Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Amantadine and cognitive flexibility: decision making in Parkinson's patients with severe pathological gambling and other impulse control disorders
Authors Cera N, Bifolchetti S, Martinotti G, Gambi F, Sepede G, Onofrj M, Di Giannantonio M , Thomas A
Received 13 September 2013
Accepted for publication 3 February 2014
Published 17 June 2014 Volume 2014:10 Pages 1093—1101
DOI https://doi.org/10.2147/NDT.S54423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Nicoletta Cera,1,2 Stefania Bifolchetti,1,3 Giovanni Martinotti,1,2 Francesco Gambi,1,2 Gianna Sepede,1,2 Marco Onofrj,1,3 Massimo Di Giannantonio,1 Astrid Thomas1,3
1Department of Neuroscience and Imaging, “G. d'Annunzio” University, Chieti, Italy; 2ITAB-Institute for Advanced Biomedical Technologies, Chieti, Italy; 3Aging Research Centre, Ce.S.I., “G. d'Annunzio” University Foundation, Chieti, Italy
Introduction: Dopamine replacement therapy for Parkinson's disease (PD) was recently linked to the development of impulse control disorders such as pathological gambling (PG), hypersexuality, compulsive shopping, and binge or compulsive eating. Antiglutamatergic agents including amantadine (Ama) reduce these behaviors in PD and non-PD patients. The aim of our study is to evaluate the changes in executive functions, emotions, and reward/loss processing during Ama treatment in PD patients.
Methods: Thirty-three patients affected by idiopathic PD were selected from a cohort of 1,096 PD patients and categorized in three different groups: ten affected by PG (PD-PG); nine PD patients with other impulse control disorder (PD-ICD); and 14 PD patient without any psychiatric disorder (PD-CTR-controls). For the neuropsychological evaluation, the following behavioral tasks where administered: the Stroop, the emotional Stroop, and the monetary reward/loss risk-taking tasks.
Results: During Ama treatment, PD-PGs showed a decrease in risky choices and an increase in non-risky choices (t(9)=−2.40, P<0.05 and t(9)=2,67, P<0.05 uncorrected, respectively). Between-group comparison showed a significant decrease in risky choices for PD-PG with respect to PD-CTR (t(22)=−4.16, P<0.01), and a decreased accuracy for positive words in comparison between PD-PG and PD-ICD (t(17)=−7,49, P<0.01) and PD-PG and PD-CTR (t(22)=−4.29, P<0.01). No within- and between-group differences were observed for Stroop task.
Discussion: Our data showed that Ama add-on therapy reduces hypersensitivity to reward and sustains activation toward uncertainty in PD-PG patients. These finding might explain the behavioral mechanism underlying the effect of antiglutamatergic drugs.
Keywords: Parkinson’s disease, executive functions, emotion
Introduction
Nonmotor symptoms occur across all Parkinson’s disease (PD) stages. The symptoms are unrecognized, underestimated, and under-treated or not treatable.1
Dopamine replacement therapy for PD was recently related to aberrant or excessive dopamine receptor stimulation resulting in the development of a number of nonmotor behavioral control problems,2 which consist of a set of complex disinhibitory pathologies such as impulse control disorders (ICDs). ICDs, and in particular pathological gambling (PG), represent a significant clinical concern in PD patients, as described by Housden et al.3
These behavioral addictions are clinical entities in which repetitive impulsive behaviors occur, with negative effects on the patients’ and their relatives’ lives; the prominent features of such conditions are: cognitive salience, as the activity dominates the subject’s thoughts and behaviors; conflict with other persons or activities; euphoria or relief, a feeling of short term pleasure from engaging in the behavior; tolerance or loss of control over the behavior; withdrawal, as experiencing unpleasant feelings when unable to engage in the behavior; and relapse and reinstatement, indicated when people unsuccessfully attempt to cut down on the behavior and subsequently engage in similar or higher levels of the activity than they had previously. Behavioral addictions are rather common in the general population, with a wide diffusion from adolescents4 to geriatric age.5 The impairment of functioning, with the presence of impulsiveness and other psychiatric symptoms, can be relevant and distressing in PD.6
