Back to Journals » Local and Regional Anesthesia » Volume 10
Ambulatory anesthetic care in children undergoing myringotomy and tube placement: current perspectives
Authors Robinson H, Engelhardt T
Received 13 December 2016
Accepted for publication 15 March 2017
Published 19 April 2017 Volume 2017:10 Pages 41—49
DOI https://doi.org/10.2147/LRA.S113591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Hal Robinson, Thomas Engelhardt
Department of Anaesthesia, Royal Aberdeen Children’s Hospital, Aberdeen, UK
Purpose: Myringotomy and tube placement is one of the most frequently performed ear, nose and throat (ENT) surgeries in the pediatric population. Effective anesthetic management is vital to ensuring successful ambulatory care and ensuring child and parental satisfaction.
Recent findings: This review summarizes recently published studies about the long-term effects of general anesthesia in young children, novel approaches to preoperative fasting and simplified approaches to the assessment and management of emergence delirium (ED) and emergence agitation (EA). New developments in perioperative ambulatory care, including management of comorbidities and day care unit logistics, are discussed.
Summary: Long-term follow-up of children exposed to general anesthesia before the age of 4 years has limited impact on academic achievement or cognitive performance and should not delay the treatment of common ENT pathology, which can impair speech and language development. A more liberal approach to fasting, employing a 6–4–0 regime allowing children fluids up until theater, may become an accepted practice in future. ED and EA should be discriminated from pain in recovery and, where the child is at risk of harm, should be treated promptly. Postoperative pain at home remains problematic in ambulatory surgery and better parental education is needed. Effective ambulatory care ultimately requires a well-coordinated team approach from effective preassessment to postoperative follow-up.
Keywords: myringotomy, ventilation tubes, anesthesia, pediatrics, ambulatory, day case
Introduction
Myringotomy and tube placement is one of the most frequently performed ear, nose and throat (ENT) surgeries in the pediatric population. It is performed for persistent otitis media with effusion (OME) associated with documented hearing loss or where the impact of hearing loss on a child’s developmental, social or educational status is judged to be significant. This review summarizes critical points when assessing, preparing and anesthetizing children for this procedure by targeting the unique challenges faced in pediatric ENT day-case surgery.
Pediatric myringotomy
Anatomy and indications
In children, the eustachian tube is shorter, more horizontally oriented and less functionally mature. These differences can predispose children to developing OME.1 Approximately 90% of children experience one episode of OME by the age of 10 years, which normally resolves spontaneously in 6–12 weeks. Active treatment with steroids, antibiotics and decongestants has no effect on clinical outcomes and is not recommended. Ongoing follow-up may be necessary, as 30%–40% of children will have recurrent or relapsing episodes.
Hearing loss at any level is known to impact a child’s social and educational development, and should signs or symptoms persist, the child should be referred for hearing tests. Surgical treatment with myringotomy and ventilation tubes is reserved for children in whom chronic inflammation is present together with documented hearing loss.2 The National Institute for Clinical Excellence issued guidance for the management of OME and recurrent acute otitis media (RAOM) outlining indications for surgery.3 These include persistent bilateral OME over a period of 3 months with a hearing level in the better ear of 25–30 dB or bilateral OME with a hearing loss of <25–30 dB where the impact is judged to be significant. The rationale for surgical treatment is to rapidly restore normal auditory function and, therefore, minimize the impact on the child’s speech, language and intellectual development.4,5
Surgical procedure
Myringotomy and tube placement is performed by making a small incision using a myringotomy knife through the layers of the tympanic membrane. This permits direct access to the middle ear space and allows the release of middle ear fluid. The placement of tubes allows the incised drum to continue to drain and facilitates resolution of the condition, while also improving the auditory deficit. The surgery is usually quick and ideally suited to a day-case procedure. Repeat procedures are sometimes required and can be more technically challenging; however, they do not preclude a same-day discharge.
When to operate?
