Back to Journals » Drug Design, Development and Therapy » Volume 12
Alpha lipoic acid combined with epalrestat: a therapeutic option for patients with diabetic peripheral neuropathy
Authors Wang XT , Lin HX , Xu S, Jin YL, Zhang R
Received 21 March 2018
Accepted for publication 2 May 2018
Published 7 September 2018 Volume 2018:12 Pages 2827—2840
DOI https://doi.org/10.2147/DDDT.S168878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Xiaotong Wang,1,* Haixiong Lin,2,* Shuai Xu,3 Yuanlin Jin,1 Ren Zhang4
1Shenzhen Bao’an Traditional Chinese Medicine Hospital Group, Guangzhou University of Chinese Medicine, Shenzhen 518133, People’s Republic of China; 2The First School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510405, People’s Republic of China; 3School of Chinese Materia Medica, Guangzhou University of Chinese Medicine, Guangzhou 510006, People’s Republic of China; 4The College of Basic Medical Science, Guangzhou University of Chinese Medicine, Guangzhou 510006, People’s Republic of China
*These authors contributed equally to this work
Background: Alpha lipoic acid (ALA), a type of antioxidant, is used in combination with epalrestat in the treatment of diabetic peripheral neuropathy (DPN). However, whether combined treatment is superior to epalrestat monotherapy is controversial.
Methods: We conducted a systematic search of PubMed, Cochrane Library and Chinese databases to identify all randomized controlled trials (RCTs) up to October 31, 2017. Data were extracted to evaluate methodological quality and analyzed using Review Manager 5.3.0 software.
Results: Twelve studies were included. Compared to epalrestat monotherapy, ALA 600 mg/d once a day (qd) combined with epalrestat 50 mg three times a day (tid) augmented the total effectiveness rate (14 days – risk ratio [RR]: 1.40, 95% CI: 1.16–1.69, P=0.0005; 28 days – RR: 1.48, 95% CI: 1.27–1.72, P<0.00001); at the same, it could improve the median motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV), peroneal MNCV, and SNCV after 14, 21, and 28 days of treatment and could reduce the Toronto Clinical Scoring System (TCSS) (weighted mean difference [WMD]: -1.60, 95% CI: (-2.91, -0.29), P=0.02) and Total Symptom Score (TSS) (WMD: -0.93, 95% CI: -1.27, -0.60, P<0.00001) after 21 days of treatment. The treatment strategy of ALA 300 mg/d qd combined with epalrestat 50 mg tid had the same effects in regard to the total effectiveness rate (RR: 1.37, 95% CI: 1.18–1.59, P<0.0001), median MNCV (WMD: 6.12, 95% CI: 5.04, 7.20, P=0.00001), median SNCV (WMD: 6.70, 95% CI: 5.75, 7.65, P=0.00001), peroneal MNCV (WMD: 6.68, 95% CI: 5.82, 7.55, P=0.00001), and peroneal SNCV (WMD: 4.27, 95% CI: 3.34, 5.20, P=0.00001) after 28 days of treatment.
Conclusion: ALA combined with epalrestat is an effective option for DPN patients. Future large-sample RCTs should be conducted to further confirm this finding.
Keywords: alpha lipoic acid, diabetic peripheral neuropathy, epalrestat, meta-analysis
Introduction
Diabetic neuropathy (DN) is a very common, symptomatic, long-term complication of diabetes mellitus, affecting nearly 50% of patients with type 1 and/or type 2 diabetes.1 According to International Diabetes Federation data, ~592 million people worldwide will be diagnosed with diabetes by 2035.2 DPN affects ~236 million people, primarily in low- and middle-income countries, causing a very large financial burden.3,4 Diabetic peripheral neuropathy (DPN) is closely associated with high morbidity, mortality, and diminished quality of life.5 Diabetic damage due to hyperglycemia and metabolic imbalance – primarily, oxidative stress – may appear in the neurons (axons or myelin sheaths) of DPN patients.6 Therefore, the typical clinical manifestations of DPN are tingling, burning, pain, cramps, paresthesia, and numbness.3 With regard to oxidative stress and the related pathways, some drugs, such as alpha lipoic acid, aldose reductase inhibitors (ARIs; eg, epalrestat), and protein kinase C inhibitors, are being investigated.6 These drugs have been widely used to treat DPN in clinical or intensive long-term comparative trials.
