Back to Journals » Journal of Inflammation Research » Volume 12
Alginate encapsulation for bupivacaine delivery and mesenchymal stromal cell immunomodulatory cotherapy
Authors Davis MS , Marrero-Berrios I , Perez I , Rabolli CP , Radhakrishnan P , Manchikalapati D, SchianodiCola J , Kamath H, Schloss RS, Yarmush J
Received 30 October 2018
Accepted for publication 28 December 2018
Published 12 March 2019 Volume 2019:12 Pages 87—97
DOI https://doi.org/10.2147/JIR.S192749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Mollie S Davis, 1 Ileana Marrero-Berrios, 1 Isabel Perez, 1 Charles P Rabolli, 1 Palangat Radhakrishnan, 2 Devasena Manchikalapati, 2 Joseph SchianodiCola, 2 Hattiyangangadi Kamath, 2 Rene S Schloss, 1 Joel Yarmush 2
1Department of Biomedical Engineering, Rutgers University, Piscataway, NJ, USA; 2Department of Anesthesiology, New York Methodist Hospital, Brooklyn, NY, USA
Purpose: Mesenchymal stromal cells (MSCs) are used to treat various inflammatory conditions. In parallel, to mitigate pain associated with inflammation, analgesics or opioids are prescribed, often with significant side effects. Local anesthetics (LAs) offer a promising alternative to these medications. However, their short duration and negative effects on anti-inflammatory MSCs have limited their therapeutic effectiveness. To mitigate these negative effects and to move toward developing a cotherapy, we engineered a sustained release bupivacaine alginate-liposomal construct that enables up to 4 days of LA release. By encapsulating MSC in alginate (eMSC), we demonstrate that we can further increase drug concentration to clinically relevant levels, without compromising eMSC viability or anti-inflammatory function.
Materials and methods: MSCs were freely cultured or encapsulated in alginate microspheres ± TNFα/IFN-γ and were left untreated or dosed with bolus, liposomal, or construct bupivacaine. After 24, 48, and 96 hours, the profiles were assessed to quantify secretory function associated with LA–MSC interactions. To approximate LA exposure over time, a MATLAB model was generated.
Results: eMSCs secrete similar levels of IL-6 and prostaglandin E2 (PGE2) regardless of LA modality, whereas free MSCs secrete larger amounts of IL-6 and lower amounts of anti-inflammatory PGE2. Modeling the system indicated that higher doses of LA can be used in conjunction with eMSC while retaining eMSC viability and function. In general, eMSC treated with higher doses of LA secreted similar or higher levels of immunomodulatory cytokines.
Conclusion: eMSCs, but not free MSC, are protected from LA, regardless of LA modality. Increasing the LA concentration may promote longer and stronger pain mitigation while the protected eMSCs secrete similar, if not higher, immunomodulatory cytokine levels. Therefore, we have developed an approach, using eMSC and the LA construct that can potentially be used to reduce pain as well as improve MSC anti-inflammatory function.
Keywords: MSC cytokine secretion, local anesthetics, encapsulated MSC, drug diffusion model
Introduction
Inflammatory diseases affect all organs and tissue types. The mechanism of action for these diseases varies by type and tissue; however, it is defined as the immune system’s response to pathogenic organisms or tissue trauma.1 Myeloid cells recognize these pathogens or injured tissue and trigger the release of proinflammatory cytokines, such as tumor necrosis factor (TNF) and interferon gamma (IFN-γ).1,2 These cytokines in turn activate other myeloid cells and epithelial cells, which help facilitate the infiltration of additional immune or inflammatory cells and also play a role in inflammatory symptoms, such as fever, redness, swelling, and pain.1
Local anesthetics (LAs) are commonly used to mitigate pain3 by reversibly blocking the sodium-gated channels on axons, thereby halting the progression of the pain signal to the brain.3–5 Reduced pain following surgical procedures has been shown to reduce healing time6,7 and hospital stays and costs.7,8 However, despite their advantages, LAs, such as bupivacaine, have been shown to have negative effects on various non-target cell types, including chondrocytes,9,10 cardiomyocytes,11,12 and various muscle cell types.13 Therefore, as therapies involving mesenchymal stromal cells (MSCs) become more prevalent as a treatment option for immunological and inflammatory diseases,14 understanding how LAs affect MSCs has become critically important.9,15,16
MSCs are multipotent, adult-like stem cells that can be isolated from several tissue sources17–21 and can control multiple physiological functions via paracrine secretion.17,19,22,23 However, LAs, in a time- and potency-dependent manner, affect MSC proliferation, secretion,16 viability,9,15,16,22,24 differentiation potential,10 and adhesion.9 For example, our laboratory has previously shown that in the presence of bolus bupivacaine, MSCs secrete reduced levels of IL-6 and prostaglandin E2 (PGE2), a constitutively secreted cytokine and a key mediator responsible for MSC immunomodulatory function, respectively. Therefore, to preserve MSC immunomodulatory function, as well as provide sustained and controlled pain relief, new LA delivery methods must be engineered.