Current studies have demonstrated an improvement in short-term hearing function by operating early in OME; however, this has yet to translate into better long-term developmental outcomes.6 Concerns of anesthetic agent toxicity on the developing human brain have been expressed and are widely publicized. This speculative risk must be weighed against the existing evidence of delayed speech and language development associated with poor hearing. Recent studies indicate that anesthetic exposure before the age of 4 years has minimal or no effect on academic achievement or cognitive performance.7,8 Even multiple exposures at this age were shown to be less significant than gender, maternal education level or month or year of birth. Earlier surgical intervention in the course of OME may, therefore, have significant benefits in children with hearing deficits, minimizing the impact on the development of writing and language skills.
Facilities and organization of pediatric ambulatory surgery
Ambulatory surgery is becoming increasingly popular with a wider range of procedures and patients now considered suitable. It is defined as a surgery that does not require an overnight stay and has a number of advantages for the child, family and hospital (Table 1). The package of day-case surgery increases patient comfort, reduces hospital stay and achieves higher child and parental satisfaction. There are few disadvantages, which arise from the inability to control and manage factors after discharge and include postoperative pain, nausea and vomiting and the potential deterioration of previously stable chronic conditions.
  | Table 1 Principal advantages of ambulatory surgery Note: Data from Bowen and Thomas.51 |
Day surgery should be provided whenever practical, enabling children to have safe surgery as close to their home as possible. Providing this service requires specialist consultant input and dedicated pediatric day care facilities with trained staff. Guidelines are necessary to assist in the smooth running of theater lists and coordinate safe patient discharge.
Specifically designed day-case units should coordinate pediatric day-case surgery. Children should be seen in preoperative assessment clinics to identify and optimize comorbidities and assess individual suitability for day-case surgery.
All children should have a named consultant surgeon and anesthetist, although list management may be delegated to nonconsultant grades and trainees.9 A named pediatrician should be contactable at all times. Registered pediatric nurses lead in the care of patients on the ward from the time of admission until discharge. Other important members of the team include play specialists and translators.
In smaller hospitals, where no on-site medical pediatric services exist, care should only be delivered by consultant surgeons and anesthetists experienced in the condition and with pediatric expertise. All team members should have up-to-date basic pediatric life support skills, with at least one member possessing advanced life support skills. Should unforeseen complications arise these sites must have agreed arrangements for managing and transferring children to specialist pediatric centers.10
Continued audit of day care units is essential to ensure that the above standards continue to be met. Important measures for evaluation include overall day-case rates, postoperative pain, and unplanned readmission rates. Questionnaires for parents and children are additional tools to ensure that services continue to develop and improve (Table S1). The atmosphere should be friendly and environment colorful and bright. Dedicated play areas should be incorporated to improve the child’s experience.
Preoperative assessment services are vital to delivering day-case surgery. Clinics are usually nurse led with consultant input but vary if consent for anesthesia is also required.11 This visit permits the identification of preexisting medical conditions and anesthetic risk factors and enables the child and parents to ask questions and alleviate any concerns. Written guidance about general anesthesia, including pre- and postoperative care arrangements and policies, should be available for each family.
Patient considerations for ambulatory care
Children with significant coexisting diseases should undergo further assessment and referred to a specialist pediatric center. Common conditions encountered in children with ENT pathology include upper respiratory tract infection (URTI), asthma, obstructive sleep apnea (OSA) and craniofacial disorders. The majority of these conditions can be optimized with specialist input and should not impede the child from having a day-case procedure. The advent of improved surgical and anesthetic techniques means that there remain very few absolute exclusion criteria for ambulatory surgery; however, certain cases including neonates, morbid obesity and complex chronic conditions must be evaluated on a case-by-case basis (Table 2).
  | Table 2 Absolute contraindications to pediatric ambulatory surgery Note: Data from Brennan and Atul.52 Abbreviation: URTI, upper respiratory tract infection. |
URTIs
Perioperative adverse respiratory events are responsible for three quarters of the critical incidents recorded in pediatric anesthesia. This encompasses laryngospasm, bronchospasm, desaturation <95%, persistent coughing, airway obstruction and postoperative stridor. The risk is greatest when active URTI is present and remains elevated for up to 6 weeks following resolution of the illness.
