Back to Journals » Infection and Drug Resistance » Volume 13
Alteration of Clinical Chemistry Parameters Among Visceral Leishmaniasis Patients in Western Tigrai, Ethiopia, 2018/2019: A Comparative Cross-Sectional Study
Authors Tesfanchal B, Gebremichail G , Belay G, Gebremariam G , Teklehaimanot G , Haileslasie H , Kahsu G, Gebrewahd A, Mardu F , Adhanom G , Berhe B , Teame H , Tsegaye A , Wolde M
Received 12 May 2020
Accepted for publication 4 August 2020
Published 26 August 2020 Volume 2020:13 Pages 3055—3062
DOI https://doi.org/10.2147/IDR.S261698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Brhane Tesfanchal,1 Gebremedhin Gebremichail,2 Getachew Belay,1 Gebreslassie Gebremariam,3 Gebreyohannes Teklehaimanot,4 Hagos Haileslasie,2 Getachew Kahsu,1 Aderajew Gebrewahd,5 Fitsum Mardu,6 Gebre Adhanom,5 Brhane Berhe,6 Hirut Teame,7 Aster Tsegaye,8 Mistire Wolde9
1Unit of Clinical Chemistry, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 2Unit of Hematology and Immuno-Hematology, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 3Unit of Clinical Chemistry, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Mekelle University, Mekelle, Ethiopia; 4Unit of Hematology and Immuno-Hematology, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Mekelle University, Mekelle, Ethiopia; 5Unit of Medical Microbiology, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 6Unit of Medical Parasitology and Entomology, Department of Medical Laboratory Science, College of Medicine and Health Science, Adigrat University, Adigrat, Ethiopia; 7Department of Public Health, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 8Unit of Hematology and Immuno-Hematology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 9Unit of Clinical Chemistry, College of Medicine and Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Brhane Tesfanchal Tel +251-949604754
Email [email protected]
Background: Visceral leishmaniasis causes alterations of lipid metabolism and it is associated with hypocholesterolemia and severe hypertriglyceridemia. Hepatic dysfunction and life-threatening hepatitis are associated with visceral leishmaniasis. Kidney damage is frequently associated with increased morbidity and mortality in visceral leishmaniasis patients.
Methods: A cross-sectional study was carried out to assess the alterations of clinical chemistry parameters among visceral leishmaniasis patients attending Kahsay Abera and Mearg hospitals, Northwest Ethiopia. A total of 100 visceral leishmaniasis patients and 100 healthy controls without visceral leishmaniasis were selected by using convenient sampling techniques. Data were entered and analyzed using statistical package for social sciences (SPSS) version 23.
Results: Results were showed that the mean value of serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, and triglyceride was significantly higher in visceral leishmaniasis patients than in apparently healthy controls, but the mean value of serum urea and total cholesterol was significantly lower in visceral leishmaniasis patients than healthy controls.
Conclusion: The finding of this study concluded that visceral leishmaniasis causes significant alterations of clinical chemistry tests like liver and lipid profile tests compared to healthy controls.
Keywords: kidney function test, lipid profile test, liver function test, visceral leishmaniasis
Introduction
Visceral leishmaniasis (VL) is a severe protozoan systemic disease that leads to 100% mortality if left untreated.1,2 It is a vector-borne potentially fatal parasitic disease caused by the Leishmania (L.) donovani/L. infantum/L. chagasi complex and transmitted to humans by phlebotomine sand flies.3 It is a common disease in many tropical and subtropical regions. Annually about 200,000 to 400,000 new cases of VL were reported worldwide and more than 90% of these VL cases were reported from six countries like Bangladesh, Brazil, Ethiopia, India, South Sudan, and Sudan. In Ethiopia, 4000 VL cases were reported annually and 60% of cases were reported from the main endemic areas of Humera and Metema plains in the northwest of Ethiopia.1 The onset of symptoms is usually fever, general weakness, anorexia, and weight loss. It is also manifested by hepato-splenomegaly, anemia, pallor, leukopenia, and thrombocytopenia as well as hypergammaglobulinaemia. Later symptoms include cachexia, hepatic dysfunction with jaundice, hypoalbuminemia, and edema.4,5
Visceral leishmaniasis causes alterations of liver function and rarely patients manifested a severe life-threatening hepatitis.6 It also causes hepatic dysfunction, such as coagulation defects, increased serum concentrations of several liver-specific enzymes, and changes in the cholesterol biosynthesis.7 Visceral leishmaniasis patients presented with enlarged liver and shows higher value of ALT, AST, ALP,8 and the disease is also characterized by severe hypertriglyceridemia with reduced levels of total cholesterol, LDL-cholesterol, and HDL-cholesterol in adult patients.9
Visceral leishmaniasis also affects the kidney and kidney involvement in chronic leishmaniasis is frequently associated with increased morbidity and mortality. Acute kidney injury was found in a significant proportion of VL patients. Loss of kidney function and interstitial nephritis with glomerular changes can be seen from VL patients. Antibodies produced in response to infection can be trapped in glomeruli by different mechanisms, such as immune complexes and leads to cause damage to the glomerulus of the kidney.10
Even though different studies were performed in clinical chemistry alterations of VL patients in different parts of the world; there is no published study in Ethiopia particularly in the study area. Therefore, this study was aimed to assess the alterations of clinical chemistry tests among VL patients attending at Kahsay Abera and Mearg Hospitals, Northwest Ethiopia.
