Back to Journals » OncoTargets and Therapy » Volume 12
Altered circulating levels of adipokine omentin-1 in patients with prostate cancer
Authors Zhou L, He W, Wang W, Zhou D
Received 8 December 2018
Accepted for publication 30 March 2019
Published 1 May 2019 Volume 2019:12 Pages 3313—3319
DOI https://doi.org/10.2147/OTT.S197507
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gaetano Romano
Lin Zhou,1,* Weiming He,2,* Wenjun Wang,1 Daoping Zhou3
1Department of Medical Record and Statistics, Anhui No.2 Provincial People’s Hospital, Hefei, Anhui, 230041, People’s Republic of China; 2Department of Public Health, Guangdong Medical University, Zhanjiang, Guangdong 524023, People’s Republic of China; 3Department of Oncology, Anhui No.2 Provincial People’s Hospital, Hefei, Anhui, 230041, People’s Republic of China
*These authors contributed equally to this work
Background: Prostate cancer (PCa), one of the most common cancers in men, accounts for nearly 20% of adult malignant neoplasms. Omentin-1 is synthesized in visceral adipose tissue and its concentration in plasma changes with cancers. However, the association between omentin-1 and PCa was rarely studied. Thus, we investigated the plasma omentin-1 levels in PCa patients in Chinese population.
Materials and methods: Ninety cases of PCa and 90 matched healthy controls were enrolled in this study. We used ELISA technique to determine the concentration of omentin-1.
Results: The concentration of omentin-1 was higher in patients with PCa compared to controls (P<0.001). Additionally, positive correlations were uncovered between omentin-1 with body mass index (r=0.240, P=0.001), waist-hip ratio (r=0.228, P=0.002), and prostate-specific antigen (r=0.589, P<0.001). Receiver operating characteristic curve analysis indicated that plasma omentin-1 differentiated PCa patients from controls with a sensitivity of 85.9% and a specificity of 83.7%.
Conclusion: Our study demonstrated that the levels of plasma omentin-1 were increased in PCa patients. Meanwhile, omentin-1 may be a possible biomarker for diagnosing PCa. For validation, more studies should focus on and elucidate the potential mechanism underlying this change.
Keywords: omentin-1, prostate cancer, obesity, body mass index, adipokines
Background
Prostate cancer (PCa) is one of the most common urological neoplasms, which accounts for nearly 20% of malignant tumors in male in America.1 Although People's Republic of China reported a lower incidence rate of PCa, the past decades had witnessed the increasing morbidity of PCa in Chinese population.2 Thus, there is a pressing need that more studies should focus on Pca including early diagnosis. Gradually, accumulative proofs have verified that genetic alternations and other etiology risk factors, such as processed fat intake, red meat consumption, superfluous nutrients, and obesity are considered to add the risk of PCa in a complicated style.3
In the past few decades, it had been discovered that the potential role of obesity promoted the process of carcinogenesis. And roughly 20% of all cancers were related to excess weight gain, and this data may be underestimated.4,5 For example, the relationships between breast cancer, pancreatic cancer, rectal cancer, and obesity had been found.6,7 Although studies reported controversial correlations between obesity and PCa, obesity was still considered as a relevant risk factor in giving rise to the higher morbidity of PCa,8 which was also confirmed by several large meta-analyses.9,10 Many factors, such as insulin, insulin-like growth factor-I, and adipokines might take critical parts in the relationship between obesity and cancers.11,12 Among the many factors, the effects of adipokines in promoting tumorigenesis and progression have been put forward recently. Adipokines, including visfatin, adiponectin,leptin, and resistin are bioactive molecules secreted by adipose cells and have been implied to be involved in obesity’s association with PCa.13,14
Recently, one of the adipokines, omentin-1, was investigated extensively. Omentin-1 was originally discovered named “intelectin”, which was separated from intestine paneth cells at first.15 Omentin-1 is a 34 kDa protein. It was a kind of depot-specific adipokine with higher expression and release from visceral depots compared with subcutaneous adipose tissue.16 Several related researches reported that altered circulating concentrations of omentin-1 in colorectal cancer and renal cell cancer patients, which indicated that omentin-1 might play a potential role in carcinogenesis.17,18 However, to our knowledge, plasma omentin-1 levels in PCa were rarely explored, especially in Asian population.19,20 Herein, we conducted this case-control study to test the plasma omentin-1 levels in PCa in Chinese population and to explore its potential role in diagnosing PCa patients.