A sound history and examination during the preoperative assessment are the most useful tools to identify children at high risk of an adverse event (Box 1).12,13 Those with two or more risk factors or evidence of active infection may benefit from rescheduling of surgery; however, this needs to be balanced against the increased frequency of URTI in children with unresolved ENT pathology. When cancellation is not appropriate, use of nonirritant volatile agents and avoidance of airway manipulation and endotracheal intubation are favorable.13
  | Box 1 Independent risk factors for perioperative adverse respiratory events in children with an URTI Abbreviation: URTI, upper respiratory tract infection. |
Asthma
Worldwide, 130 million children have asthma, with the prevalence 8–10 times higher in developed countries. Its incidence continues to increase in the pediatric population. Studies show that well-controlled asthmatics tolerate anesthesia much better and have a reduced rate of adverse perioperative respiratory events. Poorly controlled asthma should be identified at the preassessment clinic in order to optimize therapy prior to elective surgery.14 Elective surgeries should be deferred if there is evidence of active respiratory infection or an exacerbation of asthma within the preceding 2–4 weeks.
Anesthetic management of children should include continuation of oral and inhaled medication, including a dose of bronchodilator prior to surgery.
Obesity
Nearly one-third of children in the UK are classed as obese. Airway management is more difficult in this population, with an increased incidence of OSA, perioperative acute respiratory adverse events and aspiration.15 Although myringotomy is usually a short procedure, decreased lung compliance and atelectasis may result in unacceptable hypoventilation and desaturation, and intubation with positive pressure ventilation may need to be considered. Obese children also have an increased incidence of postoperative complications including airway obstruction and may require a longer duration of stay in the postoperative care unit (PACU).
Genetic abnormalities
Structural abnormalities of the eustachian tube and middle ear mean that children with Down’s syndrome and craniofacial defects are at higher risk of developing OME. Inflammation of the mucosa of the eustachian tube orifice and improper functioning of the eustachian tube musculature lead to negative middle-ear pressure and impaired ventilation, which when sustained leads to retraction of the tympanic membrane and sequestration of fluid into the middle ear space.16
Perioperative care
On the day of admission, the child should be reviewed to ensure that no new or immediate contraindications to anesthesia have arisen. Commonly encountered conditions include URTIs or other intercurrent infections. A full set of observations should be recorded, and fasting status checked. In addition, the preoperative visit allows the opportunity to establish a rapport with the child, educate parents and impart information regarding postoperative care. Anesthesia screening questionnaires may be given to parents on admission to the ward. On high turnover lists with a large number of patients, this can help reduce the administrative burden and avoid the duplication of paperwork.
Fasting and fluids
Prolonged fasting can lead to a number of adverse complications including hypoglycemia, dehydration and postoperative nausea and vomiting (PONV). This is especially important in the younger children, where these problems occur more rapidly. Recent studies have also demonstrated increased ketone body formation and mean arterial pressure variations on induction of anesthesia.17 In addition to the physiological benefits of minimizing fasting times, children are more cooperative and comfortable.18
Preoperatively, families should be given verbal and written information regarding fasting times. Some ambulatory units have moved toward staggered admissions, which may allow more individualized fasting information to be disseminated. Anesthetic and surgical teams should assess and structure theater lists to identify children at high risk, placing them earlier on the theater schedule where possible.
Current guidelines for fasting for general anesthesia includes 2 hours for clear fluids, which includes nonpulp-based fruit juices, 4 hours for breast milk and 6 hours for solids. However, shortening to a 6–4–0+ fasting regime may become a more widely accepted pediatric practice.19–21 Delivering a 2-hour absolute fasting time can be challenging, even in scheduled elective surgery. Utilizing a 6–4–0+ regime, however, reduces the risk of miscommunication with nursing staff and allows greater flexibility should a change to the theater schedule be required.
Managing anxiety
Preoperative anxiety is a significant and challenging problem. Poorly managed anxiety is linked with adverse perioperative and postoperative outcomes. Anxiety may lead to reduced compliance at induction and combative behavior, which is linked with negative behavioral changes and can impact future health care interactions.22 Risk factors for anxiety and distress include preschool age, shy temperament, parental anxiety and previous negative or distressing hospital experiences. In addition, the hearing deficits associated with OME may increase anxiety.
