Back to Journals » OncoTargets and Therapy » Volume 13
Alternative Treatment Options in Patients with Colorectal Cancer Who Encounter Fluoropyrimidine-Induced Cardiotoxicity
Authors Saif MW
Received 23 May 2020
Accepted for publication 7 September 2020
Published 9 October 2020 Volume 2020:13 Pages 10197—10206
DOI https://doi.org/10.2147/OTT.S264156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Leo Jen-Liang Su
Muhammad Wasif Saif
NorthwellHealth Cancer Institute, Donald and Barbara Zucker School of Medicine at Hofstra, Feinstein Institute for Medical Research, Lake Success, New York, NY, USA
Correspondence: Muhammad Wasif Saif
NorthwellHealth Cancer Institute, Donald and Barbara Zucker School of Medicine at Hofstra, Feinstein Institute for Medical Research, Lake Success, New York, NY 11042, USA
Tel +1 516-321-2238
Fax +1 516-321-2272
Email [email protected]
Abstract: 5-Fluorouracil (5-FU) remains to be the backbone of chemotherapy regimens approved for treatment of colorectal cancer and other gastrointestinal cancers and breast cancer. The incidence of cardiotoxicity associated with 5-FU ranges from 1.5– 18%. Previous studies also concluded that rechallenging a patient with previous 5-FU cardiotoxicity with either lower dose or another mode of administration could result in repeat of cardiac complication in up to 45% of patients. Nearly 13% of patients died upon re-exposure to 5-FU. Clinical manifestations of cardiac complications of fluoropyrimidines including angina, myocardial infarction, arrhythmias, hypotension, Tako-Tsubo syndrome, heart failure, cardiogenic shock, pericarditis, and even sudden death have been reported. Cardiotoxicity is unpredictable and no alternative chemotherapeutics have been defined so far. The author describes here treatment options for patients with metastatic colorectal cancer who have encountered fluoropyrimidine-induced cardiotoxicity, including switching to a different fluoropyrimidine, switching to a different schedule of intravenous 5-FU, or switching to a non-fluoropyrimidine-containing chemotherapy regimen if one exists. Switching to a non-fluoropyrimidine-containing chemotherapy regimen is usually the most feasible choice for patients with metastatic disease as data on adjuvant setting is usually a fluoropyrimidine or its combination with oxaliplatin at present.
Keywords: 5-FU, cardiotoxicity, fluoropyrimidines, 5-fluorouracil, DPD, dihydropyrimidine dehydrogenase, FBAL, fluoro-beta-alanine, TAS-102, uridine triacetate, S-1, capecitabine, UFT
Introduction
Fluoropyrimidines remain to be the backbone of regimens to treat many common solid tumors, including head and neck (H&N), breast, pancreas, stomach, anus, skin, small bowel, and especially colorectal cancer.1 As we continue to use these agents commonly, recognition of its related uncommon or under-recognized toxicities such as cardiac toxicity has also improved. Cardiotoxicity associated with either 5-FU or capecitabine is of utmost significance for many reasons. 5-FU is usually given orally or intravenously as a bolus or by continuous intravenous infusion and as a topical application. Intravenous 5-FU is administered to nearly 275,000 cancer patients per year and capecitabine is taken by an additional 30,000 patients per year in the US. Moreover, 5-FU is usually administered for a series of cycles up to 6 months in adjuvant setting and till progression in advanced stages. Additionally, 5-FU administration continues in the second-line after progression in combination with other agents, eg FOLFOX to FOLFIRI. These statistics further underline the importance of recognizing and managing the cardiac toxicity associated with 5-FU and its analogs.