Materials and Methods
Study Design, Period, and Area
An institutional-based comparative cross-sectional study was employed from November 2018 to March 2019 to assess selected clinical chemistry alterations among visceral leishmaniasis patients attending at Kahsay Abera and Mearg Hospitals Western Tigrai, Northern Ethiopia. Western zone of Tigrai is the biggest in terms of geographic and territorial possessions that stretches along the border of Sudan in the North West, the Amhara Administrative region in the south, and the Eritrea border in the north where people live in clusters over a wide range of areas. It is subdivided into three districts; from north to south they are Kafta Humera, Wolqayt, and Tsegede. It is one of the agriculture surplus areas in Ethiopia known for producing sorghum and exportable products like sesame which makes it a net contributor to the national economy. Based on the 2007 Census conducted by the Central Statistical Agency of Ethiopia (CSA), this Zone has a total population of 356,598, of whom 71,823 or 20.14% are urban inhabitants.
Kahsay Abera Hospital is the district Hospitals found in Kala-azar endemic region in northern Ethiopia with close to 210 beds and an estimated 742,000 catchment population including the migrant population from Sudan, and Eritrea. Mearg hospital is the district hospital found in the kala-azar endemic area in Tsegede Wereda, Western Tigrai which has an estimated 299,594 catchment population including migrant population and have 134 beds at emergency, medical, surgical, gynecology, and pediatrics wards.11
Study Participants
Case and control groups were involved in the study. Case groups include all VL patients confirmed at Kahsay Abera and Mearg Hospital laboratories during the study period. Visceral leishmaniasis patients were diagnosed by an experienced physician and VL patients who have a history of any other chronic disease (kidney disease, liver disease, cancer, HIV/AIDS, diabetic Mellitus, hypertension tuberculosis, and malaria) were excluded. Patients under treatment of antikala-azar were also excluded from the study.
The Control group includes all healthy patient attendants of Kahsay Abera and Mearg Hospitals, who were matched with cases in age and sex without having VL. Control groups were screened using rK39-based immune chromatographic tests to rule out VL and experienced physician-diagnosed healthy controls for any clinical illness. Controls who have a history of any chronic disease (kidney disease, liver disease, cancer, HIV/AIDS, diabetic Mellitus, hypertension tuberculosis, and malaria) were also excluded from the study.
Sample Size and Sampling Techniques
Sample size needed for comparing the means of two normally distributed samples is calculated by using a two-sided test with significance level α and power 1 – β and a 95% confidence level and 80% power were used to calculate the appropriate sample size. From the study conducted in Brazil mean value of creatinine among VL patients and healthy controls (0.89 ± 0.28; 0.99 ± 0.14) was used to calculate sample size using the following formula:
n = (s12+s22)/d2*(Zα+Zβ) 2 where n= desired sample size, s1= standard deviation of case group =0.28, s2= standard deviation of control group =0.14 from previous study, Zα=1.96, Zβ = power = 0.84, d= difference between two means = 0.89–0.99= −0.01 from previous study conducted in Brazil. 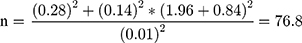 round up to 77 10% nonresponse rate = 0.1*77= 7.7 therefore the minimum sample size was 77+7.7 = 85 for each groups. Increasing sample size can give greater power to detect a significant difference between control and case groups. Therefore, 100 case groups and 100 control groups were studied in this study depending on time and available cost. A Convenient sampling technique was employed to select study participants.