Strategies to manage anxiety can be psychological and pharmacological. Prehospital programs, which include tours of the hospital and interactive learning, have been implemented successfully in a number of units; however, these are labor intensive and are less efficacious in younger children. The use of play therapy on admission to hospital has been shown to reduce anxiety and is an important part of the perioperative process. Distraction techniques including the use of tablet devices are being increasingly employed in the anesthetic room and can produce comparable levels of anxiolysis.23
Parental presence at induction remains controversial. Current studies have failed to demonstrate a benefit to the child but improve parental satisfaction and reduce parental anxiety. Limitations of available studies are the exclusion of children with chronic illness or those undergoing repeat procedures.
Common pharmacological agents used include benzodiazepines, alpha-2 receptor agonists and ketamine. These agents are usually used on their own, although reduced dose combinations have been utilized to improve efficacy and reduce medication side effects. Intranasal and intramuscular preparations may be considered as alternatives if medication is refused orally. The alpha-2 agonist clonidine has the advantages of supplementing perioperative analgesia and reducing the incidence of emergence delirium (ED).24
Induction and maintenance
The choice between an intravenous or gas induction depends on patient and parental factors as well as anesthetic preference. Neither intravenous nor gas induction has any benefit in terms of adverse reactions or influence on postoperative recovery in the ambulatory setting.
Airway management
Options for airway management include tracheal tubes, supraglottic airway devices (SADs) or facemask ventilation. Pediatric SADs are now perceived standard in day-case surgery and have a number of advantages including reduced airway manipulation, ease of insertion and reduced incidence of sore throat. Disadvantages mainly occur in younger children below the age of 1 year where anatomical and physiological changes are associated with an increased incidence of airway obstruction, malposition and laryngospasm. This can partly be overcome by insertion with a partially inflated cuff and insertion with a 180° rotation to avoid epiglottic entrapment.25 Care should also be taken with cuff volumes, as overinflation may lead to mucosal ischemia and compromise ventilation. Tracheal intubation for myringotomy and ventilation tube surgery is warranted due to patient factors or SAD failure.
Maintenance of anesthesia
General anesthesia can be maintained with either an intravenous or an inhalational technique. Potential advantages of total intravenous anesthesia (TIVA) with propofol include less PONV, laryngospasm and ED during recovery from anesthesia.26 Disadvantages include pain on injection during induction with propofol and risk of awareness. Propofol infusion syndrome (PIS) is an additional concern; however, this has never been reported during the use of TIVA for routine anesthesia. Inhaled agents, mainly sevoflurane, mitigate the need for intravenous access before induction and demonstrate no difference in time to recover from anesthesia or hospital discharge when compared with TIVA.27 Inhalational agents may be favored in centers electing to proceed with anesthesia without intravenous access. Myringotomy can be a very quick procedure, and proceeding using inhalational agents without access may be preferred if operating as a sole practitioner. This technique relies on sufficient anesthetic experience, vigilance and meticulous airway management.28
Analgesia considerations
The tympanic membrane is mainly innervated by the auriculotemporal nerve, a branch of the mandibular nerve, although also receives contributions from the auricular branch of the vagus nerve and the facial nerve. The myringotomy incision can be very stimulating, and periprocedural analgesic requirements in children undergoing surgery are very variable. Indicators of higher analgesic requirements include repeat procedures and active infection or inflammation.
The best analgesic approach for myringotomy and tube placement is yet to be confirmed.29,30 Oral paracetamol, ibuprofen or diclofenac should be administered 30 minutes prior to the procedure where possible.31 Ketorolac is an alternative nonsteroidal analgesic and has been shown to provide superior immediate postoperative analgesia when compared with paracetamol alone; however, up to 30% of these patients may still require additional analgesia in the immediate postoperative period.31,32 Combinations of paracetamol and a nonsteroidal or intranasal fentanyl have also been studied but in combination do not provide any additional analgesic benefit in the immediate postoperative period.33 Systemic opioids are an alternative used by some centers and have the additional advantage of reducing the incidence of emergence agitation (EA) or ED.34 The intranasal route is an option for units not routinely establishing intravenous access. Lack of intravenous access makes the treatment of perioperative complications more difficult, and these risks need to be balanced against the actual and perceived benefits of not establishing intravenous access.