Controlled and sustained release methods for LA delivery have been designed to help mitigate the negative effects of bolus LA delivery. These methods include LA nano- and microparticles,26,27 injectable liquid polymers,28,29 hydrogels,30,31 and Exparel®, a Food and Drug Administration (FDA)-approved sustained release liposomal bupivacaine.8,32–34 Exparel has been used in several procedures, including knee arthroscopy,35,36 colectomy,32 breast reconstruction,34,37 and hemorrhoidectomy,38 and has been shown to effectively reduce postoperative dependence on opioid-based drugs.32,35,37 However, liposomal bupivacaine, while effective, can be susceptible to dose-dumping39 and may not remain at the site of injury.27
In order to develop an improved LA delivery approach, we have engineered an alginate-liposomal bupivacaine construct (construct), which slows down LA release while enabling positional control at the injury site.39,40 This construct improved in vitro monolayer MSC viability compared to bolus doses,39 as well as IL-6 and PGE2 secretion levels in both normal and inflammatory environments.40 Moreover, the construct also enabled sustained release of bupivacaine for 96 hours, longer than the liposomal-bupivacaine formulation alone.39,40
Since the overall goal is to create a sustained release LA system to enable a cotherapy of LA pain mitigation and MSC inflammation modulation, a method to stabilize the cells at the site of injury is necessary. Encapsulating the MSCs (encapsulated MSC [eMSC]) in alginate microspheres provides positional control of the cells at the injected location, may protect them from the adverse effects of direct implantation at the injury site,41 and preserves MSC anti-inflammatory function.17 Our group has previously shown that eMSCs can remain viable for at least 2 months without differentiating or proliferating significantly17 while maintaining their constitutive secretion of regulatory cytokines and growth factors, such as PGE2. The current studies were designed to investigate the effect of bupivacaine delivery method on eMSC immunomodulatory function. The results of our studies indicate that alginate encapsulation provides several therapeutic benefits including 1) limiting drug penetration into the eMSC microenvironment thereby preserving eMSC function and 2) providing positional control of co-administered LA and cells.
Materials and methods
Chemicals and reagents
Bupivacaine and other chemicals were purchased from Sigma-Aldrich Co. (St Louis, MO, USA), unless otherwise stated. All cell culture reagents and growth factors were purchased from Thermo Fisher Scientific (Waltham, MA, USA), unless otherwise stated.
Liposomes containing bupivacaine
Liposomes were created using a dehydration–rehydration protocol, as previously described by Maguire et al.39 Hydrogen soy phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL, USA) and cholesterol were combined in a 7:3 molar ratio to create a lipid in chloroform, which was placed on a rotovap to evaporate the solvent and create a lipid film. The film was rehydrated with water and snap frozen and lyophilized overnight. The lipid was resuspended in 70 mg/mL bupivacaine-HCl and extruded through a 200 µm membrane to produce small unilamellar liposomes. To purify the liposomes, the solution was eluted through a sephadex G-50 size exclusion column with 0.9% saline. Bupivacaine concentration was determined using a UV/Vis spectrophotometer (DU730 Life Science UV/Vis Spectrophotometer; Beckman Coulter, Brea, CA, USA) at a wavelength of 264 nm.
Alginate encapsulation of liposomes
As previously described in the study by Maguire et al,39 various concentrations of bupivacaine-loaded liposomes were mixed with filtered 2.2 wt% ultrapure alginic acid (MW: 100,000–200,000 g/mol, G content: 65%–70%; FMC Biopolymer AS, Sandvika, Norway). The alginate–liposome mixture was left to polymerize for 10 minutes at room temperature in a bath of 100 mM CaCl2, 145 mM NaCl, and 10 mM MOPS.