In PD, typical ICDs include hypersexuality, PG, compulsive shopping, and/or compulsive eating; 6%–7% of PD patients meet criteria for one of these disorders.3 Lifetime prevalence of ICDs is 6.1% in all PD patients, and 13.7%–17.1% in PD patients on dopamine agonists (DA).2,7 The burden of these symptoms results in a negative impact on quality of life for patients and relatives.1
Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) recently inserted PG among the group defined as “Addiction and related problems”; it refers to persistent and recurrent gambling behavior characterized by preoccupation with gambling, increasing amounts of money spent, unsuccessful attempts to control gambling, restlessness or irritability when cutting down on gambling, lying to others about gambling, work or education, and relying on others for money. PG is the most extensively studied ICD in both PD and non-PD populations.8,9
Pathophysiology and evaluation
Neuroimaging studies observed alterations in the ventromedial prefrontal cortex (VM-PC) and the corticobasal-ganglionic-thalamic circuit in PD patients. Single-photon emission computed tomography (SPECT) studies found a dysfunction of the mesolimbic regions in PD with PG,10–12 and alteration of these pathways were shown in non-PD patients with PG, showing a reduced activity during rewarding events and monetary-reward tasks.13–15 Furthermore, the Stroop color word task16 showed reduced activity in VM-PC in poor PG patients. Stroop and memory task alterations were shown in PD patients with hypersexuality. PD patients with ICDs were impaired on spatial planning and set-shifting tests compared with healthy controls.17 The involvement of the prefrontal cortex (PC) suggests that tasks to assess executive functions are the best method to study PG and ICD in patients.
Recent studies hypothesize that levodopa-induced dyskinesias (LIDs) and behavioral alterations observed in dopamine dysregulation syndrome and ICD2,18 depend on common mechanisms involving alterations of glutamate homeostasis with combined activation of sensitized dopamine and NMDA glutamatergic receptors.19 Pre- and postsynaptic mechanisms are implicated in the devolvement of LIDs and ICDs, the first based on alterations in dopamine transmission after chronic administrations and the second because of excessive expression and sensitization of D1 receptors in striatonigral neurons.2,20 The neuronal adaption underlying the imbalance between synaptic and nonsynaptic glutamate might result in failure of PC control.
Yet no treatments are at present validated for ICDs or dopamine dysregulation syndrome; reduction or withdrawal of DA are considered possible options, but may induce severe worsening of motor control and DA withdrawal syndrome.21,22
Amantadine (Ama) is L-amino-adamantanamine, a salt of the symmetric 10-carbon primary amine. Its mechanism of action is based on interaction with dopamine, by enhancing release and inhibiting its reuptake and by changing dopamine receptor affinity, and on NMDA glutamate receptor blockade, normalizing the activity of the glutamatergic corticostriatal and subthalamic-pallidal pathways. As shown for other antiglutamatergic drugs, Ama reduces LIDs to the point that its effect on LIDs is considered by Cochrane reviews and a recent treatment guideline23 as having the only class I level A evidence of efficacy23,24 for Ama.
In a recent study, we investigated the possible efficacy of Ama in the control of PG associated with PD.9 The study was conducted in a double-blind cross-over manner with Ama 200 mg/day versus placebo and an open follow-up. Assessments included PG specific scales (Yale–Brown obsessive compulsive scale, South Oaks Gambling Screen [SOGS]) as well as assessment of expenditures and time spent gambling. Ama abolished or reduced PG in all treated patients, as confirmed by scale score and daily-expenditures reduction.
Also, the antiglutamatergic drug acamprosate ([Aca] Ca-acetyl homotaurine), a drug clinically used to reduce alcohol dependence and craving, attenuates glutamate release within the nucleus accumbens by binding to GluR5 AMPA receptors and interacting with NMDA receptors. A recent study showed that acamprosate significantly reduced obsessive-compulsive behavior in binge eating and food craving, and improved quality of life in patients affected by binge eating disorder.