If opioids are used, shorter acting agents should be preferentially selected. Morphine has not shown to produce any additional analgesic benefits in this patient group when compared with fentanyl.35 Fentanyl can be administered intravenously or intranasally and has the additional benefits of reducing EA or ED in the immediate postoperative period. It does not delay recovery and hospital discharge or increase the rate of PONV.36
Alternative methods of analgesia are topical lidocaine or regional nerve block. Topical lidocaine 2–4% applied to the tympanic membrane has been shown to reduce early postoperative analgesic requirements and provide equivalent pain relief to paracetamol.29 Blockade of the auricular branch of the vagus nerve (nerve of Arnold), which supplies the external auditory canal and inferior portion of the tympanic membrane by injecting 0.2 mL of local anesthetic (0.25% levobupivacaine) with a 30 G needle into the everted tragus, is another option.37,38
Postoperative analgesia can be provided using a variety of methods. Oral analgesia should be encouraged where possible; however, this route is often not tolerated in young patients immediately postoperatively. In patients with established intravenous access, options include paracetamol, non-steroidal anti-inflammatory drugs and fentanyl. The advantages of the latter include rapid onset and may help ameliorate behavioral issues arising on emergence from anesthesia. Intranasal, sublingual or subcutaneous routes remain alternatives in units undertaking the procedure without intravenous access. The intranasal route may also be efficacious in the management of acute ED.
PONV
PONV occurs twice as frequent in children compared to the adult population with an incidence of 13–42%.39 Effective prophylaxis and management is important to minimize the detrimental effects of severe PONV including dehydration and electrolyte disturbances. In addition, PONV is a significant reason for parental dissatisfaction and readmission after ambulatory surgery. Effective management of PONV includes identifying high-risk patients, administering prophylactic antiemetics and minimizing the exposure to established causative agents. Risk factors for PONV are summarized in Table 3.40 Single prophylaxis should be given to all children at risk of PONV, and high-risk patients should receive dual therapy. Alternatives to opioid analgesia and use of intravenous anesthesia should also be considered.41
  | Table 3 Risk factors for postoperative nausea and vomiting Abbreviations: ENT, ear, nose and throat; PONV, postoperative nausea and vomiting. |
ED
ED and EA are well-recognized problems in the immediate postoperative period occurring in up to 80% of children following general anesthesia. Grouped under the term early postoperative negative behavior (e-PONB), it is characterized by a dissociative state of consciousness in which the child is irritable, uncooperative and inconsolably crying, kicking or thrashing, Failure to recognize and manage this complication promptly can result in self-injury, disruption to surgical sites, delayed recovery and compromise safe postanesthetic care.
Identified risk factors for e-PONB include preschool age, male gender, ENT surgery and preoperative anxiety. Diagnosis can be challenging, and features of ED can be hard to distinguish from other pathology such as acute pain (Figure 1). ED and pain are separate entities, and although they may be present simultaneously, they have different trends and clinical courses.42 Common features for e-PONB include lack of eye contact and awareness of surroundings.43,44
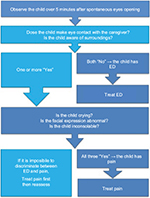  | Figure 1 Simplified ED algorithm. Note: Copyright © 2016. Adapted from Somaini M, Sahillioğlu E, Marzorati C, Lovisari F, Engelhardt T, Ingelmo PM. Emergence delirium, pain or both? A challenge for clinicians. Paediatr Anaesth. 2015;25(5):524–529.43 Abbreviation: ED, emergence delirium. |
Management of ED should be targeted at prevention. This includes nonpharmacological strategies such as preoperative preparation, parental cooperation and ensuring a calm environment prior to induction. Postoperative aims include a smooth emergence and calm, quiet recovery environment. Pharmacological strategies include intraoperative analgesics, such as clonidine and fentanyl, or short-acting sedative agents administered at the time of emergence.
