Back to Journals » OncoTargets and Therapy » Volume 13
ALK Rearrangement Adenocarcinoma with Histological Transformation to Squamous Cell Carcinoma Resistant to Alectinib and Ceritinib
Authors Kaiho T, Nakajima T , Iwasawa S, Yonemori Y, Yoshino I
Received 30 October 2019
Accepted for publication 17 January 2020
Published 19 February 2020 Volume 2020:13 Pages 1557—1560
DOI https://doi.org/10.2147/OTT.S236706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Taisuke Kaiho,1 Takahiro Nakajima,1 Shunichiro Iwasawa,2 Yoko Yonemori,3 Ichiro Yoshino1
1Department of General Thoracic Surgery, Chiba University Graduate School of Medicine, Chiba, Japan; 2Department of Respirology, Chiba University Graduate School of Medicine, Chiba, Japan; 3Department of Pathology, Chiba Rosai Hospital, Chiba, Japan
Correspondence: Takahiro Nakajima
Department of General Thoracic Surgery, Chiba University Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan
Tel +81-43-222-7171
Fax +81-43-226-2172
Email [email protected]
Abstract: Specific tyrosine-kinase inhibitors (TKIs) are widely used for the treatment of non-small-cell lung cancers with anaplastic lymphoma kinase (ALK) translocations. However, most treated patients eventually develop resistance to the TKIs. The histological transformation into small cell carcinoma is well known to be the underlying mechanism for acquired resistance; however, transformation to squamous cell carcinoma is extremely rare. We, herein, report a case of ALK rearrangement-positive adenocarcinoma that transformed to squamous cell carcinoma after administration of alectinib, and was found to be resistant to ceritinib.
Keywords: lung adenocarcinoma, anaplastic lymphoma kinase, transformation, squamous cell carcinoma, acquired resistance
Case Presentation
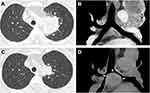
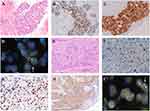
A 53 year old female, never-smoker, was referred to our hospital for an abnormal chest shadow with bloody sputum. The chest computed tomography (CT) scan revealed an 8.0 cm mass adjacent to the mediastinum in the left upper lobe (Figure 1A). Moreover, multiple lymph nodes (LNs) (mediastinal and right subclavicular) and the right adrenal gland were found to be enlarged (Figure 1B). Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed on the right superior mediastinal LN (station 4R), resulting in a diagnosis of adenocarcinoma (Figure 2A). Immunohistochemistry revealed positivity for thyroid transcription factor-1 (TTF-1) (Figure 2B) and anaplastic lymphoma kinase (ALK) (Figure 2C). The status of p40 immunohistostaining was judged as negative (weakly and sporadically positive (<10%)) in the first biopsy sample. Finally, the left lung adenocarcinoma was evaluated as c-T4N3M1c, c-stage IVB possessing ALK gene rearrangement, and the patient was administered alectinib.
Four months after this administration, bloody sputum and enlargement of a subcarinal LN were noted. The chest CT scan showed stenosis of the bilateral main bronchus due to the enlarged subcarinal node, suggesting therapeutic failure (Figure 1C and D). During bronchoscopy, it was revealed that an endobronchial tumor arising from the carina could have caused the stenosis. Emergently, the endobronchial tumor was mechanically cored out using a rigid bronchoscope, and the bronchial lumen was secured with a tracheobronchial stent (AERO stent; Merit Medical, Salt Lake City, UT, USA). After endobronchial treatment, the bronchial symptoms subsided, and ceritinib was administered instead of alectinib. The cored-out tumor was histologically diagnosed as being transformed to squamous cell lung carcinoma (Figure 2E) that was negative for TTF-1 (Figure 2F) and positive for p40 (Figure 2G). Additional immunohistochemical analysis showed positivity for ALK (Figure 2H), and subsequent fluorescence in situ hybridization (FISH) using break-apart probes (Vysis ALK Break Apart FISH Probe Kit; Abbott, Abbott Park, IL, USA) confirmed ALK rearrangement, which was consistent with the findings from the primary LN biopsy. The ALK-FISH positivity was 92.0% in both the samples (Figure 2D and I). After ceritinib administration, the tumor progressed. Despite one cycle of cytotoxic chemotherapy and radiation, the patient’s respiratory condition deteriorated due to tumor extension to the airway, and she died six months after starting alectinib treatment.