Therefore, a possible option for ICDs and LIDs treatment might be to consider antiglutamatergic drugs.23 However, few molecules are characterized by efficient antiglutamatergic activity;25 for example, memantine,26,27 Dextrometrophan,28 and budipine are considered antiglutamatergic drugs, yet only anecdotal reports showed LIDs or ICDs reduction. Lamotrigine, oxcarbazepine, and topiramate represent a class of anticonvulsant compounds with glutamate-release-inhibiting properties that shows encouraging evidence as novel medications for alcoholism.29,30 However, their potential in ICDs still needs to be demonstrated.
In our study, which considers a subsample of PD patients with ICDs previously described elsewhere,9,31 we tested before (T0) and after (T1) Ama add-on medication (200 mg/day) to assess its effect on executive functions, emotion, and reward and loss processing. The color word Stroop task, emotional Stroop task (EST), and monetary reward/loss task slot machine-like game, respectively, were used to test the effect of Ama in PD patients with PG (PD-PG) and PD patients with ICDs (PD-ICD).
Materials and methods
Study population
A cohort of 1,096 patient with diagnosis of idiopathic PD according to UK-BBC32 were regularly followed and treated from January 2011 to March 2012 in our Movement Disorder Clinic; they were asked to participate in the study. Inclusion criteria were: idiopathic PD; aged between 18 and 80 years; ascertained dopaminergic response; patients willing and able to give written informed consent; patients willing and able to comply to the study procedures; stable dose of dopaminomimetic drugs for at last 28 days; diagnosis of PD-PG for group A, and diagnosis of PD-ICD for group B (diagnosis according to DSM, 4th edition [DSM-IV] text revision, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale, a rating scale designed to measure severity of symptoms and support a diagnosis of impulse control disorders and related disorders in PD).33 Group C was PD control (PD-CTR) without PG or ICD.
Exclusion criteria were: history or signs suggesting atypical or secondary parkinsonism; history of stereotactic brain surgery for PD; mini–mental state examination (MMSE) score less than 24 at screening; any medical or psychiatric condition (other than ICD) that may compromise the patient’s participation in the study; women with child-bearing potential; history of antipsychotics or anticholinergics medication and previous treatment with Ama.
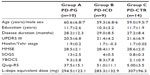
Thirty-three patients (six females) with idiopathic PD met the inclusion/exclusion criteria. PG was identified according to DSM-IV (rule of five of nine items) and SOGS criteria.34 PD patients were grouped on the basis of DSM-IV text revision criteria for ICDs and PG. PD patients were divided into three different groups: group A consisted of ten PD patients affected by pathological gambling (PD-PG; mean age 60.6±6.8 years); group B consisted of nine PD patients with other ICDs such as compulsive shopping, hypersexuality, and binge eating (ICD-PD; mean age 59.3±6.8 years); and group C consisted of 14 PD patients without PG or ICD (PD-CTR; mean age 59.0±9.5 years). Patients were matched for age, education, disease stage and duration, and dopaminomimetic therapy according to their need. None of the patients presented with LID.
According to our clinical procedures, all patients were evaluated with the Unified Parkinson’s Disease Rating Scale,35 PD stage was assessed with the Hoehn/Yahr scale,36 and all patients underwent MMSE neuropsychiatric evaluation.37 The study was approved by the University of Chieti ethics committee and was carried out according to the declaration of Helsinki and subsequent revisions. Patients (or caregivers) signed a written informed consent.
In order to understand whether the effect of antiglutamatergic drugs in ICD is dependent on modulation of executive functions or on processing of emotions and reward/loss, we assessed the effect of Ama as add-on therapy with the color word Stroop task, EST, and monetary reward/loss task slot machine-like game in PD-PG, ICD-PD, and PD-CRT. The patients were evaluated at T0 and T1, add-on medication (200 mg/day), following an opportune up and down titration. The neuropsychological tasks were performed at T0 and T1, also at the same time when the patients presented optimal motor performance and in “on” phase.
Tasks and stimuli
Tasks were given to all participants in a randomized order and performed at T0 and T1. Each participant performed tasks after the task had been explained in-depth. Task stimuli were presented on a computer by using MATLAB software.