Nerve conduction velocity (NCV) is an objective indicator of neuronal damage in the distal segment of the peripheral nerves and is accepted as an essential part of the diagnosis of DPN.7–9 Previous studies have shown that motor nerve conduction velocity (MNCV) or sensory nerve conduction velocity (SNCV) are significantly reduced with the development of DPN.10,11 ALA – a coenzyme in the tricarboxylic acid cycle – is an antioxidant that may prevent and reduce diabetic micro- and macrovascular complications12 and is an effective treatment of DPN.13 Experimental studies have proved that ALA can improve nerve blood flow, reduce oxidative stress, and improve distal nerve conduction.14 One meta-analysis reported that infusions of ALA (600 mg IV/day) ameliorated the symptoms of neuropathy after 3 weeks,15 with IV therapy being more effective than oral treatment (SMD =−2.8 vs SMD =−1.8).16 Epalrestat is an ARI that relieves oxidative stress and suppresses the polyol pathway, which delays the progression of DPN and effectively and safely improves both DN symptoms and the MNCV in the context of neuropathy.17–19
Accumulating evidence has shown that ALA combined with epalrestat may be a viable option for patients with DPN because of its marked beneficial effect on clinical symptoms and nerve conduction velocity (NCV).20 However, the available randomized controlled trials (RCTs) examining this combination therapy have not been systematically retrieved and evaluated. We conducted a meta-analysis to assess the efficacy and safety of ALA combined with epalrestat for patients with DPN.
Materials and methods
This systematic review was registered in PROSPERO CRD42017081310, and we strictly followed the Cochrane Collaboration framework guidelines21 and the PRISMA Statement22 to conduct the review (Table S2).
Selection criteria
Studies that satisfied the following criteria were selected. 1) Patients: Patients with DPN. The diagnostic criteria for diabetes mellitus and its complications in the trials were in accordance with the criteria of the 1999 World Health Organization (WHO) guideline.23 The diagnostic criteria for DPN were in accordance with a statement by the American Diabetes Association24 or the guidelines for the prevention and treatment of Type 2 diabetes (2013 version) of the Chinese Diabetes Society.25 Patients with other types of peripheral neuropathy, such as cerebral infarction, Guillain–Barre syndrome, severe venovascular disease, cervical spondylosis, and lumbar lesions, were excluded. There were no restrictions on patient race, region, age, sex, or on the severity or duration of DPN. 2) Interventions: Combined treatment was compared to epalrestat monotherapy or on the basis of co-intervention for the treatment of DPN patients. 3) Comparisons: Subjects received ALA combined with epalrestat vs epalrestat alone. 4) Outcomes: Clinical effectiveness rate, Toronto Clinical Scoring System (TCSS), Total Symptom Score (TSS), adverse reactions, MNCV, and SNCV were measured. The total effectiveness rate was calculated on the basis of following criteria: subjective symptom alleviated, tendon reflex improved, and NCV increased by ≥3 m/s after treatment. 5) Study design: RCTs that determined the clinical utility of ALA combined with epalrestat for the treatment of DPN were selected. Reviews, cross-sectional studies, cohort studies, animal experiments, and commentaries were excluded.