round up to 77 10% nonresponse rate = 0.1*77= 7.7 therefore the minimum sample size was 77+7.7 = 85 for each groups. Increasing sample size can give greater power to detect a significant difference between control and case groups. Therefore, 100 case groups and 100 control groups were studied in this study depending on time and available cost. A Convenient sampling technique was employed to select study participants.
Method of Data Collection
Questionnaire
An interviewer-based semi-structured questionnaire was used to collect socio-demographic characteristics and data collectors fill the questionnaire by direct interview of the study participants and information concerning the clinical history was obtained from the clinical log sheet.
Serological Method (rk39)
It used for early diagnosis of visceral leishmaniasis at both peripheral and central levels. The RDTs detect specific antibodies against the kinesin-related antigen that is present in Leishmania donovani. This test is performed by placing a specified amount of patient specimen on the bottom of the strip and adds a specified amount of buffer provided. The result was recorded after 10–20 minutes based on the manufacturer's instruction.
Parasitological Method
A spleen aspirate was collected by an experienced physician and transported to the laboratory. In the laboratory, a blood film was prepared and stained by Giemsa for 10–15 minutes. The stained aspirates were examined by using a light microscope to detect the presence or absence of amastigote of Leishmania donovani.
Sample Collection, Storage, and Transportation
About 3–5 mL venous blood sample was collected using serum separator tube from both VL patients and healthy controls. Serum sample was separated by centrifuge at 4000 rpm for 5 minutes and the sample was stored at a temperature of −20 up to −30°c in deep freezer before laboratory analysis. The serum sample was transported to Adigrat general Hospital laboratory department for clinical chemistry analysis.
Clinical Chemistry Tests
Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Alkaline phosphatase, total serum bilirubin, serum direct bilirubin, serum creatinine, serum urea, total cholesterol, triglycerides, HDL, and LDL were estimated following the instructions of commercial kits provided by biosystem reagents.
Statistical Analysis
All statistical analysis was conducted with the statistical package for the social sciences (SPSS) version 23 software. Descriptive statistics were used to describe the descriptive data in the form of tables. Comparisons between VL cases and control groups were made using Independent Student’s t-test. The significance level was fixed at 5% or the corresponding p-value.
Data Quality Assurance
Data collectors were trained to ensure the quality of the data and the questionnaires were pre-tested to ensure clarity, length, logical sequence, and skip patterns of the questions. Experienced laboratory personnel have participated in the proper collection, processing, and transportation of the sample. Standard operating procedures (SOPs) were used strictly followed to assure the quality of laboratory examination of samples. Both normal and pathological quality controls were analyzed before the sample analysis to ensure the proper function, validity, and reliability of the instrument. The samples were analyzed after both controls were accepted.
Operational Definition
Liver Function Tests: - are groups of blood tests useful in the evaluation and management of patients with hepatic dysfunction. Commonly used tests to check liver function are the alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, and bilirubin tests.
Renal Function Tests: – are groups of blood tests useful in the evaluation and management of patients with kidney dysfunction. Some of the blood tests are urea, creatinine, and uric acid.
Lipid Profile Tests: – are a group of tests that include total cholesterol, triglyceride, HDL-C, LDL-C, VLDL-C, and other Apolipoproteins.
Clinical Chemistry Alterations: – it is the alteration of different tests includes RFT, LFT, and Lipid profiles.
Cases: – are individuals who have visceral leishmaniasis.
Controls: – are individuals who are healthy and unlikely to share visceral leishmaniasis.
Chronic Disease: – is a human health condition or disease that is long-lasting in its effect which includes hypertension, heart disease, kidney disease, liver disease, HIV/AIDS, cancer, Diabetic Mellitus, etc.
Normal: – is the test value lies within the established reference ranges.
Low: – is the value of the test lies below the established reference ranges.
High: – is the value of the test lies above the established reference ranges.