Monetary reward/loss risk-taking task
Given the participants, we chose to make the task very simple to avoid stress derived from difficult instructions. Based on previous studies on PG,14,15 we chose a non-realist gambling task. The task, is able to observe processes underlying addictive behavior and to measure indirectly the functioning of the front-striatal pathway in PD-PG.38
The task we used was a version of a slot-machine game where participants were asked to bet 50€ (high reward) or 5€ (low reward) on a random drawn square. There were two squares: a red square representing a winning bet and a black square representing a losing bet. The goal of the game was to win as much money as possible. The task was divided in three within-subjects randomized subsessions, the first with a 75% probability of winning, the second with a 50% probability of winning, and the third with a 25% probability of winning. For data analysis, we considered risky choices the bet of 50€ on losing squares. Choices and reaction times were collected.
Stroop task
The classic Stroop test relies on the observation that color naming can be slowed by the concomitant presence of a color word (competing information). The task requires participants to respond to the ink color and suppress the more familiar word identity. Whilst responses in congruent settings are relatively automatic, incongruence between the letters and ink color requires keen attention and leads to slower responses.39,40
Each trial consisted of a fixation cross at the center of a grey screen (500 ms), followed by word stimulus (500 ms), and then by an interstimulus interval ([ISI] 3,000 ms). During ISI, participants were instructed to press a key corresponding to the color (red or green) of the word that appeared on the screen with both speed and accuracy. The task comprised four blocks (100 trials), with half of the trials being incongruent (eg, the word “red” printed in the color green) and half being congruent (eg, the word “red” printed in the color red). Color words and ink colors selected for use in this study included red, green, and black. Presented trial type was randomized, though each participant completed 40 of each possible trial type (both congruent and incongruent trials using each of the three possible colors) until the end of the task. Participants were initially exposed to a 20-trial practice block.
Emotional Stroop task
EST is widely used to investigate attentional bias and interference caused by emotionally salient stimuli.40 The task presented neutral and emotionally charged stimuli (neutral and emotionally-negative/-positive words) with three different colors (black, red, and green), and the patients were asked to press the button corresponding to the color of the word as quickly as possible.
Color naming EST was designed based on previous studies.41–43 Neutral, negative, and positive words were selected on the basis of norms in the Italian language, ie, emotionality, familiarity, and statistical frequency. The task contained 111 trials presented in three blocks of 37 words each (three different words for each word type). The word stimuli were presented for 500 ms and the ISI was 3,000 ms. The color-identification response latency for each stimulus was recorded (Figure 1).
  | Figure 1 Psychological tasks. |
Data analysis
Statistical analysis was performed using Statistica®, Version 6.0 (StatSoft, Inc., Tulsa, OK, USA). Results of the Stroop task were analyzed as follows: the mean reaction times and percentage of correct responses (accuracy) for each stimulus (congruent and incongruent, respectively) was computed; then, three different two-tailed t-tests were computed for each group for the T0 and T1 to assess the effect of add-on therapy.
Results for EST Task were analyzed, computing mean reaction times and accuracy for positive and negative words. Two tailed t-tests were computed for each PD group at T0 and T1.
Results for the monetary reward/loss task were analyzed by computing the percentage of risky and non-risky choices. Three different two-tailed t-tests were performed to assess the effect of add-on therapy.
Between-group comparison was performed for T0 and T1 separately for each task. Where possible, Bonferroni correction was applied to t-test results.
Results
Thirty-three patients (six females) with idiopathic PD met the inclusion/exclusion criteria. PD patients were divided into three different groups: ten PD-PG (mean age: 60.6±6.8 years); nine PD-ICD (mean age 59.3±6.8 years) ; and 14 PD patients without PG or ICD (PD-CTR) (mean age 59.0±9.5). As shown in Table 1, patients were not different in age, education, disease stage and duration, and dopaminomimetic therapy according to their need. UPDRS III scores were not different between groups. MMSE evaluation did not show a significant cognitive decline (MMSE >24). SOGS scores indicated the presence of pathological gambling in group A, while in the other group the scores were under the cut-off for pathological gambling. On the YBOCS, scores indicating the presence of obsessive-compulsive behaviors were reported in the PD-PG and PD-ICD groups. On the QUIP-R scale, scores showed impulse-control behavior in the PD-PG and PD-ICD groups.