Materials and methods
Patients
The present study was conducted in accordance with the Declaration of Helsinki and authorized by the Ethics Committee of the First Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, People's Republic of China. All candidates provided informed consent to allow analysis of data for research purposes. In the calculation, the minimum total sample size was measured as 52 and a minimum of 26 for PCa and control group, respectively. In total, between July 2016 with July 2017, 90 patients newly diagnosed with PCa who underwent 12-core trans-rectal ultrasound (TRUS) guided prostate biopsy at the First Affiliated Hospital of Guangdong Medical University were recruited in our study. All patients were assessed for elevated prostate-specific antigen (PSA) (>4 ng/mL) or abnormal digital rectal examination for TRUS prostate biopsy. In all patients, prostate was routinely biopsied near base, mid-gland, and apex, bilaterally, with six biopsies per side. Patients with PCa were arranged to three groups by Gleason score: well differentiation (Gleason score<7), moderately differentiation (Gleason score=7), and poorly differentiation (Gleason score>7). Meanwhile, 90 age-matched volunteers were selected as healthy controls from people who visited the Affiliated Hospital of Guangdong Medical University for a routine check-up. All patients were recruited using regular criteria: no curative medication for PCa; no history of malignancy or prostate operations; no diagnosis of acute infectious diseases; and no impairment of heart, liver or kidney. Peripheral venous blood samples were collected from all patients and controls after fasting for at least 12 hrs. Whole blood was centrifuged with 8000 rpm at 4°C for 30 mins and the supernatants were collected. All the samples were put into 1.5 mL eppendorf tubes and preserved at −80°C until testing.
Physical and biochemical measurements
Anthropometric measurements were collected in the present study, including height, weight, waist and hip circumference. The body mass index (BMI) was calculated as the weight divided by the square of the height (kg/m2). Waist–hip ratio (WHR) was calculated as waist circumference divided by hip circumference. Biochemical parameters were detected in previously stored plasma samples. The plasma levels of total cholesterol (TC), triacylglycerol, high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC) concentrations, fasting blood glucose (FBG), creatinine, blood urea nitrogen (BUN) and PSA were measured using the standard methods by a chemistry analyzer (Shimadzu, cl8000, Japan).
Plasma omentin-1 concentration determination
Levels of omentin-1 detected in plasma samples reserved through an ELISA assay according to the user manual (omentin-1: CUSABIO, CSB-E09745h, People's Republic of China). The detection range of the assay was 1.56–100pg/mL (omentin-1). The precision of this assay: intra-assay precision <8% and inter-assay precision <10%.
Statistical analysis
The software used in the study was IBM SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA) for statistical analyses. Quantitative characteristics were described as mean±SE . One-way analysis of variance and the nonparametric Kruskal–Wallis test were used for comparisons of groups and subgroups. Subsequently, Pearson’s (normally distributed) or Spearman’s correlation analyses were performed to test the correlations between omentin-1 levels and previously mentioned clinical and biochemical parameters. The accuracy of omentin-1 to differentiate PCa from control was assessed by receiver operating characteristic (ROC) curve analysis. For all analyses, a two-tailed P<0.05 was considered significant.
Results
Studied groups
The general characteristics information including anthropometric measurements and biochemical parameters of PCa individuals (90 subjects) and the healthy individuals (90 subjects) were included in Table 1. No statistically significant difference was found in age (P=0.469), BMI (P=0.083), WHR (P=0.057), FBG (P=0.064), HDLC (P=0.896), LDLC (P=0.169), creatinine (P=0.468), and BUN (P=0.264) between the groups. TC (4.68±0.81 vs 4.41±0.94; P=0.035), triacylglycerol (1.32±0.60 vs 1.51±0.55; P=0.034), and PSA (1.13±0.44 vs 36.80±35.44; P<0.001) levels were significantly lower in the healthy controls group than the PCa patients.
 | Table 1 Comparison of general characteristics and biochemical parameters |
Plasma levels of omentin-1
Plasma concentrations of omentin-1 in PCa group and healthy controls were all detectable. Plasma omentin-1 concentrations were detected to range from 0.014 to 21.854 ng/mL with a mean of 4.96 ng/mL for healthy controls and range from 0.377 to 40.393 ng/mL with a mean of 12.94 ng/mL for PCa patients (Table 1). As shown in Figure 1, PCa patients had significantly higher plasma omentin-1 levels compared to the healthy individuals (P<0.001). To exclude the probable impact of BMI on omentin-1 levels, we tested the statistical difference of BMI between three groups divided by tumor grade. As a result, no statistical differences were observed between the groups about the BMI (F=0.039, P=0.962). In addition, we did not find a significant relationship between the tumor grades of PCa and the omentin-1 level (P=0.873) (Table 2).