Search strategy
A systematic literature search of multiple databases for relevant trials was undertaken. All Chinese databases, including the Chinese Biomedical Database, Wanfang Data, the VIP Chinese Science and Technology Journals Database, the China National Knowledge Infrastructure, as well as English databases, such as PubMed and Cochrane Library, were searched from their inception to October 31, 2017. For the Chinese databases, free-text terms such as “epalrestat” or “lipoic acid” and “diabetic peripheral neuropathy” or “peripheral neuropathy” or “diabetic neuropathy” or “DPN” and “randomized controlled trial” or “randomized” and “blind” were used; there were no restrictions on subheadings. For the English databases, the following mesh terms were used, with no restriction on subheadings: ((diabetic AND peripheral neuropathy) OR (diabetic neuropathy OR diabetic peripheral neuropathy)) AND ((thioctic acid) OR (thioctic AND acid) OR (lipoic acid) OR (lipoic AND acid)) AND (epalrestat) AND (randomized OR randomized controlled trial) AND blind. The search strategies of PubMed and CNKI were showed in Table S1. Other electronic databases will be search using the similar strategy. Publication languages were confined to Chinese and English. Clinical trials published in abstract form were selected only if sufficient data could be retrieved from the abstract or authors. The reference lists of the potentially eligible studies were also reviewed to discover additional clinical trials missed by the initial search.
Data selection
All retrieved results were imported into NoteExpress 3.2.0. Duplicate data from different databases were identified by NoteExpress 3.2.0, and two reviewers (Wang XT and Lin HX) independently screened the remaining abstracts and full texts of potentially eligible trials. Any disagreements were settled by discussion among four authors (Wang XT, Lin HX, Jin YL and Zhang R). SX checked the final data set. Treatment strategies that were not repeated were eliminated.
Data extraction and risk of bias
For each eligible study, two reviewers (Wang XT and Lin HX) extracted study information (first author names, publication year), patient data (age, sample sizes of the treatment group and control group), therapeutic strategy (intervention methods, intervention time), and DPN outcome. Risk assessment and quality evaluation, as determined by the Cochrane criteria, were used to evaluate each eligible study.26
Statistical analysis
The clinical effectiveness rate, TCSS, TSS, adverse reactions, MNCV, and SNCV were evaluated and merged. Review Manager (version 5.3.0) software was utilized to analyze the data. The data are presented as RR or weighted mean difference (WMD) and 95% CI for the included studies. Statistical heterogeneity was assessed using the Chi-squared test and I2 values.27 I2>75% indicated significant heterogeneity, 50% <I2≤75% was regarded as mildly significant heterogeneity, and 0% ≤ I2≤50% was defined as indicating no heterogeneity. A random-effects model and sensitivity analysis was undertaken when mild or significant heterogeneity (P<0.05, I2>50%) was detected among the analyzed studies. Moreover, subgroup analysis was conducted on the basis of different therapeutic methods or follow-up durations. A fixed-effects model was used in the absence of heterogeneity. When possible, comparisons of studies that used the same treatment strategy for different durations were made using the chi-squared test and the associated P-value. Funnel plots, Begg’s test, and Egger’s test were conducted on clinical outcomes to further analyze the potential publication bias. Begg’s test P>0.05 and Egger’s test P>0.05 indicated no significant publication bias.
Results
Eligible studies
Our initial search strategy yielded 240 potential articles, and Figure 1 shows the search process. Duplicate records were removed in NoteExpress 3.2.0 (n=135), and 59 studies were excluded after screening of the titles and abstracts. The full text of 46 studies was screened. Twenty-eight trials using other drugs, three trials with suspected duplicated data, and three trials28–30 that did not use the same treatment strategy were removed. Twelve clinical trials were included in the meta-analysis. The detailed characteristics of the included studies are presented in Table 1.
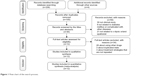  | Figure 1 Flow chart of the search process. |
Risk of bias of the included studies
The quality of the included studies was high in terms of randomization, completeness of outcome data, selective reporting, and other potential biases. However, the studies conducted by Fang32 and Huang41 did not mention a randomized design. In addition, the poor allocation concealment, inadequate blinding of participants or personnel, and inadequate blinding of outcome assessment should be considered (Figure 2).