No significant between- and within-group differences were observed in mean reaction times for the three different tasks. Data for within-group results are shown in Table 2.
At T0, results from the risk-taking task, Stroop task, and EST were not different between groups.
At T1, results from the risk-taking task showed a decrease in risky choices and increase in non-risky choices (t(9)=−2.40, P<0.05 and t(9)=2.67, P<0.05 uncorrected, respectively) in PD-PG patients. Between-group comparison of PD-PG versus PD-CRT showed a significant decrease in risky choices for the PD-PG group (t(22)=−4.16, P<0.01).
At T1, results from the Stroop task did not show within- and between-group differences. Interestingly, we did not observe modifications in the accuracy.
At T1, our results for the EST showed a significant decrease in accuracy for positive words in the PD-PG and PD-ICD groups (t(9)=−15.83, P<0.05 corrected and t(8)=−2.7, P<0.05 uncorrected, respectively), while a significant increase was observed to negative words in the PD-ICD group (t(9)=−2.40, P<0.03 uncorrected). PD-PG patients showed a decrease in accuracy for positive words compared to the PD-ICD group (t(17)=−7.49, P<0.01) and PD-CTR group (t(22)=−4.29, P<0.01). Results are shown in detail in Figure 2.
Discussion
The purpose of our study was to assess the effect of Ama add-on therapy on executive functions, emotions, and processes underlying reward and loss in PD-PG and PD-ICD. Ama is one of the possible pharmacological treatments for PD and non-PD patients with PG as has been shown in recent studies.9,44 The main outcome of the present study was to evaluated the efficacy of Ama in improving ICDs in PD-PG and PD-ICD patients using neuropsychological tasks at time T0 and T1 in the three patient groups.
The main outcome of this study was to assess the efficacy of Ama in improving the selection of non-risky choices in the risk-taking task in PD-PG patients and the increase in accuracy to negative words in the PG-ICD group.
The significant reduction of risky choices after treatment observed in this study may represent a protective factor that is able to reduce the number of relapses in subjects with PG. Reduction of risky choices could be considered the focal process responsible for the efficacy of Ama in PD-PG, previously described in other studies.9,44
Our data highlight a modified behavior in PD-PG patients after introduction of Ama; in other PD patients (PD-ICD/PD-CTR), performance was unchanged and executive function, specifically inhibition processing, was not affected.
In all PD patients, the Stroop task we performed did not show changes in accuracy, indicating that there is no deficiency in inhibition of interference, as shown by Kertzman.45 Previous literature shows contradictory results regarding executive functions, PD-PG and PD-ICD patients data, and the relationship between executive functions and PG or other ICDs in PD patients.46,47
The differences observed in the Stroop task and EST at T1 indicate the involvement of different inhibitory processes during the execution of the task.48 For the EST, we found at T1 in PD-ICD a decrease in accuracy for positive words and an increase in accuracy for negative words, while PD-PG showed a decrease in accuracy for positive words.
Previous studies where EST was used indicated that the level of activation or performance for emotional words was higher than for neutral words, resulting in a decreased ability to inhibit the processing of emotional meaning.49,50 The higher performance relative to negative words may depend on the automatic vigilance effect.51 The automatic vigilance effect is an innate mechanism that normally allows a person to direct attention to negative or dangerous stimuli, having a high adaptive value. Automatic vigilance seems to be more active during experimental tasks like EST in healthy subjects.51
Between-group comparison for the post-treatment period showed decreased accuracy in recognizing positive words in PD-PG and PD-ICD patients. The possible effect of Ama may be the enhancement of the automatic vigilance effect in PD-PG and PD-ICD patients. This is in line with our results.
A relevant limitation of this study is the small sample size and the consequent low statistical power, but levels of significance have been found, confirming the strength of the data.