Results
Socio-Demographic Characteristics of Study Participants
A total of 200 study participants were included in this study comprised 100 VL confirmed patients (91 of them were males) and 100 healthy control groups (90 of them were males). The mean age ± SD of VL patients and healthy controls were 27.98 ± 9.634 years (range 15–54 years) and 27.64 ± 4.758 years (range 17–41 years) respectively. There was a male predominance in the VL patients and control groups in this study, and there was no significant difference (p=0.752) in the mean age of the VL patients and the control groups. More than half (51%) of VL patients and 56% of healthy controls were attended primary school. About 60% of VL patients and 56% of healthy controls were daily labors. The majority (94%) of VL patients and 91% of healthy controls were from rural residents. This study was showed that the distribution of VL among age groups, so that the majority of cases 28 (28%) patients within (15–20 years), 23 (23%) patients within (26–30), 20 (20%) within 21–25years, 20 (20%) above 35 years and 9 (9%) within 31–35 years (Table 1).
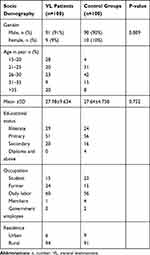 |
Table 1 Socio-Demographic Characteristics of Visceral Leishmaniasis Patients and Healthy Controls in Western Tigrai, Northern Ethiopia November 2018 to April 2019 (n=200) |
Clinical Features of Visceral Leishmaniasis Patients
The main clinical symptoms and signs presented at the initial evaluation of VL patients were: fever (100%), splenomegaly (100%), general weakness (85%), skin mucosal pallor (72%), bleeding (67%), weight loss (65%), anorexia (52%) and hepatomegaly (36%) (Figure 1).
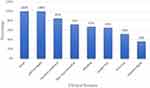 |
Figure 1 Clinical features of visceral leishmaniasis patients in Western Tigrai, Northern Ethiopia from November 2018 to April 2019 (n = 100). |
Abnormal Clinical Chemistry Test Result of Visceral Leishmaniasis Patients
From 100 VL patients an increased level of serum AST, ALT, ALP, direct bilirubin, total bilirubin, creatinine, urea, and triglyceride was found in 9 (9%), 10 (10%), 15 (15%), 18 (18%), 25 (25%), 6 (6%), 4 (4%) and 16 (16%) of VL patients, respectively; meanwhile, a decreased serum urea, cholesterol and HDL-C have been reported from 33 (33%), 35 (35%) and 20 (20%) of VL patients, respectively.
Comparison of Clinical Chemistry Tests Among VL Patients and Healthy Controls
Liver enzymes like ALT, AST, ALP and total bilirubin were significantly higher in VL patients than in healthy controls with means ± SD was (25.3 ± 19.3 versus 17.58 ± 6.38, p<0.001), (28.34 ± 29.17 versus 19.09 ± 7.94, p=0.003), (95.3 ± 60 versus 76.02 ± 19.22, p=0.002) and (0.759 ± 0.413 versus 0.62 ± 0.23, p=0.004) respectively. Similarly, the mean value of serum triglyceride was significantly higher among VL patients compared to healthy controls (146.36 ± 63.64 versus 111.46 ± 33.3, p<0.001).
The mean value of serum urea and total cholesterol was significantly lower in VL patients than healthy controls (23.11±10.72 versus 29.19 ± 9.32, p<0.001) and (130.37 ± 36.69 versus 145.1± 20.07, p=0.001) respectively. However, there was no significant difference between VL patients and healthy controls in the mean value of serum direct bilirubin, creatinine, HDL-cholesterol, and LDL-cholesterol (Table 2).