Discharge from ambulatory care
Several key areas ensure adequate postoperative analgesia in pediatric ambulatory surgery. These include suitable perioperative pain relief, parental guidance on the management of pain at home and supply of appropriate discharge analgesia. Despite this, postoperative pain at home remains a problem in ambulatory surgery. Postoperative pain continues following discharge, as parental analgesic administration does not correlate with children’s reported pain scores.44,45 Even studies employing parental training into the use of pain scores failed to demonstrate an improvement in analgesia administration.44,46
Postoperative pain associated with myringotomy is rarely severe, with most children reporting mild-to-moderate pain scores.47 Higher pain scores may be associated with bilateral or repeat procedures. As indicated earlier, pain duration following the procedure is short lived, with mild pain rarely persisting beyond 48 hours. Parental instructions should focus on regular administration of simple analgesia and regular assessment of their child’s pain at home.
Effective discharge following simple day-case procedures requires experienced pediatric trained staff and stringent protocols. Checklists facilitate safe nurse led discharge in pediatric day-case units.48 The patient should have stable vital signs, and their pain and nausea controlled. The resumption of oral intake may be difficult in some children, and refusal to take oral fluids is no longer considered a contraindication to discharge. Suitable social and geographical factors should also be present, and the family should have easy access to medical care should complications arise. Parents and guardians should receive written and verbal instructions containing information about analgesia, postoperative complications, emergency contact numbers and follow-up arrangements.
Telephone follow-up should be attempted whenever possible.49 This improves parental satisfaction, can provide local audit data and enables troubleshooting of any acute complications, which may reduce hospital readmission and burden on primary care. Pain and uncontrolled nausea and vomiting are the two most common reasons for hospital readmission.50
Summary
Effective ambulatory anesthesia for children requiring myringotomy and ventilation tubes is key to improving the child’s experience and overall parental satisfaction. Several clinical issues affect outcomes and include the timing of surgery, fasting strategies and management of immediate postoperative problems such as ED and PONV. It is essential that pediatric services and local protocols are well established in order to achieve optimal clinical care.
Disclosure
The authors report no conflicts of interest in this work.
References
Zielhuis GA, Rach GH, van den Broek P. Predisposing factors for otitis media with effusion in young children. Adv Otorhinolaryngol. 1988;40:65–69. | ||
National Institute for Health and Care Excellence. Clinical Knowledge Summaries. Otitis Media with Effusion. Available from: https://cks.nice.org.uk/otitis-media-with-effusion. Accessed October 10, 2016. | ||
Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: tympanostomy tubes in children – executive summary. Otolaryngol Head Neck Surg. 2013;149(1):8–16. | ||
Wallace IF, Berkman ND, Lohr KN, Harrison MF, Kimple AJ, Steiner MJ. Surgical treatments for otitis media with effusion: a systematic review. Pediatrics. 2014;133(2):296–311. | ||
Mui S, Rasgon BM, Hilsinger RL Jr, Lewis B, Lactao G. Tympanostomy tubes for otitis media: quality-of-life improvement for children and parents. Ear Nose Throat J. 2005;84(7):418–424. | ||
Browning GG, Rovers MM, Williamson I, et al. [homepage on the Internet]. Grommets (Ventilation Tubes) for Hearing Loss Associated with Otitis Media with Effusion in Children (Cochrane Review). 2010. Available from: www.thecochranelibrary.com. Accessed November 4, 2016. | ||