Clinical manifestations of cardiac complications of fluoropyrimidines may include angina, myocardial infarction, arrhythmias, hypotension, Tako-Tsubo syndrome, heart failure, cardiogenic shock, pericarditis, and even sudden death,2,4 as summarized in Table 1.
 |
Table 1 The Most Frequent Cardiac Complications Related to 5-FU Administration2 |
The underlying pathophysiological mechanisms to explain5-FU-induced cardiotoxicity remain undefined, but its association with mode and schedule of administration and genuine reproducibility have been well-recognized.2,3 It is proposed to be multifactorial, and many mechanisms proposed include coronary spasm, direct myocardial ischemia due to endothelial damage, changes in platelet agreeability, abnormalities of coagulation proteins, an autoimmune reaction, result of pharmacogenetics related to 5-FU, such as dihydropyrimidine dehydrogenase (DPD) enzyme abnormality, direct effect of the catabolite, especially fluoro-beta-analine (FBAL) on the myocardium, or cardiotoxic impurities in 5-FU formulation2,5 (Table 2 and Figure 1).
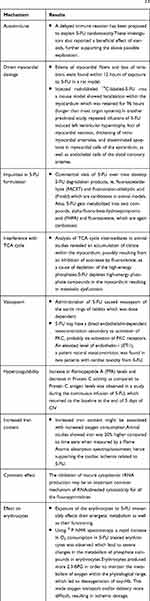 |
Table 2 Potential Mechanisms Underlying 5-FU Cardiotoxicity2,5 |
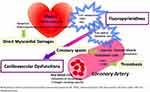 |
Figure 1 Mechanisms of cardiac toxicities associated with 5-FU/capecitabine Adapted from Shiga, T, Hiraide, M Cardiotoxicities of 5-Fluorouracil and Other Fluoropyrimidines. Curr. Treat. Options in Oncol. 2020;21(27). This article is licensed under a Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/ .6 |
Several studies have also attempted to identify factors that might alter the incidence or severity of 5-FU-induced cardiac toxicity, including age, prior history of coronary artery disease, presence of comorbidities (smoking, hypertension, diabetes), and concomitant administration of other chemotherapeutic agents or radiation therapy.2–4,7 However, the majority of the cases reported previously had no such risk factors identified except few proposing an increased risk with concomitant administration of certain chemotherapeutics, such as 5-FU when used with cisplatin, or prior chest radiation, or combination of capecitabine with oxaliplatin and bevacizumab.2–8 We presented a met-analysis in 2001 which revealed that cardiac toxicity was associated with the longer duration of 5-FU administration and later we found similar toxicities associated with capecitabine, that mimics medium duration of the infusion. No relationship to dose was found.2,4 Reports include both chemo-naïve patients as well as those rechallenged after developing cardiac toxicity to 5-FU.9
Previous studies have alarmed that rechallenging a patient with previous 5-FU cardiotoxicity with either a lower dose or another mode of administration could result in repeat of cardiac complication in up to 45% of patients.2,–7–9 Additionally, approximately 13% of patients died upon being re-exposed to 5-FU.2 Investigators have also evaluated the use of anti-anginal drugs with 5-FU and capecitabine. Two older studies looked at nitroglycerine and nifedipine and diltiazem. In one study, seven out of 300 patients manifested cardiac toxicity after administration of 5-FU with prophylactic nitroglycerin which failed to prevent EKG changes suggestive of myocardial ischemia during repeat infusion.10 A similar lack of protective efficacy was seen with either nifedipine 60 mg/day, or diltiazem 80 mg/day administered with simultaneous intravenous nitroglycerin at therapeutic doses.11 Eskilsson and Albertsson treated 58 patients receiving fluorouracil infusions with verapamil 120 mg three times a day. They found evidence of ischemia in 12% of patients, compared with 13% in a previously studied comparable group not receiving prophylaxis.12 They concluded that calcium-channel blockade does not protect against cardiotoxicity. These data underline the fact that a rechallenge with 5-FU is not without risk and should be reserved only for those patients in whom there is no reasonable alternative therapy while observing aggressive prophylaxis and close monitoring.
The previous experience in investigating the cardiac toxicities of fluoropyrimidines, contribution to the clinical trials associated with development of S-1, TAS-102, and research in 5-FU pharmacogenetics of our group maintains my interest in managing 5-FU associated cardiotoxicity.8–10 The treatment options for patients who have encountered fluoropyrimidine-induced cardiotoxicity in patients with CRC can be broadly divided into three groups:
- Switch to a different fluoropyrimidine,
- Switch to a different schedule of intravenous 5-FU, or
- Switch to a non-fluoropyrimidine-containing chemotherapy regimen if one exists.