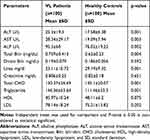 |
Table 2 Comparison of Clinical Chemistry Tests Among VL Patients and Healthy Controls in Western Tigrai, Northern Ethiopia, November 2018 to April, 2019 (n=200) |
Discussion
Visceral leishmaniasis causes derangement of liver functions and rarely VL patients also can present with severe life-threatening hepatitis.6 Leishmania donovani causes lipid profile changes and affects the kidney which leads to alteration of glomerular and tubular functions.4,9
In the present study, the mean of serum ALT, AST, and ALP was significantly higher in VL patients than healthy controls. This finding was in line with the previous study conducted in Sudan, Iraq, and India6,8,12,13 which was reported a significantly higher value of AST, ALT, and ALP in VL patients. Furthermore, different case reports supported the present study and reported liver dysfunction with the raised value of serum AST, ALT, and bilirubin from VL patients.14–19 Similarly, studies conducted in India and Bangladesh also reported abnormally high liver inflammatory enzymes like ALT, AST, and ALP in VL patients.6,20 This may be attributed to the levels of particular circulating cytokines during the inflammatory processes observed in active VL disease could be a determinant and consequence of severe liver disease.17 The liver function alteration might be due to hepatomegaly and the generation of immune complex in VL patients. The production of reactive oxygen species from the activated macrophage may also contribute to the alterations of liver function.21 In contrast, a study conducted in was reported no significant difference in the mean value of serum ALT, and AST between VL patients and healthy controls.22 This variation might be due to differences in sample size, the difference in study design, the difference in geographical area, and the difference in the genetic makeup of study participants.
The mean value of direct bilirubin and serum total bilirubin showed a slight increment in VL cases than healthy controls. However, only the mean value of serum total bilirubin was significantly higher in VL patients compared to healthy controls. This finding was in line with the study conducted in Bihar, India13 which showed that serum bilirubin was significantly higher in VL patients than in healthy controls. Besides different case reports conducted in India, Turkey, and a cohort study conducted in Brazil showed that elevated total and direct bilirubin in VL patients.18,19,23 This may attribute to hemolysis of red blood cells and sequestration and destruction of red blood cells (RBC) in enlarged spleen of VL patients.24 However, this study was not in agreement with the study conducted in Iraq and which were reported no significant difference in the mean value of serum bilirubin between VL patients and healthy controls. This variance might be attributed due to differences in reference intervals of clinical chemistry tests, lifestyle differences among study participants, and geographical variations.8,22
The mean value of Serum Triglyceride was significantly higher and serum total cholesterol was significantly lower among VL patients compared to healthy controls. These results are in agreement with studies conducted in Iraq, Brazil, and Greece.9,25,26 A case report conducted in Greece and other studies conducted in California have reported decreased serum cholesterol levels, severe hypocholesterolemia, mild hypertriglyceridemia from VL patients.27,28 This lipid alteration may be due to the extraction of membrane cholesterol by L. donovani and the changes in the levels of lipoproteins may be directly related to the modulation of the immune response.29 Endothelial lipoprotein lipase, an enzyme responsible for cleavage of triglyceride may be impaired in patients with VL. This can be decreasing the metabolism of triglyceride which leads to increased triglyceride and decreases the formation of HDL-c and LDL-c from VLDL.9,26
There was no significant difference in the mean of HDL-c and LDL-c between VL patients and healthy controls. In contrast, different studies conducted in Iraq, Greece, and Brazil were reported significantly low value of serum HDL-c and LDL-c among VL patients than healthy controls.9,25-27,29,30 This difference might be due to genetic variability among study participants, differences in diet among study participants, the difference in environmental temperature, and seasonal variation.
The mean value of serum creatinine had no significant difference among VL patients compared to healthy controls. This finding was in line with another study conducted in Brazil which was reported no significant difference in creatinine level between VL patients and healthy controls.31 Whereas the mean of serum urea was significantly lower in VL patients compared to the healthy controls. This finding was in agreement with the study conducted in Brazil.31 This significantly decreased urea may be attributed to a low protein diet and reduced production in the liver.
Conclusions
Visceral leishmaniasis patients showed significant alterations of liver function tests and lipid profile tests compared to healthy controls. This may indicate that VL is one of the causes of liver dysfunction and lipid profile disorders. Even though the renal function tests show a slight increment in VL patients than healthy controls, there was no statistical difference between VL patients and healthy controls.
Limitation of the Study
Different tests like electrolyte, GFR, uric acid, protein, albumin, and endocrine tests were not done in this study due to lack of budget.
Abbreviations
ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase HDL, High-density lipoprotein; LDL, Low-density lipoprotein; SOP, Standard operating procedures; VL, Visceral leishmaniasis.
Data Sharing Statement
All datasets generated and/or analyzed during the current study are summarized in the manuscript.