MSC culture and treatment conditions
Human bone marrow-derived MSCs (Institute for Regenerative Medicine, Texas A&M College of Medicine) were cultured as previously described by Maguire et al.39 Briefly, MSCs at passage 2 were thawed and cultured as a monolayer at 1,714 cells/cm2 in a T-175 flask in a humidified 37°C, 5% CO2 incubator.18 Minimum Essential Medium α containing no deoxy- or ribonucleotides and supplemented with 10% FBS (Atlanta Biologicals, Flowery Branch, GA, USA), 2 mM l-glutamine, 1 ng/mL basic fibroblast growth factor, 100 U/mL penicillin, and 100 mg/mL streptomycin was added to the cells and was allowed to grow until 70% confluence. For monolayer cell controls (free MSC), the cells were then trypsinized and replated at 12,000 cells/well in a 24-well plate (6,000 cells/cm2) and allowed to attach overnight. For encapsulated cells, see “Alginate encapsulation of MSCs”.
Fresh media, with or without activating cytokines (25 ng/mL of TNF-α and IFN-γ; R&D Systems, Minneapolis, MN, USA), replaced the previous media. Transwell inserts (Corning Incorporated, Corning, NY, USA) containing the different treatment conditions (media, bolus bupivacaine, bupivacaine-loaded liposomes, or alginate–liposome construct) were added to the wells containing MSCs, both free and encapsulated, and incubated for 24, 48, or 96 hours in the same conditions as described earlier (37°C, 5% CO2 incubator). At the end of each time point, the supernatants were removed and saved for cytokine analysis (see “Cytokine measurement”), and the cells were stained via Hoechst and LIVE/DEAD cell staining, as described previously (see “Cell viability”).17,22
Alginate encapsulation of MSCs
MSCs were thawed and expanded as described earlier. At 70% confluence, cells at passage 2 were then trypsinized and seeded into 225 cm2 flasks for further expansion. The cells were grown to 70% confluence, trypsinized, and encapsulated in alginate at passage 4 as described previously.17,22 Briefly, MSCs were suspended in nonsupplemented Ca2+-free DMEM and subsequently mixed with 2.2% (w/v) alginate in Ca2+-free DMEM to achieve a final cell density of 4×106 cells/mL and a final alginate concentration of 1.98% (w/v).22 These parameters were chosen specifically to maintain high cell viability and the undifferentiated MSC phenotype.17 Alginate-MSC microspheres (450–550 µm in diameter) were produced by an electrostatic bead generator (Nisco Engineering AG, Zurich, Germany) and gelled by ionic crosslinking. The microspheres were incubated for 10 minutes in a crosslinking solution containing 100 mM calcium chloride, 145 mM sodium chloride, 10 mM MOPS, and 5 g/L glucose in deionized water (pH 7.2). eMSCs were then washed with PBS and coated with poly-l-lysine (PLL) by incubation in a 0.05% (w/v) solution. After a final PBS wash, eMSCs were suspended in fully supplemented minimum essential medium eagle alpha (MEM-α) and transferred to a 25 cm2 flask overnight.
Cell viability
Before use, eMSC viability was determined by Hoechst 33342 and LIVE/DEAD cell staining, as described previously.17,22 Briefly, eMSCs were incubated for 30 minutes with fresh media containing calcein AM (Molecular Probes), ethidium homodimer (Molecular Probes, Eugene, OR, USA), and Hoechst 33342 following vendor’s instructions. eMSCs were washed three times with media before imaging with an Olympus IX81 spinning disk confocal microscope. Five hundred-micrometer Z stacks at 20 µm intervals were acquired for 10 capsules per condition and analyzed for live cells (green fluorescence) and dead cells (red fluorescence) using Slidebook 5.0 (3i; Intelligent Imaging Innovations, Denver, CO, USA) and ImageJ (NIH) software. The total number of live and dead cells per capsule was quantified, and the percentage viability (%) was calculated by dividing the live cell number by the total cell number in the capsule. Finally, the percentage viability (%) was averaged among the 10 capsules. In experiments, eMSCs were cultured in 24-well plates at 12,000 cells/well.