Switching to a non-fluoropyrimidine-containing chemotherapy regimen is usually the most feasible choice for patients with metastatic disease because data on adjuvant setting supports only the use usually of a fluoropyrimidine monotherapy, such as 5-FU or capecitabine or its combination with oxaliplatin at present. Table 3 summarizes other agents, including novel fluoropyrimidines and non-fluoropyrimidines, as alternative treatment options with cancer.
 |
Table 3 Switch to a Different Fluoropyrimidine |
Switch to a Different Fluoropyrimidines
Table 3 summarizes the novel and other analogs of different fluoropyrimidines which may be considered as an alternative treatment for colorectal cancer patients who encountered cardiotoxicity to 5-FU or capecitabine. The composition, data on cardiac toxicity, potential mechanism of action responsible for lower incidence of cardiac toxicities, any comparison to 5-FU/capecitabine if available, and availability are summarized below.
Switch to a Different Schedule of Intravenous 5-FU
Previous data indicated that the incidence of 5-FU related cardiotoxicity is lower with a bolus schedule than with a continuous infusion schedule or oral capecitabine. Based on these observations, we further investigated the feasibility and safety that bolus 5-FU can be an alternative for patients who have developed cardiotoxicity while receiving 5-FU or capecitabine. To date, we have treated up to 13 patients safely with bolus 5-FU. Table 4 summarizes the published cases of successful rechallenge with bolus 5-FU in patients who developed cardiotoxicity with infusional or oral fluoropyrimidine.24,25
 |
Table 4 Summary of Few Cases Rechallenged with Bolus5-FU |
Interestingly, capecitabine was rechallenged in few of these patients in our experience but sadly all of them developed similar symptoms, leading to cessation of the drug. It is of utmost importance to understand that the experience with this strategy is limited to only a few cases and that bolus 5-FU has also been associated with cardiac toxicity as well. We believe that 5-FU is rapidly cleared from the blood stream following bolus 5-FU (half-life of 15–20 minutes) and probably a direct effect of drug on cardiac systems is unlikely, as seen in these cases.24,25 However, at present we do not endorse use of bolus 5-FU unless done in a vigorous environment in consultation with the cardiology team and discontinue 5-FU immediately if a cardiac event occurs. It is also important to remember that a delayed onset cardiotoxicity has also been reported in the literature and demands a close follow-up.
Switch to a Non-Fluoropyrimidine Containing Chemotherapy Regimen
Switching to a non-fluoropyrimidine-containing chemotherapy regimen is the most viable option for patients with mCRC. Table 5 summarizes the data on these regimens.
 |
Table 5 Non-Fluoropyrimidine Containing Chemotherapy Regimen |
Summary
To sum up, 5-FU cardiotoxicity is an infrequent, but a real phenomenon. It is probable that 5-FU cardiotoxicity may be much more common and clinically significant than previously reported as awareness has risen due to continued use, many 5-FU based regimens, longer duration on therapy, and availability of novel agents.2,42,43 Although the history of pre-existing coronary artery disease may increase the risk of cardiac toxicity associated with 5-FU/capecitabine, the published data does not seem to underline the predictive value of the presence of cardiac risk factors for the development of 5-FU-induced cardiac side-effects. Therefore, caution must be taken in treating these patients and if any signs or symptoms suggest cardiotoxicity, the drug should be suspended and a thorough work-up must be performed with multidisciplinary approach.
Despite a known benefit of nitrates and calcium channel blockers in ischemic heart disease, the effectiveness of this prophylactic therapy in patients receiving 5-FU/capecitabine has not been consistent.10–12 Few other reports indicated that beta-blockers should be avoided as they can be spasmogenic. Use of prophylactic use of anti-anginal agents has not been consistent. Cianci et al44 reported their experience with three cases of 5-FU-associated cardiotoxicity who received prophylactic transepidermal nitroglycerin. In this case series, they reported that the patients did not develop ischemic symptoms, such as angina. Kinhult et al45 showed that dalteparin, an antithrombotic, can protect against thrombogenic effects of 5-FU, secondary to its direct toxic effect on the vascular endothelium.