 | Table 2 One way ANOVA analysis on omentin-1 levels with PCa status |
 | Figure 1 The omentin-1 levels in the healthy control (HC) subjects (n=90) and prostate cancer (PCa) patients (n=90). ** P<0.001. |
Spearman correlation analysis
As a result, there was a positive correlation between omentin-1 level and BMI value in PCa group (r=0.291, P=0.005) and whole group (r=0.240, P=0.001), respectively. Another obesity-related parameter WHR was also found to be positively correlated with omentin-1 level in healthy controls (r=0.216, P=0.038), PCa patients (r=0.219, P=0.036), and whole (r=0.228, P=0.002). Besides, positive correlation was also observed between omentin-1 level and PSA (r=0.589, P<0.001) (Table 3). Our result demonstrated that plasma levels of omentin-1 were not significantly correlated with other parameters, including age, FBG, HDLC, LDLC, TC, Triacylglycerol, creatinine, and BUN levels in the whole group.
 | Table 3 Pearson’s or Spearman’s correlation coefficient analysis of omentin-1 levels with general clinical characteristics and biochemical parameters |
ROC curve analysis
ROC curve was applied to assess the performance of plasma omentin-1 level in distinguishing PCa individuals from healthy controls. In Figure 2, we showed the ROC curve of the investigated plasma omentin-1. The area under the ROC curve is 0.872. With a cutoff point of 8.196 ng/mL for plasma omentin-1 concentrations, we were able to distinguish PCa patients from controls with a sensitivity of 85.9%, and a specificity of 83.7%.
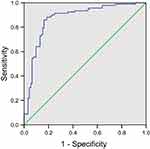 | Figure 2 ROC curve analysis assessed the performance of omentin-1 levels in diagnosing prostate cancer. The area under the ROC curve (AUC) for omentin-1 was 0.883, P<0.001. |
Discussion
As is known to the public, PCa is becoming an increasingly noticeable public health problem. Described in the very recent cancer statistics, PCa was thought to account for about 19% of all cases of cancers newly diagnosed and 9% of cancer-related deaths in men in America.1 And there was a gradual rise in the incidence rates ofPCa and obesity in many countries, with a diet habits rich of high fat,sugar, and cholesterol.21 Therefore, the similar morbidity trends of PCa and obesity suggested the probable relevance between them. Yet, the correlation between obesity and PCa occurrence was complicated and indicated conflicting conclusions. Park et al reported that obesity may be an independent risk factor of higher incidence of PCa,22 which was supported by another two studies.23,24 Moreover, previous meta-analysis demonstrated the correlation between high BMI and high incidence of PCa.9,10 Interestingly, there were also some other studies arguing that BMI was not a risk factor of PCa.25,26
Many hypotheses are trying to address the questions about the correlation between cancer incidence and obesity. Among these factors, the involvement of adipokines, including resistin, apelin, and leptin played an important role in the process of microenvironment chronic inflammation. The chronic inflammation in the microenvironment could promote the tumor initiation and progression.27,28 For instance, leptin exhibited a role of promoting the incidence of PCa. The potential mechanism may be the function of leptin to induce the lesions of prostatic intraepithelial and the inhibition of apoptosis.29
In recent years, omentin-1 was known to be one of the adipokines enriched in human adipose tissue. Several studies demonstrated that patients diagnosed with polycystic ovary syndrome who indicated higher BMI showed lower concentration of omentin-1 compared to the healthy group.30,31 Moreover, other researches about colorectal cancer and renal cell carcinoma elaborated the regulatory effects of obesity.17,18 Concretely, Fazeli et al observed a higher concentration of omentin-1 in colorectal cancer patients compared with controls.17 However, Shen et al reported lower plasma concentrations of omentin-1 in renal cell carcinoma patients than healthy controls.18 Furthermore, Uyeturk et al conducted the only research about the omentin-1 in PCa in Turkey population and reported a higher plasma omentin-1 concentrations in PCa patients than benign prostatic hyperplasia (BPH) patients.19 Also, Fryczkowski et al tested the omentin level in Poland population, and found higher plasma omentin levels in PCa patients than BPH patients.20 In the present study, we observed