  | Figure 2 Risk of bias graph and bias summary. |
Total effectiveness rate
Eight studies containing total effectiveness rate data were included. The meta-analysis was conducted using a fixed-effects model based on the lack of heterogeneity (I2=0%, P>0.05). According to the subgroup analysis, although the total effectiveness rate differed by treatment strategy, the combined treatment exhibited a better total effectiveness rate than epalrestat alone (ALA 600 mg/d qd combined with epalrestat 50 mg tid for 14 days – RR: 1.40, 95% CI: 1.16–1.69, P=0.0005; for 28 days – RR: 1.48, 95% CI: 1.27–1.72, P<0.00001; ALA 300 mg/d qd combined with epalrestat 50 mg tid for 28 days – RR: 1.37, 95% CI: 1.18–1.59, P<0.0001; Figure 3).
  | Figure 3 Forest plot of meta-analysis of total effectiveness rate. |
Median MNCV
Ten trials investigated the median MNCV. A beneficial and statistically significant effect of combined treatment on median MNCV was observed in two different treatment strategies compared with epalrestat monotherapy. The pooled results of ALA 600 mg/d qd combined with epalrestat 50 mg tid were significantly higher than that of epalrestat monotherapy (14 days – pooled WMD: 7.98, 95% CI: 6.17–9.80, P<0.00001; 21 days – WMD: 2.74, 95% CI: 1.50–3.98, P<0.0001; 28 days – WMD: 9.01, 95% CI: 3.46–14.56, P=0.001). Significant differences were identified between these different treatment durations (χ2=24.41, P<0.00001). Moreover, ALA 300 mg/d qd combined with 50 mg tid epalrestat for 28 days indicated the benefit of the combined-treatment regimen (WMD: 6.12, 95% CI: 5.04–7.20, P<0.00001; Table 2).
Peroneal MNCV
Eleven trials investigated peroneal MNCV. The pooled results suggested a significant difference in peroneal MNCV between the combined treatment and epalrestat-alone groups in two different treatment strategies (ALA 600 mg/d qd combined with epalrestat 50 mg tid: for 14 days – WMD: 9.77, 95% CI: 7.78–11.75, P<0.00001; for 21 days – WMD: 4.18, 95% CI: 1.49–6.88, P=0.002; for 28 days – WMD: 7.13, 95% CI: 3.94–10.32, P<0.0001. ALA 300 mg/d qd combined with epalrestat 50 mg tid for 28 days – WMD: 6.68, 95% CI: 5.82–7.55, P<0.00001; Table 2). There were significant differences between the results obtained using ALA 600 mg/d qd combined with epalrestat 50 mg tid for different durations (χ2=10.84, P=0.004).
Median SNCV
Ten studies provided median SNCV data of patients with DPN. The analysis revealed a significant improvement in median SNCV in combined-treatment patients compared to epalrestat-treated patients in two different treatment strategies (ALA 600 mg/d qd combined with epalrestat 50 mg tid: for 14 days – WMD: 7.12, 95% CI: 5.13–9.11, P<0.00001; for 21 days – WMD: 3.88, 95% CI: 3.08–4.68, P<0.00001; for 28 days – WMD: 9.97, 95% CI: 1.56–18.38, P=0.02. ALA 300 mg/d qd combined with epalrestat 50 mg tid for 28 days – pooled WMD 6.70, 95% CI: 5.75–7.65, P<0.00001; Table 2). There were significant differences between the results obtained using ALA 600 mg/d qd combined with epalrestat 50 mg tid for different periods of time (χ2=10.51, P=0.005).
Peroneal SNCV
Eleven trials were included for a comparison of peroneal SNCV results. The pooled results indicated that ALA combined with epalrestat significantly improved peroneal SNCV in different treatment strategies (ALA 600 mg/d qd combined with epalrestat 50 mg tid: for 14 days – WMD: 7.76, 95% CI: 5.77–9.76, P<0.00001; for 21 days – WMD: 3.30, 95% CI: 0.45–6.14, P=0.02; for 28 days – WMD: 6.29, 95% CI: 3.01–9.58, P=0.0002. ALA 300 mg/d qd combined with epalrestat 50 mg tid for 28 days – WMD: 4.27, 95% CI: 3.34–5.20, P<0.00001; Table 2). There were significant differences between the results obtained using ALA 600 mg/d qd combined with epalrestat 50 mg tid for different periods of time (χ2=6.34, P=0.04).