Cytokine measurement
Cell culture supernatants were thawed and tested for IL-6 and PGE2 using ELISA (Biolegend, San Diego, CA, USA and Cayman Chemical, Ann Arbor, MI, USA). All were run according to manufacturer’s instructions, and absorbance was read using a microplate reader (DTX880 Multimode Detector; Beckman Coulter). Supernatants were also used for a bead-based multiplex immunoassay (Bio-Plex Pro™ Human Cytokine Standard 27-Plex, Group I; Bio-Rad Laboratories Inc., Hercules, CA, USA), which measured 27 human cytokines, chemokines, and growth factors. Analysis was conducted according to manufacturer’s instructions and read using the Bio-Plex 200 system (Bio-Rad Laboratories Inc.).
Computational model
HPLC data presented in Maguire et al39 was used to determine the overall release profile of bupivacaine from liposomes and the engineered construct, as shown in Davis et al40 Using a half-life of 2.7 hours,42 the diffusion of bupivacaine from the LA modality (bolus, liposome, or construct) to MSCs encapsulated in alginate microspheres was modeled using MATLAB (The MathWorks Inc, Natick, MA, USA). A one-dimensional Implicit Finite Difference model of diffusion was conducted using corning 24-well plate and transwell dimensions, with an alginate height of 1.3 mm within the transwell. Construct was considered to have drug equally dispersed throughout, as demonstrated in the study by Maguire et al,39 and a diffusivity constant of 8.5e–15 m2/s.39 The diffusivity constants for bupivacaine within media and alginate are 6.71e–10 m2/s43 and 2.77e–7 cm2/s,44 respectively. The LA was placed in a transwell, and the distance between the transwell and the top of the alginate microspheres is 0.4 mm. The computational model used the center of the 500 µm alginate microspheres as the measurement point, with an average of 100 live cells per microspheres and 115 microspheres per well. Gravity was assumed to be negligible.
Statistics
Statistical differences among data sets were determined using ANOVA followed by Fisher’s least significant difference (LSD) post hoc analysis. Significance level was α=0.05 in Kaleidagraph software version 4.1 (Synergy Software, Reading, PA, USA). Data points represent mean ± standard error of the mean (SEM) for the indicated number of independent observations (n).
Results
Effect of engineered hydrogel liposome construct on basal eMSC secretion
Our ultimate goal is to develop a coadministered therapy that would include both pain mitigation and immunomodulation. We previously developed an alginate eMSC construct with superior anti-inflammatory function relative to free MSC. Therefore, we evaluated the effect of bupivacaine exposure on eMSC function. The effect of bupivacaine presentation on IL-6 and PGE2 eMSC secretory function was examined. IL-6 is a cytokine that is constitutively secreted in large amounts by MSC. As seen in Figure 1A, the concentration of eMSC-secreted IL-6, regardless of LA modality, remained unchanged relative to media control. Free monolayer MSCs continue to proliferate in culture and secreted significantly higher levels of IL-6 compared to eMSCs at 96 hours.
MSC immunomodulatory functions are of key importance for use in a cotherapy with LAs. Therefore, PGE2 secretion was analyzed. We previously demonstrated that PGE2 is a key anti-inflammatory mediator of inflammatory M1 macrophage attenuation. As seen in Figure 1B, regardless of LA modality, all eMSCs produced similar PGE2 concentrations. Consistent with our previous studies and despite the difference in MSC proliferation,17 monolayer MSCs produce significantly less PGE2 than their encapsulated counterparts at all time points.
Effect of engineered hydrogel liposome construct on activated eMSC secretion
The engineered construct has been designed for use after injury, where the environment contains inflammatory stimuli. Therefore, IL-6 and PGE2 levels were analyzed in activated conditions to mimic the inflammatory environment. As seen in nonstimulated conditions, eMSCs produce similar amounts of IL-6 regardless of LA mode, but in all cases lower than the amount secreted by free monolayer MSCs (Figure 2A). Next we examined PGE2 secretion, as our previous studies indicated that this molecule was solely responsible for MSC attenuation of M1 macrophages and promotion of the alternative M2 phenotype. Stimulated eMSC PGE2 production follows a similar trend to nonstimulated PGE2 production, where the eMSCs produce similar amounts at all time points regardless of LA modality. Notably, eMSC PGE2 secretion is significantly higher than free monolayer MSC production at all time points (Figure 2B).