Ethics Approval and Consent to Participate
The study was conducted after the study protocol was reviewed and approved by the Institutional Review Board (IRB) of the Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University DRERC/381/18/MLS. Official letters were also obtained from Tigrai Regional Health Bureau and the Chief Executive Officer of Kahsay Abera and Mearg Hospitals. The purposes, procedures, and significance of the study were explained to the study participants, and written informed consent was obtained from each study participant with parents’ consent for aged less than 18 years. The study was conducted in accordance with the Helsinki declaration and physicians working in the hospitals were informed about the study and responsible to protect the life, health, dignity, privacy, and confidentiality of personal information of study participants. The researcher also respects the privacy and confidentiality of any information obtained from the participant using unique codes given for each questionnaire and corresponding specimen. The participant or his/her legal guardian has the right to withdraw consent to participate in research at any time during the preceding without any reason. The predictable risk was continuously monitored by the researcher and all study participants were no faced any associated risk with the study. Study participants with pathologic chemistry test results were communicated with respective physicians for appropriate treatment.
Acknowledgments
We are grateful to Kahsay Abera and Mearg Hospital staff for their support. We thank Addis Ababa University for facilitating and partially funding this study. We would also like to thank Addis Ababa University electronic thesis library for uploading our thesis as grey literature to become easily available for readers. Our thanks also extend to the study participants for their willingness for participation.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, revising the manuscript critically for important intellectual content, read and comments the manuscript for submission, gave final approval of the manuscript version to be published and agreed to be accountable for every aspect of the work.
Disclosure
The authors declared they have no competing interests. This manuscript’s thesis was uploaded to Addis Ababa University electronic thesis library based on an author’s thesis requirement for academic purposes and available online at: http://213.55.95.56/handle/123456789/21270
References
1. Leta S, Dao THT, Mesele F, Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Tropica Dis. 2014;8(9):e3131. doi:10.1371/journal.pntd.0003131
2. Boakye D, Wilson M, Kweku M. A review of leishmaniasis in West Africa. Ghana Med J. 2005;39(3):94.
3. Palatnik-de-Sousa CB, Day MJ. One Health: the global challenge of epidemic and endemic leishmaniasis. Parasit Vectors. 2011;4(1):197. doi:10.1186/1756-3305-4-197
4. Clementi A, Battaglia G, Floris M, Castellino P, Ronco C, Cruz DN. Renal involvement in leishmaniasis: a review of the literature. NDTPlus. 2011;4(3):147–152.
5. Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Research. 2017;6.
6. Mathur P, Samantaray JC, Samanta P. High prevalence of functional liver derangement in visceral leishmaniasis at an Indian tertiary care center. Clin Gastroenterol Hepatol. 2008;6(10):1170–1172. doi:10.1016/j.cgh.2008.04.033
7. de Freitas EO, Leoratti F, Freire-de-Lima CG, Morrot A, Feijó DF. The contribution of immune evasive mechanisms to parasite persistence in visceral leishmaniasis. Front Immunol. 2016;7:153. doi:10.3389/fimmu.2016.00153
8. Naseralla BA, Al-Quraishi MA, Jebur MS. Serological detection and liver functions of pediatric visceral leishmaniasis in Baghdad hospitals. Int J Curr Microbiol App Sci. 2015;4(1):100–107.
9. Gatto M, Abreu M, Tasca KI, et al. Biochemical and nutritional evaluation of patients with visceral leishmaniasis before and after treatment with leishmanicidal drugs. Rev Soc Bras Med Trop. 2013;46(6):735–740. doi:10.1590/0037-8682-0198-2013
10. Silva Junior G, Barros EJG, Daher EDF. Kidney involvement in leishmaniasis – a review. Braz J Infect Dis. 2014;18(4):434–440. doi:10.1016/j.bjid.2013.11.013
11. HMIS of Kahsay Abera and Mearg Hospitals. 2018.
12. El Hag I, Hashim F, El Toum I, Homeida M, El Kalifa M, El Hassan A. Liver morphology and function in visceral leishmaniasis (Kala-azar). J Clin Pathol. 1994;47(6):547–551. doi:10.1136/jcp.47.6.547
13. Kumar S, Singh R. Observation of deviations and comparisons of liver function:test in different stages of Kala-azar. IOSR J Dental Med Sci. 2018;17(7):34–40.