TCSS
Three trials of 188 patients were included in the TCSS data. A random-effects model was developed because of the high heterogeneity (I2=80%, P=0.006). The combined WMD was −1.60 (95% CI: −2.91, −0.29; P=0.02), revealing that the TCSS was significantly lower in the group receiving a combination of ALA 600 mg/d qd and epalrestat 50 mg tid than in the group receiving epalrestat monotherapy after 21 days of treatment (Table 2).
TSS
A fixed-effects model was created because no heterogeneity was found between the three studies (I2=0%, P=0.69). The pooled data demonstrated that TSS was significantly lower in the combination treatment group (ALA 600 mg/d qd and epalrestat 50 mg tid) than in the epalrestat-alone group after 21 days of treatment (WMD: −0.93, 95% CI: −1.27, −0.60, P<0.00001; Table 2).
Adverse reactions
Only Han’s study38 reported an allergic reaction (1/55) in the combined-treatment group. Three trials31,35,40 reported no adverse reactions in either the combination treatment or epalrestat group.
Sensitivity analysis
In the sensitivity analysis, the pooled data of the above outcomes did not show significant changes, indicating that the pooled results were steady. With respect to the ALA 600 mg qd combined with epalrestat 50 mg tid for the 28-day subgroup, the observed heterogeneity between therapies for the median MNCV and SNCV was absent after removing the study by Liu33 (I2=17%, P=0.27; I2=34%, P=0.22). In this scenario, based on the results of a fixed-effects model, the combined-treatment regimen dramatically improved the median MNCV and SNCV compared to epalrestat alone (median MNCV: WMD: 6.64, 95% CI: 5.52, 7.36, P<0.00001; median SNCV: WMD: 5.72, 95% CI: 4.98, 6.47, P<0.00001; Table 3).
Publication bias
The funnel plot was essentially symmetrical, indicating no obvious publication bias in the total effectiveness rate. The Begg’s test score was Z=1.11 (P=0.266), and the Egger’s test score was t=1.31 (P=0.238), suggesting no evidence of publication bias (Figure 4).
  | Figure 4 Funnel plot of total effectiveness rate. |
Discussion
DN includes disorders of peripheral nerves and is a diabetic microvascular complication.6 The prevalence of DPN in patients is approximately 30%, but up to 50% of these patients develop neuropathy during the course of the disease.43 The total annual economic burden associated with DPN and its complications is estimated to be $4.6–13.7 billion (US), with approximately 27% of the direct medical expense of DM being attributed to DPN.44 The pathogenesis of DPN is not clear; however, defects in metabolic and vascular pathways combined with oxidative stress play important roles in the onset and progression of nerve injury.45 Many potential treatments, such as the curcumin derivative J147 and the methanolic extract of Juglans regia L. leaf, are effective in animal models of DPN; however, these treatments have not been validated in clinical trials.46,47 ALA and epalrestat are efficacious in DPN patients and are popular in clinical use.48,49 Many RCTs, especially in recent years, have confirmed that a combination of ALA and epalrestat improves MNCV and SNCV in DPN patients more efficiently than monotherapies.29,33
In our meta-analysis, 12 studies met our inclusion criteria. The data suggested that combination therapy was superior to epalrestat alone in improving the total effectiveness rate. Furthermore, the combination of ALA plus epalrestat increased the MNCV and SNCV of the median nerve and the nervus peroneus communis compared to epalrestat monotherapy. Notably, combination therapy was better in reducing the TCSS and TSS of patients with DPN. The results of the present meta-analysis are partially consistent with another recent meta-analysis published in September 2015,50 which demonstrated that ALA significantly improved the remission rate of clinical symptoms and nerve conduction velocity. However, combination therapy was not considered in this previous meta-analysis.50 In addition, the Cochrane methodological criteria were not adequately fulfilled, and studies with different intervention times were not included, resulting in lower quality results.50 More clinical trials were published in different databases in recent years, providing us with an opportunity to conduct a higher quality meta-analysis involving trials that considered different ALA doses and durations of intervention.