that PCa patients had significantly higher circulating omentin-1 levels of 12.94±6.15 ng/mL than the healthy control group of 4.96±4.71 ng/mL (P<0.001). Then, Pearson or Spearman correlation analysis revealed that circulating omentin-1 concentrations positively correlated with PSA, BMI, and WHR. Previous studies were conflicted regarding the role of omentin-1 in cancers and its association with obesity. Fazeli et al suggested a significant correlation between circulating omentin-1 concentrations and colorectal cancer, which was independent of obesity.17 Shen et al argued plasma omentin-1 concentrations were obviously decreased in renal cell carcinoma patients, and negatively correlated with BMI.18 Our result found that omentin-1 concentrations were positively correlated with BMI and WHR, which were intrinsic indicators of obesity, in PCa patients.19
In our study, patients with high-grade tumors were not found to have significant elevation of omentin-1 levels. Similarly, Uyeturk et al also reported no significant association between the differentiation grades of PCa and the omentin level,19 suggesting omentin-1 may not be a marker of aggressiveness. Consistent with our study, Shen et al also indicated that plasma omentin-1 concentrations were not correlated with the TNM staging (T1–4N0M0) of renal cancer.18 Besides, Fazeli et al also demonstrated that colorectal cancer patients’ circulating omentin-1 concentrations were not related to the TNM staging.17 Thus, there was a similarity in studies regarding the function of omentin-1 in pathogenesis of cancer with different studies on plasma omentin-1 with respect to grades of the disease.
Up till now, the definite pathogenesis of omentin-1 in tumorigenesis has not been illuminated clearly. Zhang et al revealed a possible mechanism about cancer and omentin-1. In their study, hepatocellular carcinoma proliferation was suppressed. They also found p21 protein upregulated in hepatocellular carcinoma cells. And in return, the expression of a tumor suppresser gene-related protein p53 increased.32 In addition, omentin-1 promoted hepatocellular carcinoma apoptosis by upregulating the bax-to-bacl-2 ratio and inhibiting capases-3 activation.32 Besides, recent studies demonstrated omentin-1’s effect on enhancing Akt phosphorylation/activation.16,33 Meanwhile, the PI3K/Akt-eNos Ras pathway participated in the process of colorectal tumor incidence.34 Obviously, what can be hypothesized is that omentin-1 may facilitate the activation of the Akt signaling pathway and subsequently modulate eNOS, thereby contribute to the process of pathogenesis of colorectal cancer.35–37 So, more studies will be needed to illuminate the definite roles of omentin-1 in PCa and other malignancies.
Conclusion
In summary, omentin-1 concentrations were observed to be significantly elevated in PCa patients. Circulating omentin-1 concentrations positively correlated with obesity-related markers BMI and WHR. These findings indicated that omentin-1 may have a potential role in the development of PCa through mechanisms that play an important part in the association of obesity and PCa. This study suggested that the plasma omentin-1 may assist in the diagnosis of PCa. For validation, more studies would be needed to shed light on the potential mechanisms about this increase and to evaluate the interplay between PCa and obesity, which may further provide promising and novel pharmacological insights for PCa diagnosis and therapy in the near future.
Acknowledgments
We are grateful to Dr Souvik Mendal at University of Science and Technology of China for participating in the critical revision of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Stefani ED, Boffetta PL, Ronco A, Deneo-Pellegrini H. Meat consumption, related nutrients, obesity and risk of prostate cancer: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2016;17(4):1937–1945.
4. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi:10.1056/NEJMoa021423
5. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–565. doi:10.1634/theoncologist.2009-0285
6. Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136. doi:10.1093/epirev/mxt010
7. Carreras-Torres R, Johansson M, Gaborieau V, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a mendelian randomization study. J Natl Cancer Inst. 2017;109(9):djx012. doi:10.1093/jnci/djx007
8. Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91(3):421–430.