A theoretical limitation is represented by two previous studies using a cross-sectional or retrospective design,8,52 which did not evidence a statistical reduction of PG in patients treated with Ama. Yet, cross-sectional or retrospective studies are subject to treatment and recall bias, and can only identify the prevalence and clinical correlates of ICDs, but not their incidence or risk factors; as such these are not sufficient evidence of efficacy or lack of efficacy of a drug.53
Any new treatment calls for validations and further study designs. Taking as an example the history of L-3,4-dihydroxyphenylalanine treatment, the initial evidence of L-3,4-dihydroxyphenylalanine benefits in PD were challenged by reports showing its inefficacy or side effects; the key to efficacy was patient selection and dose finding.54 The lack of studies due to inappropriate patient selection is misleading and represents one of the major problems in clinical/pharmacological research,8,55,56 particularly in PD, where the clinical presentation and the variety of symptoms – ranging from hallucinations to motor and sleep-behavior problems – is large.31,57 In our recent study,9 the obvious prerequisite was the absence of prior exposure to Ama, whereas in cross-sectional observational studies, the history of Ama intake, of psychosis and tachyphylaxis are well known effects of Ama, were not considered.
Further research with larger clinical trials are needed to assess the role of antiglutamatergic drugs in the treatment of ICDs in PD.
Disclosure
The authors report no conflicts of interest in this work.
References
Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. | |
Voon V, Fernagut PO, Wickens J, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8(12):1140–1149. | |
Housden CR, O’Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology. 2010;35(11):2155–2164. | |
Villella C, Martinotti G, Di Nicola M, et al. Behavioural addictions in adolescents and young adults: results from a prevalence study. J Gambl Stud. 2011;27(2):203–214. | |
Pietrzak RH, Petry NM. Severity of gambling problems and psychosocial functioning in older adults. J Geriatr Psychiatry Neurol. 2006;19(2):106–113. | |
Martinotti G, Andreoli S, Giametta E, Poli V, Bria P, Janiri L. The dimensional assessment of personality in pathologic and social gamblers: the role of novelty seeking and self-transcendence. Compr Psychiatry. 2006;47(5):350–356. | |
Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. | |
Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. | |
Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010;68(3):400–404. | |
van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson’s disease? Neuropsychopharmacology. 2009;34(13):2758–2766. | |
Cilia R, Cho SS, van Eimeren T, et al. Pathological gambling in patients with Parkinson’s disease is associated with fronto-striatal disconnection: a path modeling analysis. Mov Disord. 2011;26(2):225–233. | |
Cilia R, Ko JH, Cho SS, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiol Dis. 2010;39(1):98–104. | |
Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8(2):147–148. | |
van Holst RJ, van Holstein M, van den Brink W, Veltman DJ, Goudriaan AE. Response inhibition during cue reactivity in problem gamblers: an fMRI study. PloS One. 2012;7(3):e30909. | |
Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol Psychiatry. 2012;71(8):749–757. | |
Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101 Suppl 1:142–151. | |
Vitale C, Santangelo G, Trojano L, et al. Comparative neuropsychological profile of pathological gambling, hypersexuality, and compulsive eating in Parkinson’s disease. Mov Disord. 2011;26(5):830–836. | |
Silveira-Moriyama L, Evans AH, Katzenschlager R, Lees AJ. Punding and dyskinesias. Mov Disord. 2006;21(12):2214–2217. | |
Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. | |
de la Fuente-Fernández R, Sossi V, Huang Z, et al. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain. 2004;127(Pt 12):2747–2754. | |
Pondal M, Marras C, Miyasaki J, et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J Neurol Neurosurg Psychiatry. 2013;84(2):130–135. | |
Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol. 2010;67(1):58–63. | |
Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003467. | |
Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(1):141–143. | |
Allers KA, Bergstrom DA, Ghazi LJ, Kreiss DS, Walters JR. MK801 and amantadine exert different effects on subthalamic neuronal activity in a rodent model of Parkinson’s disease. Exp Neurol. 2005;191(1):104–118. | |