ALA directly eliminates free radicals, inhibits peroxidation, enhances blood flow, increases nerve Na+ −K+ ATPase activity, protects endothelial function, and increases the speed of nerve conduction.14,45,51,52 Moreover, these data indicate that ALA benefits the vascular abnormalities of DPN and remarkably improves peripheral nerve function. In addition, ALA increases insulin sensitivity.53 Therefore, ALA is a good option as a treatment for DPN that targets the pathogenic origins of the condition. Epalrestat prevents peripheral nerve injury by reducing expression of antioxidant enzyme and aldose reductase, relieving oxidative stress, and suppressing the overactive polyol pathway.17 Epalrestat is, therefore, a valid choice for DPN treatment. The combination of ALA with epalrestat further improved the symptoms of DPN, NCV, and the peripheral blood levels of high sensitivity C-reactive protein.54 The hospitalization cost study in 58 patients revealed that epalrestat was more economical than other drugs, such as alprostadil, and no difference in the total effectiveness rate was observed for the treatment of DPN for 1 month.55 These data provide a promising option for DPN patients, especially those who have a poor clinical response to epalrestat monotherapy.
Limitations
There are some limitations of the present meta-analysis. First, most of the included clinical trials in this review were conducted in China, given that few combined-treatment regimens were undertaken outside of China. Data from other countries were not retrieved because of language and database limitations. Treatment strategies that were not repeated were also eliminated, which may have excluded some reliable treatment options. In addition, there were no placebo groups in any of the included trials, making it impossible to eliminate the possibility that the observed responses were due to the placebo effect.
Second, heterogeneity among clinical trials was observed in the analyses of the MNCV and SNCV of the median nerve, specifically for the treatment strategy consisting of ALA 600 mg qd combined with epalrestat 50 mg tid for 28 days. The sensitivity analysis of the pooled data demonstrated that the median MNCV and SNCV were not significantly altered with or without the inclusion of Liu’s study,33 indicating that the pooled results were steady. However, a contribution of Liu’s study33 to heterogeneity was identified, demonstrating that different doses of ALA and different intervention times may be the sources of heterogeneity.
Third, most of the included clinical trials had relatively poor methodological quality. Fang32 and Huang41 did not mention details on randomization and reported no differences in age, gender, course of the disease, or fasting blood glucose between the two groups. Data from Deng’s study36 were inconsistent in terms of wording and should have been proofread by the author. We attempted to contact other authors via email or telephone for more detailed information regarding each trial, but little useful information was received. Considering the limitations of this review, CONSORT statements56 are recommended for the reporting of future clinical trials.
Conclusion
Evidence from this analysis suggests that ALA combined with epalrestat is an efficient therapeutic option for patients with DPN. Future large-sample RCTs should be conducted to verify this finding.
Acknowledgments
The authors thank the teachers of Yilong Education, Guangzhou, China, for their advice on statistics. This study was supported by the College Students’ Innovative Entrepreneurial Training Program of Guangzhou University of Chinese Medicine (grant no 201710572271) and National Natural Science Foundation of China (grant no 31700288).