9. Hu MB, Liu SH, Jiang HW, Bai P-D, Ding Q, Culig Z. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: a dose-response meta-analysis of 29,464 patients. PLoS One. 2014;9(9):e106677. doi:10.1371/journal.pone.0106677
10. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi:10.1016/S0140-6736(08)60269-X
11. De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi:10.1155/2013/291546
12. Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: cancer metabolism. J Clin Onco. 2016;34(35):4277–4284. doi:10.1200/JCO.2016.67.9712
13. Arisan ED, Arisan S, Atis G, Palavan-Unsal N, Ergenekon E. Serum adipocytokine levels in prostate cancer patients. Urol Int. 2009;82(2):203–208. doi:10.1159/000200801
14. Baillargeon J, Platz EA, Rose DP, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1331–1335. doi:10.1158/1055-9965.EPI-06-0082
15. Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem Biophys Res Commun. 1998;251(3):759–762. doi:10.1006/bbrc.1998.9513
16. Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–E1261. doi:10.1152/ajpendo.00572.2004
17. Fazeli MS, Dashti H, Akbarzadeh S, et al. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine. 2013;62(1):81–85. doi:10.1016/j.cyto.2013.02.012
18. Shen XD, Zhang L, Che H, et al. Circulating levels of adipocytokine omentin-1 in patients with renal cell cancer. Cytokine. 2016;77:50–55. doi:10.1016/j.cyto.2015.09.004
19. Uyeturk U, Sarici H, Kin Tekce B, et al. Serum omentin level in patients with prostate cancer. Med Oncol. 2014;31(4):923. doi:10.1007/s12032-014-0374-0
20. Fryczkowski M, Bułdak RJ, Hejmo T, Kukla M, Żwirska-Korczala K. Circulating levels of omentin, leptin, VEGF, and HGF and their clinical relevance with PSA marker in prostate cancer. Dis Markers. 2018;2018:3852401. doi:10.1155/2018/3852401
21. Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015;175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
22. Park J, Cho SY, Lee SB, Son H, Jeong H. Obesity is associated with higher risk of prostate cancer detection in a biopsy population in Korea. BJU Int. 2014;114(6):891–895. doi:10.1111/bju.12600
23. Barrington WE, Schenk JM, Etzioni R, et al. Difference in association of obesity with prostate cancer risk between US African American and non-Hispanic white men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015;1(3):342–349. doi:10.1001/jamaoncol.2015.0513
24. Hu MB, Bai PD, Wu YS, et al. Higher body mass index increases the risk for biopsy-mediated detection of prostate cancer in Chinese men. PLoS One. 2015;10(4):e0124668. doi:10.1371/journal.pone.0124668
25. Benn M, Tybjærg-Hansen A, Smith GD, Nordestgaard BG. High body mass index and cancer risk-a Mendelian randomisation study. Eur J Epidemiol. 2016;31(9):879–892. doi:10.1007/s10654-016-0147-5
26. Bonn SE, Sjolander A, Tillander A, Wiklund F, Grönberg H, Bälter K. Body mass index in relation to serum prostate-specific antigen levels and prostate cancer risk. Int J Cancer. 2016;139(1):50–57. doi:10.1002/ijc.30052
27. Liao LM, Schwartz K, Pollak M, et al. Serum leptin and adiponectin levels and risk of renal cell carcinoma. Obesity (Silver Spring). 2013;21(7):1478–1485. doi:10.1002/oby.20138
28. Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflam. 2011;2011:376909. doi:10.4061/2011/376909
29. Stattin P, Soderberg S, Hallmans G, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86(3):1341–1345. doi:10.1210/jcem.86.3.7328
30. Tan BK, Adya R, Farhatullah S, et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57(4):801–808. doi:10.2337/db07-0990
31. Choi JH, Rhee EJ, Kim KH, et al. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur J Endocrinol. 2011;165(5):789–796. doi:10.1530/EJE-11-0375
32. Zhang YY, Zhou LM. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol. 2013;698(1–3):137–144. doi:10.1016/j.ejphar.2012.11.016
33. Kataoka Y, Shibata R, Ohashi K, et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase-and Akt-dependent mechanisms. J Am Coll Cardiol. 2014;63(24):2722–2733. doi:10.1016/j.jacc.2014.03.032
34. Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452(187):646–649. doi:10.1038/nature06778
35. Bergström A, Hsieh CC, Lindblad P, Lu CM, Cook NR, Wolk A. Obesity and renal cell cancer-a quantitative review. Br J Cancer. 2001;85(7):984–990. doi:10.1038/sj.bjc.6692040
36. Wada J. Vaspin: a novel serpin with insulin-sensitizing effects. Expert Opin Inv Drug. 2008;17(3):327–333. doi:10.1517/13543784.17.3.327
37. Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–1196. doi:10.1038/nm1307
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