Hansen TG, Engelhardt TE, Weiss M. Growing up – the relevance of anaesthetic drug-induced neurotoxicity. JAMA Pediatr. 2017;171(1):e163481. | ||
Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Academic performance after anesthesia and surgery during childhood – a large-scale nationwide study. JAMA Pediatr. 2017;171(1):e163470 | ||
British Association of Paediatric Surgeons. Standards for Children’s Surgery. Available from: http://www.baps.org.uk/content/uploads/2013/04/Standards-for-childrens-surgery-2013-Final.pdf. Accessed October 12, 2016. | ||
Mudumbai SC, Honkanen A, Chan J, et al. Variations in inpatient pediatric anesthesia in California from 2000 to 2009: a caseload and geographic analysis. Paediatr Anaesth. 2014;24(12):1295–1301. | ||
Rushforth H, Burge D, Mullee M, Jones S, McDonald H, Glasper EA. Nurse-led paediatric assessment: an equivalence study. Paediatr Nurs. 2006;18(3):23–29. | ||
Tait AR, Malviya S, Voepel-Lewis T, Munro HM, Seiwert M, Pandit UA. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95(2):299–306. | ||
Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet. 2010;376(9743):773–783. | ||
Rajesh MC. Anaesthesia for children with bronchial asthma and respiratory infections. Indian J Anaesth. 2015;59(9):584–588. | ||
Owen J, John R. Childhood obesity and the anaesthetist. Contin Educ Anaesth Crit Care Pain. 2012;12:169–175. | ||
Sharma RK, Nanda V. Problems of middle ear and hearing in cleft children. Indian J Plast Surg. 2009;42(suppl):144–148. | ||
Maekawa N, Mikawa K, Yaku H, Nishina K, Obara H. Effects of 2-, 4- and 12-hour fasting intervals on preoperative gastric fluid pH and volume, and plasma glucose and lipid homeostasis in children. Acta Anaesthesiol Scand. 1993;37(8):783–787. | ||
Splinter WM, Stewart JA, Muir JG. Large volumes of apple juice preoperatively do not affect gastric pH and volume in children. Can J Anaesth. 1990;37(1):36–39. | ||
Andersson H, Zarén B, Frykholm P. Low incidence of pulmonary aspiration in children allowed intake of clear fluids until called to the operating suite. Paediatr Anaesth. 2015;25(8):770–777. | ||
Schmidt AR, Buehler P, Seglias L, et al. Gastric pH and residual volume after 1 vs 2 h fasting time for clear fluids in children. Br J Anaesth. 2015;114(3):477–482. | ||
Engelhardt T, Wilson G, Horne L. Are you hungry? Are you thirsty? – fasting times in elective outpatient pediatric patients. Paediatr Anaesth. 2011;21(9):964–968. | ||
Sullivan MO, Wong GK. Pre-induction techniques to relieve anxiety in children undergoing general anaesthesia. Contin Educ Anaesth Crit Care Pain. 2013;13:196–199. | ||
Erhaze EK, Dowling M, Devane D. Parental presence at anaesthesia induction: a systematic review. Int J Nurs Pract. 2016;22(4):397–407. | ||
Johr M. Clondine in paediatric anaesthesia. Eur J Anaesthesiol. 2011;28(5):325–326. | ||
Nakayama S, Osaka Y, Yamashita M. The rotational technique with a partially inflated laryngeal mask airway improves the ease of insertion in children. Paediatr Anaesth. 2002;12(5):416–419. | ||
Gaynor J, Ansermino JM. Paediatric total intravenous anaesthesia. Br J Anaesth Educ. 2016;16(11):369–373. | ||
Ortiz AC, Atallah ÁN, Matos D, da Silva EMK. Intravenous versus inhalational anaesthesia for paediatric outpatient surgery. Cochrane Database Syst Rev. 2014;2:CD009015. | ||
Smith J, Rollin AM. The placement of an intravenous cannula is always necessary during general anaesthesia in children: a pro-con debate. Paediatr Anaesth. 2012;22(5):455–461. | ||
Howard R, Carter B, Curry J, et al. Postoperative pain. Paediatr Anaesth. 2008;18(suppl 1):36–63. | ||
Philips ML, Willis BC, Broman AJ, Lam HV, Nguyen TT, Austin TM. Bimodal analgesia vs fentanyl in pediatric patients undergoing bilateral myringotomy and tympanostomy tube placement: a propensity matched cohort study. J Clin Anesth. 2016;32:162–168. | ||
Bean-Lijewski JD, Stinson JC. Acetaminophen or ketorolac for post myringotomy pain in children? A prospective, double-blinded comparison. Paediatr Anaesth. 1997;7(2):131–137. | ||