We recommend assessment of traditional cardiovascular risk factors and optimal management of cardiovascular disease, as a part of routine care for all patients before, during, and after receiving 5-FU/capecitabine (Table 6). However, in any patient who develops symptoms suggestive of ischemia, such as angina and/or electrocardiographic evidence of myocardial ischemia during the administration of 5-FU and capecitabine, termination of chemotherapy and administration of nitrates or calcium channel blockers should be considered under close observation. Cardiology consultation must be carried out and risk stratification should be performed. It is important to keep in mind that rechallenging these patients with similar agents can result in reoccurrence of cardiac toxicity. In addition to ischemic toxicity, arrhythmias also occur in these patients. Therefore, ECG monitoring is recommended if there is any suspicion leading to cardiotoxicity of these agents.46 In addition to non-invasive diagnostic tests, coronary angiography should be considered in patients who develop ischemia during or following 5-FU/capecitabine. Cardiac toxicity with newer oral 5-FU agents seem to be of less frequency, especially TAS-102, which is more widely available compared to older agents, such as S-01 or S-1 or UFT. In the adjuvant setting, only UFT and raltitrexed as a single agent have documented activity in randomized phase III trials, and experience with combination regimens is scarce. As mentioned earlier, re-challenge with 5-FU/capecitabine after an episode of cardiac toxicity to these agents can pose a higher risk of complications, including sudden cardiac death.2 Therefore, one must consider immediate termination of these chemotherapeutic drugs and modification of the treatment regimen.
 |
Table 6 Suggested Recommendations |
It is worth-mentioning here that in 2015, uridine triacetate, an oral active prodrug of uridine, which is a naturally occurring nucleoside and competes with the 5-FU metabolite for incorporation into RNA of normal tissue, was approved by the Food and Drug Administration (FDA) as an antidote to 5-FU (or capecitabine).47 In a study, 137 of 142 overdose patients who were treated with uridine triacetate had a rapid reversal of severe acute cardiotoxicity.47 The indications included use of uridine triacetate in patients with overdose or for those who exhibit early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early onset, unusually severe adverse reactions (eg, gastrointestinal toxicity and/or neutropenia). It is worth mentioning here that overdose of 5-FU/capecitabine is not the most responsible for its cardiotoxicity. In fact, the majority is regarding to normal chemotherapy regimens and not overdose. At present, the use of this antidote to prevent or treat cardiac toxicity of 5-FU has not been studied and warrants future studies to clarify its role in the treatment of fluoropyrimidine associated cardiotoxicity.
Funding
Partly funded by R01 CA085381.
Disclosure
The author reports no conflicts of interest for this work.
References
1. Myers CE. The pharmacology of the fluoropyrimidines. Pharmacol Rev. 1981;33:1–15.
2. Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191–202. doi:10.1517/14740330902733961
3. Anand AJ. Fluorouracil cardiotoxicity. Ann Phanrmacother. 1994;28(3):374–378. doi:10.1177/106002809402800314
4. Saif MW, Tomita M, Ledbetter L, Diasio RB. Capecitabine-related cardiotoxicity: recognition and management. J Support Oncol. 2008;6(1):41–48.
5. Norwood RA, Lokich JJ, Moore C. The syndrome of 5-fluorouracil cardiotoxicity: an elusive cardiopathy. Cancer. 1993;72:2287–2288.