MATLAB modeling
Given the relative secretory stability of eMSC in the presence of all delivery modes of bupivacaine, we investigated whether the effect could be explained by diffusional exclusion out of the eMSC capsule. MATLAB modeling was used to help determine the drug concentration percentage over time that would be exposed to the encapsulated cells. As seen in Figure 3, at no time point during the construct delivery system does the bupivacaine concentration reach 10% of the initial dose (1 mM), which is considered a toxic bupivacaine level, whereas the bolus release reaches a maximum of 18% in the first 8 hours (Figure 3). For the concentration of bupivacaine to reach cytotoxic levels (0.1 mM) in eMSC during any 8-hour period, the minimum initial concentration of bupivacaine in the construct was predicted to be 9.5 mM. Using this same initial dose, the concentration of bupivacaine during the first 8 hours from the bolus release would be 1.8 mM, which would be 18 times more concentrated than the minimum concentration that we have previously shown to be toxic for MSC.
  | Figure 3 Computational model of eMSC and LA. Notes: Using HPLC data from the study by Maguire et al39 and a half-life of 2.7 hours,42 a MATLAB explicit finite difference method was generated to determine the concentration of drug in the presence of eMSCs at a given time point. By 8 hours, <1% of the initial dose of 1 mM from the construct reached the cells, whereas 18.06% of the bolus drug reached the cells in the same time period. Using the construct, <1% was present at any time point for the total 96 hours, yielding an overall total accumulation of 8.1% assuming no internalization of the drug. However, as the bupivacaine is constantly degrading over time, we were more concerned with the concentration of bupivacaine that reached the cells during any 8-hour time period. Abbreviations: eMSC, encapsulated mesenchymal stromal cell; LA, local anesthetic. |
Effect of increased LA concentration in the engineered hydrogel liposome construct on eMSC secretion
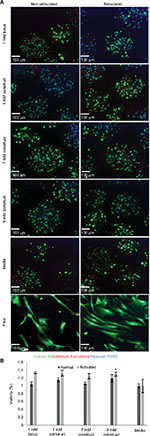
The MATLAB model indicated that larger concentrations of bupivacaine may be used in the construct in conjunction with eMSC while still retaining eMSC viability and immunomodulatory secretions. Using this model, we back-calculated to a maximum drug concentration of 9.5 mM while still retaining a cell apparent dose of 0.1 mM, the 90% viability cutoff for MSC.22,39 As can be seen in Figure 4A and B, despite being dosed with these higher LA concentrations, encapsulated cells are similarly viable to free MSCs exposed to lower LA concentrations as well as eMSC media controls.
Moreover, IL-6 secretion levels were significantly higher at 96 hours for eMSC in the presence of 7 and 9 mM LA constructs compared to bolus and media conditions, although still lower than the amount secreted by free MSC, in part due to the proliferative capabilities of free MSC vs eMSC (Figure 5A). eMSC PGE2 secretion also followed similar trends. As seen in Figure 5B, the construct, including the higher LA concentrations, yielded significantly higher levels of PGE2 compared to media and bolus conditions at 48 and 96 hours.
Under inflammatory conditions, simulated by the addition of IFN-γ and TNF-α, the same trends are present. For IL-6, 7 mM construct yielded the highest relative secretion levels, with 9 mM slightly lower (Figure 5C). PGE2 secretion levels were highest with 7 and 9 mM, significantly higher than media, bolus, and 1 mM construct at 48 and 96 hours time points (Figure 5D).
To further understand the effect of higher LA concentrations on the anti-inflammatory potential of eMSC in an inflammatory environment, a Bio-Plex Pro™ Human Cytokine Standard 27-Plex, group 1 assay was run on the stimulated conditions. Relative levels of immunomodulatory cytokines represented by this panel are similar even when the dose is increased to clinically relevant LA levels. Therefore, our data indicate that by alginate encapsulating both MSC and LA, MSC secretory and anti-inflammatory function is preserved even when higher initial LA doses are used.