14. Mukerrama SM, Kabir A, Deb SR, et al. Visceral Leishmaniasis turning into chronic liver disease. J Med. 2016;17(1):51–54. doi:10.3329/jom.v17i1.30063
15. Prakash A, Singh N, Sridhara G, et al. Visceral leishmaniasis masquerading as chronic liver disease. J Asso Physic India. 2006;54:893–894.
16. Sagnelli C, Di Martino F, Coppola N, Crisci A, Sagnelli E. Acute liver failure: a rare clinical presentation of visceral leishmaniasis. J Microbiologica Sci. 2012;35(1):93.
17. Dos Santos PL, de Oliveira FA, Santos MLB, et al. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Negl Trop Dis. 2016;10(1):e0004375. doi:10.1371/journal.pntd.0004375
18. Prajapati R, Kumar A, Sharma P, et al. A rare presentation of Leishmaniasis. J Clin Exp Hepatol. 2016;6(2):146–148. doi:10.1016/j.jceh.2016.01.001
19. Çelik Ü, Leblebisatan G, Alhan E, Aksaray N. Immune hemolytic anemia in association with visceral leishmaniasis. J Pediatr Inf. 2007;1:36–38.
20. Rashid AM, Al Mamun A, Rasul C, Afrafuzzaman M, Hossain M, Rahman MM. Jaundice in pediatric visceral leishmaniasis (kala-azar) patients. J Med. 2007;8(1):14–16. doi:10.3329/jom.v8i1.1374
21. Bankoti R, Stäger S. Differential regulation of the immune response in the spleen and liver of mice infected with Leishmania donovani. J Tropica Med. 2012;2012.
22. Kashani MN, Firooz A, Eskandari SE, et al. Evaluation of meglumine antimoniate effects on liver, kidney and pancreas function tests in patients with cutaneous leishmaniasis. Eur J Dermatol. 2007;17(6):513–515. doi:10.1684/ejd.2007.0266
23. Daher EF, Sampaio AM, Martiniano LVM, Vieira APF, Junior GBS. Acute kidney injury in visceral leishmaniasis: a cohort of 10 patients admitted to a specialized intensive care unit in northeast of Brazil. Asian Pac J Trop Dis. 2013;3(1):41–46. doi:10.1016/S2222-1808(13)60009-2
24. Varma N, Naseem S. Hematologic changes in visceral leishmaniasis/kala azar. Indian J Hematol Blood Transfus. 2010;26(3):78–82. doi:10.1007/s12288-010-0027-1
25. Sulaiman SN Role of lipid profiles in visceral Leishmaniasis activity. Republic of Iraq Ministry of Higher Education and Scientific Research University of Baghdad College of Science available from:https://www.google.com/search?client=ms-opera-mini-android $oq=role+of+lipid+profile+in+visceral+leshimaniasis+activity+pdf$aqs.
26. Soares NM, Leal T, Fiuza M, et al. Plasma lipoproteins in visceral leishmaniasis and their effect on Leishmania‐infected macrophages. Paras Immunol. 2010;32(4):259–266. doi:10.1111/j.1365-3024.2009.01187.x
27. Liberopoulos E, Alexandridis G, Bairaktari E, Elisaf M. Severe hypocholesterolemia with reduced serum lipoprotein (a) in a patient with visceral leishmaniasis. Ann Clin Lab Sci. 2002;32(3):305–308.
28. Feingold KR, Grunfeld C. Lipids: a key player in the battle between the host and microorganisms. J Lipid Res. 2012;53(12):2487–2489. doi:10.1194/jlr.E033407
29. Sviridov D, Bukrinsky M. Interaction of pathogens with host cholesterol metabolism. Curr Opin Lipidol. 2014;25(5):333. doi:10.1097/MOL.0000000000000106
30. Soares NM, de Souza JN, Leal TF, et al. Sera from Visceral Leishmaniasis patients display oxidative activity and affect the TNF-α production by macrophages in vitro. Bio Med Res Int. 2017. doi:10.1155/2017/5861453.
31. Lima FV, Lima IV, Silva GJ, Daher E, Lima EV. Evaluation of renal function in human visceral leishmaniasis (kala-azar): a prospective study on 50 patients from Brazil. J Nephrol. 2007;20(4):430–436.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