Author contributions
Ren Zhang and Xiaotong Wang conceived and designed the experiments. Haixiong Lin and Xiaotong Wang extracted the data, conducted statistical analysis, and wrote the manuscript. Shuai Xu checked the data. Yuanlin Jin and Ren Zhang interpreted the results. Ren Zhang supervised the manuscript writing. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. | ||
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. | ||
Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14. | ||
Aszmann O, Tassler PL, Dellon AL. Changing the natural history of diabetic neuropathy: incidence of ulcer/amputation in the contralateral limb of patients with a unilateral nerve decompression procedure. Ann Plast Surg. 2004;53(6):517–522. | ||
Bril V. Treatments for diabetic neuropathy. J Peripher Nerv Syst. 2012;17 Suppl 2(S2):22–27. | ||
Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev. 2013;2013:1–15. | ||
Ito A, Kunikata H, Yasuda M, et al. The Relationship between Peripheral Nerve Conduction Velocity and Ophthalmological Findings in Type 2 Diabetes Patients with Early Diabetic Retinopathy. J Ophthalmol. 2018;2018:1–7. | ||
Jurado J, Ybarra J, Romeo JH, Pou JM. Clinical screening and diagnosis of diabetic polyneuropathy: the North Catalonia Diabetes Study. Eur J Clin Invest. 2009;39(3):183–189. | ||
Perkins BA, Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin Neurophysiol. 2003;114(7):1167–1175. | ||
Hussain G, Rizvi SAA, Singhal S, Zubair M, Ahmad J. Cross sectional study to evaluate the effect of duration of type 2 diabetes mellitus on the nerve conduction velocity in diabetic peripheral neuropathy. Diabetes Metab Syndr. 2014;8(1):48–52. | ||
Luan S, Cui L, Tang X, et al. Motor nerve conduction velocity distribution of diabetic neuropathy. Chinese Journal of Neurology. 2006;39(7):436–439. | ||
Yi X, Maeda N. Alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55(8):2238–2244. | ||
Reed LJ, Debusk BG, Gunsalus IC, Hornberger CS. Crystalline alpha-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science. 1951;114(2952):93–94. | ||
Nagamatsu M, Nickander KK, Schmelzer JD, et al. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995;18(8):1160–1167. | ||
Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21(2):114–121. | ||
Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJG. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:1–8. | ||
Li QR, Wang Z, Zhou W, et al. Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen Res. 2016;11(2):345–351. | ||
Li P, Ma J, Gao J, et al. Clinical efficacy and safety of epalrestat in diabetic neuropathy-A multicenter randomized controlled clinical trial. Chinese Journal of Endocrinology and Metabolism. 2015;31(9):743–747. | ||
Hotta N, Akanuma Y, Kawamori R, et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care. 2006;29(7):1538–1544. | ||
Guo Y, Zhao Y, Liu Y, Song D. Analysis the effect of lipoic acid combined with epalrestat in Chinese people with diabetes peripheral neuropathy. Journal of Practical Diabetology. 2017;13(01):15–18. | ||
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Vol. 2011: The Cochrane Collaboration; 2017. updated March 2011. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–W64. | ||
Alberti KGMM, Zimmet PZ, Definition ZPZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. | ||
Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. | ||
Chinese Diabetes Society of Chinese Medical Association. The guideline of prevention and treatment of type 2 diabetes in China (2013 version). Chinese Journal of Diabetes. 2014;22(08):2–42. | ||
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Zhang T, Wang XM, Li X, Tang T. Clinical curative effects of epalrestat combined α-lipoic acid in the treatment of diabetic peripheral neuropathy. Chinese Journal of Modern Drug Application. 2014;8(3):138–139. | ||
Wang J, Chen YD. The clinical effect of lipoic acid combined with epalrestat in the treatment of diabetic peripheral neuropathy. Medical Information. 2014;27(10):222. | ||
Sun DJ, Gu W, Liu QQ. Clinical Observation of Epalrestat Combined with Lipoic Acid in the Treatment of Diabetic Peripheral Neuropathy. China Pharmacy. 2017;28(23):3226–3229. | ||