6. Shiga T, Hiraide M. Cardiotoxicities of 5-Fluorouracil and other fluoropyrimidines. Curr Treat Options in Oncol. 2020;21(27). doi:10.1007/s11864-020-0719-1
7. Jensen SA, Sorensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58:487–493. doi:10.1007/s00280-005-0178-1
8. Kwakman JJ, Simkens LH, Mol L, Kok WE, Koopman M, Punt CJ. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: A retrospective analysis of the CAIRO studies of the Dutch colorectal cancer group. Eur J Cancer. 2017;76:93–99. doi:10.1016/j.ejca.2017.02.009
9. Van Cutsem E, Hoff PM, Blum JL, et al. Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann Oncol. 2002;13:484–485. doi:10.1093/annonc/mdf108
10. Akpek G, Hartshorn KL. Failure of oral nitrate and calcium channel blocker therapy to prevent 5-fluorouracil-related myocardial ischemia: a case report. Cancer Chemother Pharmacol. 1999;43(2):157–161. doi:10.1007/s002800050877
11. Oleksowicz L, Bruckner HW. Prophylaxis of 5-fluorouracil-induced coronary vasospasm with calcium channel blockers. Am J Med. 1988;85:750–751. doi:10.1016/S0002-9343(88)80268-7
12. Eskilsson J, Albertsson M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncol. 1990;29(8):1001–1003. doi:10.3109/02841869009091790
13. Chen J, Han M, Saif MW. TAS-102 an Emerging Oral Fluoropyrimidine. Anticancer Res. 2016;36(1):21–26.
14. Lopez CA, Azimi-Nekoo E, Chung SY, Newman J, Shen J, Saif MW. Meta-analysis and systematic review of the cardiotoxicity of TAS-102. JCO. 2020;38:e16053e16053. doi:10.1200/JCO.2020.38.15_suppl.e16053
15. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi:10.1056/NEJMoa1414325
16. Saif MW, Syrigos KN. Katirtzoglou NA. S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs. 2009;18(3):335–348. doi:10.1517/13543780902729412
17. Saif MW, Rosen LS, Saito K, Zergebel C, Ravage-Mass L, Mendelson DS. A Phase I study evaluating the effect of CDHP as a component of S-1 on the pharmacokinetics of 5-fluorouracil. Anticancer Res. 2011;31(2):625–632.
18. Franck C, Malfertheiner P, Venerito M. Safe administration of S-1 after 5-fluorouracil-induced cardiotoxicity in a patient with colorectal cancer. BMJ Case Rep. 2017;2017:bcr2016219162. doi:10.1136/bcr-2016-219162
19. Yamamoto J, Haruno A, Yoshimura Y, et al. Effect of coadministration of uracil on the toxicity of tegafur. J Pharm Sci. 1984;73:212–214. doi:10.1002/jps.2600730217
20. Kikuchi K, Majima S, Murakami M. Clinical survey on cardiotoxicity of tegafur (FT-207) compilation of a nationwide survey. Gan to Kagaku Ryoho. 1982;9((8):):1482–1488.
21. Köhne CH, Thuss-Patience P, Friedrich M, et al. Raltitrexed (Tomudex): an alternative drug for patients with colorectal cancer and 5-fluorouracil associated cardiotoxicity. Br J Cancer. 1998;77(6):973–977. doi:10.1038/bjc.1998.160
22. Van Cutsem E, Cunningham D, Maroun J, Cervantes A, Glimelius B. Raltitrexed: current clinical status and future directions. Ann Oncol. 2002;13(4):
23. Ransom D, Wilson K, Fournier M, et al. Final results of australasian gastrointestinal trials group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol. 2014;25:117–121. doi:10.1093/annonc/mdt479
24. Saif MW, Garcon MC, Rodriguez G, Rodriguez T. Bolus 5-fluorouracil as an alternative in patients with cardiotoxicity associated with infusion 5- fluorouracil and capecitabine: a case series. In vivo. 2013;27(4):531–534.
25. Shaib W, Lee V, Saif MW. Bolus 5-fluorouracil as an alternative modality to infusion 5-fluorouracil in a patient with rectal cancer and capecitabine-induced cardiotoxicity. Vivo. 2009;23(5):821–826.