Discussion and conclusion
MSC-based therapies have been developed as viable treatment options for multiple inflammatory diseases such as rheumatoid arthritis, multiple sclerosis, acute and chronic wounds, and inflammatory skin conditions.45,46 In many of these diseases, the number of inflammatory cells increases. For instance, in rheumatoid arthritis, infiltrating T-cells stimulate other immune cells to promote more inflammation.45 MSCs have been shown to inhibit this cascade by inhibiting proliferation and activation of these T-cells.45 In multiple sclerosis, MSC treatment has been shown to reduce the severity of the disease.47 Osteoarthritis (OA) is different than these other diseases in that the inflammation is related to an increase in chemokines and cytokines released by the inflamed chondrocytes and synovial cells and not by the infiltration of additional immune cells. Nevertheless, MSCs have the ability to modulate this form of inflammation as well, and although it has been shown that MSCs from different donors secrete different cytokine concentrations, the overall cytokine trends remain constant.22,48 The cytokines they secrete can attenuate macrophage inflammation by promoting anti-inflammatory macrophages instead of their proinflammatory phenotype.22 Therefore, OA, a local disease, may be a perfect candidate for our proposed dual therapy.
OA and other inflammatory diseases exhibit cellular and cytokine influx associated with the inflammatory response, which secondarily leads to significant pain levels, especially in chronic conditions. LA offers a promising pain mitigation alternative, especially if longer duration delivery approaches can be developed. However, current pain mitigation treatments, including LA, may not allow for viable cellular therapies, and therefore there is a need for a system that can preserve MSC functionality at clinically relevant LA levels to develop a multimodal therapy.
LAs are typically injected at the local area in a liquid form, which, while fast acting, has a short duration of 30 minutes to 8 hours. The only FDA-approved LA sustained release formulation is Exparel, a liposomal bupivacaine system. Exparel claims to last for up to 72 hours; however, due to the small size of liposomal LA, the liposomes can migrate from the injection site.27 Additionally, liposomes tend to have an initial burst dose-dumping,39 which may lead to drug concentrations that are cytotoxic. Other non-FDA-approved sustained release formulations, such as Posimir®, have only been able to show pain mitigation for up to 48 hours. These sustained release systems are typically used for postoperative pain, not pain associated with inflammation.
In order to improve the localization, release profile, and duration of LA delivery, we have developed a controlled release formulation composed of liposomal bupivacaine embedded in alginate.39,40 We have previously demonstrated that our construct allowed for superior diffusivity control.39,40 Both computational and in vitro data demonstrated that incorporation of alginate provided a sustained release profile of bupivacaine for at least 4 days. When compared to other LA formulations, we have also shown that this formulation mitigates the detrimental effects of LA on monolayer MSCs,40 which could allow for the coadministration of LA for long-lasting pain control in conjunction with MSC therapies to provide tissue healing and regeneration for patients. In addition, MSCs can be coencapsulated with the construct to improve the efficacy of MSC therapy and further augment cell therapeutic outcomes. Our studies were designed to evaluate the construct’s effects on alginate-encapsulated MSC secretory function.
To evaluate the efficacy of our system as a dual therapy, we exposed eMSCs to several bupivacaine formulations and assessed their secretome function. Interestingly, the in vitro eMSC cytokine analysis indicated that regardless of LA modality, the cells are protected by the alginate bead and produce similar levels of IL-6 and PGE2 (Figure 1). The increase in PGE2 in the presence of the construct is not completely understood and further investigation is needed to determine if it is an LA or alginate-induced response. No differences were seen in inflammatory conditions, with all encapsulated conditions secreting similar amounts and monolayer-free MSCs secreting more and less IL-6 and PGE2, respectively, as expected (Figure 2). These results differ from our previous observations with monolayer MSC, where altered secretion patterns were dependent on the LA formulation and the inflammatory state of the system,25,40 which highlights the significance of alginate microencapsulation as a method to preserve MSC cell function.
To further explain the in vitro analysis and understand how the system will interact with a cell therapy, a computational model was established to determine the amount of drug exposure the cells receive. Based on previous data,22 for the cells to remain 90% viable, only 0.1 mM of bupivacaine should be present at a given time. The model shows that 8.1% of the initial dose of 1 mM bupivacaine actually reaches the cells over the 96 hours analyzed (Figure 3), which is less than the 10% margin necessary for viability. Therefore, the eMSCs would be protected from sustained released bupivacaine, whereas 18% of the initial 1 mM released in the bolus dose reaches the cells within 8 hours (Figure 3). This model indicates that a starting concentration of 9.5 mM is possible within a construct in conjunction with eMSC while retaining the cell viability; however, a 9.5 mM bolus dose would kill the cells, both free and eMSC.