He YM, Li XH, Yin Z. The efficacy and safety of epalrestat combined with α-lipoic acid in the treatment of diabetic peripheral neuropathy in elderly patients. Chinese Journal of Geriatric Care. 2013;11(3):51–52. | ||
Fang MF. Clinical observation of thioctic acid combined with epalrestat for diabetic peripheral neuropathy. Journal of Modern Medicine & Health. 2014;30(04):483–484. | ||
Liu HM. Clinical effects of epalrestat combined with α-lipoic acid in the treatment of diabetic peripheral neuropathy. Diabetes New World. 2014;34(16):17–19. | ||
Yan B. Clinical Efficacy of α-lipoic Acid Combined with Epalrestat in the Treatment of Type 2 Diabetic Peripheral Neuropathy. Medical Science Journal of Central South China. 2015;43(02):169–171. | ||
Qu P, Zeng JE. α-lipoic acid combined with epalrestat treating diabetic peripheral neuropathy: 25 cases report. Journal of Yangtze University. 2009;6(4):33–34. | ||
Deng XZ. Clinical observation of epalrestat combined with α-lipoic acid in treating diabetic peripheral neuropathy. Contemporary Medicine. 2011;17(36):135–136. | ||
Luo F, Hu JP, Li QC. Clinical observation of α-lipoic acid combined with epalrestat in the treatment of diabetic peripheral neuropathy. Chinese Journal of Practical Medicine. 2013;40(14):61–62. | ||
Han LY. Effects of combination of lipoic acid, epalrestat and Novomix 30 on diabetic perineuropathy patients. China Medicine. 2012;7(2):163–165. | ||
Wang WL, Zhu H, Wang SX, Wang JY. The efficacy of epalrestat combined with α-lipoic acid in the treatment of diabetic peripheral neuropathy in elderly patients. Chinese Journal of Gerontology. 2013;33(19):4854–4856. | ||
Yang CZ, Zhang YH. α-lipoic acid combined with epalrestat in the treatment of diabetic peripheral neuropathy: 50 cases report. World Latest Medicine Information. 2012;12(7):65–66. | ||
Huang AH. Effect of α-lipoic acid and epalrestat in treating type 2 diabetes mellitus peripheral neuropathy. Chinese Journal of Clinical Rational Drug Use. 2016;9(06):3–5. | ||
Qi JX. The efficacy of α-lipoic acid combined with epalrestat in the treatment of type 2 diabetic peripheral neuropathy. World Latest Medicine Information. 2016;16120(73):124. | ||
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. | ||
Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–1795. | ||
Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167(4):465–471. | ||
Daugherty DJ, Marquez A, Calcutt NA, Schubert D. A novel curcumin derivative for the treatment of diabetic neuropathy. Neuropharmacology. 2018;129:26–35. | ||
Nasiry D, Khalatbary AR, Ahmadvand H, Talebpour Amiri FT, Akbari E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complement Altern Med. 2017;17(1):476. | ||
Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig. 2011;2(1):33–42. | ||
Wang XT, Liin HX. Clinical Efficacy of α-Lipoic Acid versus Epalrestat in the Treatment of Diabetic Peripheral Neuropathy: A Systematic Review. China Pharmacy. 2017;28(6):786–790. | ||
Tang J, Liu C, Yan SY. Efficacy of Mecobalamin versus α-Lipoic Acid in the Treatment of Diabetic Peripheral Neuropathy: a Meta-analysis. China Pharmacy. 2015;27:3800–3802. | ||
Androne L, Gavan NA, Veresiu IA, Orasan R. In vivo effect of lipoic acid on lipid peroxidation in patients with diabetic neuropathy. In Vivo. 2000;14(2):327–330. | ||
Haak E, Usadel KH, Kusterer K, et al. Effects of alpha-lipoic acid on microcirculation in patients with peripheral diabetic neuropathy. Exp Clin Endocrinol Diabetes. 2000;108(3):168–174. | ||
Kamenova P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones. 2006;5(4):251–258. | ||
Zhang JC, Tang SL, Wang LH, Yang J, Zhang Y. Clinical effects of alpha-lipoic acid combined with epalrestat in elderly patients with diabetic peripheral neuropathy. Chinese Journal of Geriatrics. 2017;36(3):287–291. | ||
Li F. Curative effect of epalrestat or alprostadil combined with α-lipoic acid in the treatment of elderly patients with diabetic peripheral neuropathy. Guide of China Medicine. 2016;14(07):170. | ||
Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(18):18. |
Supplementary materials
  | Table S1 Represents the search strategy for PubMed, CNKI |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