Pappas AL, Fluder EM, Creech S, Hotaling A, Park A. Postoperative analgesia in children undergoing myringotomy and placement equalization tubes in ambulatory surgery. Anesth Analg. 2003;96(6):1621–1624. | ||
Rampersad S, Jimenez N, Bradford H, Seidel K, Lynn A. Two-agent analgesia versus acetaminophen in children having bilateral myringotomies and tubes surgery. Paediatr Anaesth. 2010;20(11):1028–1035. | ||
Finkel JC, Cohen IT, Hannallah RS, et al. The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesth Analg. 2001;92(5):1164–1168. | ||
Hippard HK, Govindan K, Friedman EM, et al. Postoperative analgesic and behavioral effects of intranasal fentanyl, intravenous morphine, and intramuscular morphine in pediatric patients undergoing bilateral myringotomy and placement of ventilating tubes. Anesth Analg. 2012;115(2):356–363. | ||
Galinkin JL, Fazi LM, Cuy RM, et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93(6):1378–1383. | ||
Bhananker SM, Azavedo L, MacCormick J, Splinter W. Topical lidocaine and oral acetaminophen provide similar analgesia for myringotomy and tube placement in children. Can J Anaesth. 2006;53(11):1111–1116. | ||
Voronov P, Tobin MJ, Billings K, Cote CJ, Iyer A, Suresh S. Postoperative pain relief in infants undergoing myringotomy and tube placement: comparison of a novel regional anesthetic block to intranasal fentanyl – a pilot analysis. Peadiatr Anaesth. 2008;18(12):1196–1201. | ||
Suresh S, Voronov P. Head and neck blocks in infants, children, and adolescents. Peadiatr Anaesth. 2012;22(1):81–87. | ||
The Association of Paediatric Anaesthetists of Great Britain and Ireland. Guidelines on the Prevention of Post-operative Vomiting in Children. 2009. Available from: http://www.apagbi.org.uk/sites/default/files/APA_Guidelines_on_the_Prevention_of_Postoperative_Vomiting_in_Children.pdf. Accessed November 6, 2016. | ||
Höhne C. Postoperative nausea and vomiting in pediatric anesthesia. Curr Opin Anaesthesiol. 2014;27(3):303–308. | ||
Somaini M, Sahillioğlu E, Marzorati C, Lovisari F, Engelhardt T, Ingelmo PM. Emergence delirium, pain or both? A challenge for clinicians. Paediatr Anaesth. 2015;25(5):524–529. | ||
Somaini M, Engelhardt T, Fumagalli R, Ingelmo PM. Emergence delirium or pain after anaesthesia—how to distinguish between the two in young children: a retrospective analysis of observational studies. Br J Anaesth. 2016;116(3):377–383. | ||
Homer JJ, Swallow J, Semple P. Audit of pain management at home following tonsillectomy in children. J Laryngol Otol. 2001;115(3):205–208. | ||
Fortier MA, MacLaren JE, Martin SR, Perret-Karimi D, Kain ZN. Pediatric pain after ambulatory surgery: where’s the medication? Pediatrics. 2009;124(4):588–595. | ||
Vincent C, Chiappetta M, Beach A, et al. Parents’ management of children’s pain at home after surgery. J Spec Pediatr Nurs. 2012;17(2):108–120. | ||
Wilson CA, Sommerfield D, Drake-Brockman T, von Bieberstein L, Ramgolam A, von Ungern-Sternberg BS. Pain after discharge following head and neck surgery in children. Paediatr Anaesth. 2016;26(10):992–1001. | ||
British Association of Day Surgery. Day Case and Short Stay Surgery. Available from: https://www.aagbi.org/sites/default/files/Day%20Case%20for%20web.pdf. Accessed November 5, 2016. | ||
Brenn BR, Choudhry DK, Sacks K. Outpatient outcomes and satisfaction in pediatric population: data from the postoperative phone call. Paediatr Anaesth. 2016;26(2):158–163. | ||
Whippey A, Kostandoff G, Ma HK, Cheng J, Thabane L, Paul J. Predictors of unanticipated admission following ambulatory surgery in the pediatric population: a retrospective case–control study. Paediatr Anaesth. 2016;26(8):831–837. | ||
Bowen L, Thomas M. Paediatric day case surgery. Anaesth Intensive Care Med. 2016;17(6):274–279. | ||
Brennan LJ, Atul PJ. Paediatric day-case anaesthesia. Contin Educ Anaesth Crit Care Pain. 2003;3:134–138. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