26. Fuchs CS, Moore MR, Harker G, et al. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol. 2003;21:807–814. doi:10.1200/JCO.2003.08.058
27. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi:10.1056/NEJMoa033025
28. Van Cutsem E, Joulain F, Hoff PM, et al. Aflibercept plus FOLFIRI vs. Placebo plus FOLFIRI in second-line metastatic colorectal cancer: a post hoc analysis of survival from the phase III Velour study subsequent to exclusion of patients who had recurrence during or within 6 months of completing adjuvant oxaliplatin-based therapy. Target Oncol. 2016;11(3):
29. Becouarn Y, Rougier P. Clinical efficacy of oxaliplatin monotherapy: phase II trials in advanced colorectal cancer. Semin Oncol. 1998;25(2 Suppl 5):
30. Grivicich I, Mans DR, Peters GJ, Schwartsmann G. Irinotecan and oxaliplatin: an overview of the novel chemotherapeutic options for the treatment of advanced colorectal cancer. Braz J Med Biol Res. 2001;34(9):
31. Haller DG, Rothenberg ML, Wong AO, et al. Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol. 2008;26(28):4544–4550. doi:10.1200/JCO.2008.17.1249
32. Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi:10.1056/NEJMoa071834
33. Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy- refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi:10.1200/JCO.2006.08.1620
34. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicenter, randomized, placebo-controlled, Phase 3 trial. Lancet. 2013;381(9863):303–312. doi:10.1016/S0140-6736(12)61900-X
35. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):
36. Ferrarotto R, Machado K, Mak MP, et al. A multicenter, multinational analysis of mitomycin C in refractory metastatic colorectal cancer. Eur J Cancer. 2012;48:820–826. doi:10.1016/j.ejca.2012.01.008
37. Saif MW, Kaley K, Brennan M, Garcon MC, Rodriguez G. Mitomycin-C and capecitabine (MIXE) as salvage treatment in patients with refractory metastatic colorectal cancer: a retrospective study. Anticancer Res. 2013;33(6):2743–2746.
38. Ehrlich MI, Kaley K, Smith M, Saif WM. Safety and efficacy of s-MOX regimen in patients with colorectal cancer who developed cardiotoxicity following fluoropyrimidine administration: a case series. Arch Med Case Rep. 2020;2(1):23–29.
39. Scheithauer W, Kornek GV, Brugger S, et al. Randomized phase II study of irinotecan plus mitomycin C vs. oxaliplatin plus mitomycin C in patients with advanced fluoropyrimidine/leucovorin-pretreated colorectal cancer. Cancer Invest. 2002;20(1):
40. Comella P, Biglietto M, Casaretti R, et al. Irinotecan and mitomycin C in 5-fluorouracil-refractory colorectal cancer patients. A phase I/II study of the Southern Italy cooperative oncology group. Oncology. 2001;60(2):127–133. doi:10.1159/000055309
41. Petrelli F, Barni S, Bertocchi P, Zaniboni A. TAS-102, the first “cardio-gentle” fluoropyrimidine in the colorectal cancer landscape? BMC Cancer. 2016;16:386. doi:10.1186/s12885-016-2409-8
42. Stewart T, Pavlakis N, Ward M, et al. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J. 2010;40:303–307. doi:10.1111/j.1445-5994.2009.02144.x
43. Saif MW, Smith M, Maloney A. The first case of severe takotsubo cardiomyopathy associated with 5-fluorouracil in a patient with abnormalities of both dihydropyrimidine Dehydrogenase (DPYD) and Thymidylate Synthase (TYMS) genes. Cureus. 2016;8(9):e783.
44. Cianci G, Morelli MF, Cannita K, et al. Prophylactic options in patients with 5-fluorouracil-associated cardiotoxicity. Br J Cancer. 2003;88(10):1507–1509. doi:10.1038/sj.bjc.6600967
45. Kinhult S, Albertsson M, Eskilsson JCM, et al. Antithrombotic treatment in protection against thrombogenic effects of 5-fluorouracil on vascular endothelium: a scanning microscopy evaluation. Scanning. 2001;23:1–8. doi:10.1002/sca.4950230101
46. Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol. 1989;7:509–514. doi:10.1200/JCO.1989.7.4.509
47. Ma WW, Saif MW, El-Rayes BF, et al. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer. 2017;123:345. doi:10.1002/cncr.30321
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