Moreau C, Delval A, Tiffreau V, et al. Memantine for axial signs in Parkinson’s disease: a randomised, double-blind, placebo-controlled pilot study. J Neurol Neurosurg Psychiatry. 2013;84 (5):552–555. | |
Rabey JM, Nissipeanu P, Korczyn AD. Efficacy of memantine, an NMDA receptor antagonist, in the treatment of Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1992;4:277–282. | |
Verhagen Metman L, Blanchet PJ, van den Munckhof P, Del Dotto P, Natté R, Chase TN. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov Disord. 1998;13(3):414–417. | |
Martinotti G, Andreoli S, Giametta E, Poli V, Bria P, Janiri L. Thedimensional assessment of personality in pathologic and social gamblers: the role of novelty seeking and self-transcendence. Compr Psychiatry. 2006;47(5):350–356. | |
Chen YC, Holmes A. Effects of topiramate and other anti-glutamatergic drugs on the acute intoxicating actions of ethanol in mice: modulation by genetic strain and stress. Neuropsychopharmacology. 2009;34(6):1454–1466. | |
Onofrj M, Luciano AL, Iacono D, et al. HLA typing does not predict REM sleep behaviour disorder and hallucinations in Parkinson’s disease. Mov Disord. 2003;18(3):337–340. | |
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. | |
Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX, Siderowf A. Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale. Mov Disord. 2012;27(2):242–247. | |
Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. | |
Fahn S, Elton RL. Members of the Unified Parkinson’s Disease Rating Scale Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Development in Parkinson’s Disease. Vol 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–164. | |
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. | |
Folstein NF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for clinician. J Psychiatry Res. 1975;12:189–198. | |
Limbrick-Oldfield EH, van Holst RJ, Clark L. Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? Neuroimage Clin. 2013;2:385–393. | |
Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. | |
MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. | |
Bentall RP, Kaney S. Content specific information processing and persecutory delusions: an investigation using the emotional Stroop test. Br J Med Psychol. 1989;62:355–364. | |
Newman JP. Effects of characterological anxiety and situational arousal on the solving of a color-word interference task: hemispheric processing implications. Int J Neurosci. 1990;52(1–2):1–9. | |
Eide P, Kemp A, Silberstein RB, Nathan PJ, Stough C. Test-retest reliability of the emotional stroop task: examining the paradox of measurement change. J Psychol. 2002;136(5):514–520. | |
Pettorruso M, Martinotti G, Di Nicola M, et al. Amantadine in the treatment of pathological gambling: a case report. Front Psychiatry. 2012;3:102. | |
Kertzman S, Lidogoster H, Aizer A, Kotler M, Dannon PN. Risk-taking decisions in pathological gamblers is not a result of their impaired inhibition ability. Psychiatry Res. 2011;188(1):71–77. | |
Santangelo G, Vitale C, Trojano L, Verde F, Grossi D, Barone P. Cognitive dysfunctions and pathological gambling in patients with Parkinson’s disease. Mov Disord. 2009;24:899–905. | |
Siri C, Cilia R, De Gaspari D, et al. Cognitive status of patients with Parkinson’s disease and pathological gambling. J Neurol. 2010;257(2):247–252. | |
Cothran DL, Larsen R. Comparison of inhibition in two timed reaction tasks: the color and emotion Stroop tasks. J Psychol. 2008;142(4):373–385. | |
Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev. 1990;97(3):332–361. | |
Williams JM. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. | |
Pratto F, John OP. Automatic vigilance: the attention-grabbing power of negative social information. J Pers Soc Psychol. 1991;61(3):380–391. | |
Weintraub D, Sohr M, Siderowf A, et al. Amantadine use associated with impulse control disorders in Parkinson disease. Ann Neurol. 2006;63(7):969–973. | |
Wang O, Kilpatrick RD, Critchlow CW, et al. Relationship between epoetin alfa dose and mortality: findings from a marginal structural model. Clin J Am Soc Nephrol. 2010;5:182–188. | |
Fahn S. The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord. 2008;23 Suppl 3:S497–S508. | |
Walsh RA, Lang AE. Multiple impulse control disorders developing in Parkinson’s disease after initiation of amantadine. Mov Disord. 2012;27(2):326. | |
Mestre TA, Strafella AP, Thomsen T, Voon V, Miyasaki J. Diagnosis and treatment of impulse control disorders in patients with movement disorders. Ther Adv Neurol Disord. 2013;6(3):175–188. | |
Sprenger F, Poewe W. Management of motor and non-motor symptoms in Parkinson’s disease. CNS Drugs. 2013;27(4):259–272. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.