Discussion
A histologic transformation has been reported as a mechanism of acquiring resistance to molecular-targeted drugs for lung cancer harboring aberrant driver genes. Several reports of histologic transformation, especially transformation to small cell lung cancer (SCLC), have been reported during the treatment with EGFR tyrosine kinase inhibitors (EGFR-TKIs).1 The histologic transformation, mainly to SCLC,2 has also been observed for ALK-rearranged adenocarcinomas as a mechanism of acquired resistance to ALK-TKIs, in addition to ALK amplification, ALK secondary mutation,3 and activation of bypass pathways.4
In the present case, the histologic transformation of endobronchial tumor to squamous cell carcinoma was observed with ALK aberrant fusion, just as with the initial diagnosis. ALK rearrangement was confirmed by both immunohistochemistry and FISH, despite of histologic differences. Initially, the tumor (adenocarcinoma) showed good sensitivity to alectinib, whereas the endobronchial tumor (squamous cell carcinoma) showed resistance to alectinib and ceritinib despite of harboring the same ALK fusion.
In spite of the administration of effective tyrosine kinase inhibitors, the patient showed failed response to molecular-targeted therapeutic agents. The current clinical approaches for such patients include 1) administrating third-generation ALK inhibitors, such as lorlatinib or brigatinib, 2) applying clinical sequencing to explore the unknown molecular mechanisms of resistance, 3) trying the novel combination chemotherapy, including immune-checkpoint inhibitors.
The main limitation of this case report is that the histological diagnosis was made based on the 2015 World Health Organization classification of lung tumors.5 The small biopsied specimen was diagnosed based on an immunohistochemistry panel. The initial diagnosis from the metastatic LN was adenocarcinoma with no findings of squamous cell carcinoma based on either the histological characteristics or immunohistochemistry. However, there was the possibility of a minor squamous cell carcinoma component within the tumor. Although different histological components co-existed within the tumor, ALK fusion was observed in both the mediastinal LNs and the endobronchial tumor.
The ethics committee at Chiba University accepted this study (No.3627), and written informed consent was provided by the patient’s next of kin for the case details and accompanying images to be published.
Conclusion
We report a case of ALK rearrangement-positive adenocarcinoma that transformed to squamous cell carcinoma after administration of alectinib and was found to be resistant to ceritinib as well.
Disclosure
Dr Takahiro Nakajima reports personal fees from Olympus Medical Systems, personal fees from AstraZeneca, outside the submitted work. Dr Shunichiro Iwasawa reports personal fees from Chugai Pharmaceutical Co., Ltd., during the conduct of the study; grants from Ono Pharmaceutical Co., Ltd., personal fees from Daiichi Sankyo Company, Limited, personal fees from AstraZeneca K.K., outside the submitted work. Dr Ichiro Yoshino reports consultancy and personal fees from Intuitive Surgical; Honoria from Boehringer Ingelheim; personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Johnson&Johnson, Covidien, Pfizer, Astra Zeneca, outside the submitted work. The authors report no other conflicts of interest in relation to this study.
References
1. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–e172. doi:10.1016/S1470-2045(14)71180-5
2. Cha YJ, Cho BC, Kim HR, Lee HJ, Shim HS. A case of ALK-rearranged adenocarcinoma with small cell carcinoma-like transformation and resistance to crizotinib. J Thorac Oncol. 2016;11(5):e55–e58. doi:10.1016/j.jtho.2015.12.097
3. Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734–1739. doi:10.1056/NEJMoa1007478
4. Isozaki H, Takigawa N, Kiura K. Mechanisms of acquired resistance to ALK inhibitors and the rationale for treating ALK-positive lung cancer. Cancers. 2015;7(2):763–783. doi:10.3390/cancers7020763
5. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.