions of high-dose radiation therapy decrease long- term survival of favorable patients with unresectable non-small cell carcinoma of the lung analysis of 1244 cases from 3 RTOG trials. Int J Radiat Oncol Biol Phys. 1993;27(3):493–498. | ||
Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57(3):875–890. | ||
Schild SE. Elective nodal irradiation (ENI) doesn’t appear to provide a clear benefit for patients with unresectable non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2008;72(2):311–312. | ||
McDonald S, Meyerowitz C, Smudrin T, Rubin P. Preliminary results of a pilot study using WR-2721 before fractionated irradiation of the head and neck to reduce salivary dysfunction. In J Radiat Oncol Biol Phys. 1994;29(4):747–754. | ||
Kligerman MM, Liu T, Liu Y, Scheffler B, He S, Zhang Z. Interim analysis of a randomized trial of radiation therapy of rectal cancer with/without WR-2721. Int J Radiat Oncol Biol Phys. 1992;22(4):799–802. | ||
Antonadou D, Coliarakis N, Synodinou M, et al; Clinical Radiation Oncololgy Hellenic Group. Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51(4):915–922. | ||
Komaki R, Lee JS, Kaplan B, et al. Randomized phase III study of chemoradiation with or without amifostine for patients with favorable performance status inoperable stage 2–3 non-small cell lung cancer: preliminary results. Semin Radiat Oncol. 2002;12(1):46–49. | ||
Lawrence YR, Paulus R, Langer C, et al. The addition of amifostine to carboplatin and paclitaxel based chemoradiation in locally advanced non-small cell lung cancer: long-term follow-up of Radiation Therapy Oncology Group (RTOG) randomized trial 9801. Lung Cancer. 2013;80(3):298–305. | ||
Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S86–S93. | ||
Graces YI, Okuno SH, Schild SE, et al. Phase 1 North Central Cancer treatment Group Trial-N9923 of escalating doses of twice-daily thoracic radiation therapy with amifostine and with alternating chemotherapy in limited stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;67(4):995–1001. | ||
Han HS, Han JY, Yu SY, et al. Randomized phase 2 study of subcutaneous amifostine versus epoetin-alpha given 3 times weekly during concurrent chemotherapy and hyperfractionated radiotherapy for limited-disease small cell lung cancer. Cancer. 2008;113(7):1623–1631. | ||
Johnson PW, Muers MF, Peake MD, et al. A randomized trial of amifostine as a cytoprotective agent in patients receiving chemotherapy for small cell lung cancer. Br J Cancer. 2001;84(1):19–24. | ||
Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. J Clin Oncol. 1993;11(2):336–344. | ||
Turrisi A, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited stage small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.