Higher starting concentrations of LA within a construct were analyzed in conjunction with the eMSC to determine the viability and immunomodulatory functionality of the cells. IL-6 and PGE2 secretion levels are higher in the presence of higher LA concentrations, yet the viability remains relatively constant across all conditions (Figure 4). Interestingly, we observed a modest but statistically significant higher number of viable cells in the eMSC treated with the 9 mM construct when compared to media controls. This phenomenon will need further investigation to understand the complex cellular responses in our system to LA dose and the cell microenvironment. The multiplex analysis indicated that even with increased LA concentration, even at clinically relevant levels, cytokine secretion remained relatively unchanged (Figure 6).
Overall, we have demonstrated that the construct should be able to mitigate pain over an extended period of time via bupivacaine release. Furthermore, the bupivacaine construct more effectively limited eMSC exposure and hence preserved cell viability. Although the eMSC secretory functions that were quantified in our studies were relatively stable despite the delivery mode, other functions may be more sensitive to small changes in drug exposure. In either case, alginate encapsulation of both LA-containing liposomes and MSC provides a superior dual therapy for pain mitigation and anti-inflammatory control due to its potential for 1) positional control, 2) improved drug release profiles for pain mitigation, and 3) sustained eMSC immunomodulatory secretions. Nevertheless, even though this prospective therapy presents an exciting option for further clinical studies, it is important to acknowledge that our observations are based on in vitro studies that do not consider the different in vivo cellular niches. Therefore, additional studies are necessary to draw conclusions about the clinical efficacy of this treatment. In the future, this therapy can be used together or separately to help treat various inflammatory conditions.
Acknowledgments
Research reported in this manuscript was supported by the National Institute of General Medical Sciences of the National Institute of Health under award number T32 GM008339 as well as the United States Department of Education Graduate Assistance in Areas of National Need under award number P200A150131.
Disclosure
The authors report no conflicts of interest in this work.
References
Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol. 2017;18(8):826–831. | ||
Moretto MM, Weiss LM, Cl C, Ia K, Combe CL, Khan IA. IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection. J immunol (Baltimore, Md. 1950). 2007;179(4):2485–2492. | ||
Lirk P, Picardi S, Hollmann MW, Anaesthetics L. Local anaesthetics. Eur J Anaesthesiol. 2014;31(11):575–585. | ||
Ballieul RJ, Jacobs TF, Herregods S, van Sint Jan P, et al. The peri-operative use of intra-articular local anesthetics: a review. Acta Anaesthesiol Belg. 2009;60(2):7. | ||
Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Progr. 2012;59(2):90–102. | ||
Gouin JP, Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am. 2011;31(1):81–93. | ||
Garimella V, Cellini C, Control PP. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(3):191–196. | ||
Vyas KS, Rajendran S, Morrison SD, et al. Systematic review of liposomal bupivacaine (Exparel) for postoperative analgesia. Plast Reconstr Surg. 2016;138(4):748e–756e. | ||
Dregalla RC, Lyons NF, Reischling PD, Centeno CJ. Amide-type local anesthetics and human mesenchymal stem cells: clinical implications for stem cell therapy. Stem Cells Transl Med. 2014;3(3):365–374. | ||
Breu A, Eckl S, Zink W, Kujat R, Angele P. Cytotoxicity of local anesthetics on human mesenchymal stem cells in Local Anesthetics on Human Mesenchymal Stem Cells in Vitro. Arthroscopy. 2013;29(10):1676–1684. | ||
Bourne E, Wright C, Royse C. A review of local anesthetic cardiotoxicity and treatment with lipid emulsion. Local Reg Anesth. 2010;3:11–19. | ||
Ryu HY, Kim JY, Lim HK, et al. Bupivacaine induced cardiac toxicity mimicking an acute non-ST segment elevation myocardial infarction. Yonsei Med J. 2007;48(2):331–336. | ||
Metterlein T, Hoffmann P, Späth R, Gruber M, Graf BM, Zink W. In vitro myotoxic effects of bupivacaine on rhabdomyosarcoma cells, immortalized and primary muscle cells. Cancer Cell Int. 2015; 15(1):75. | ||
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. | ||
Rahnama R, Wang M, Dang AC, Kim HT, Kuo AC. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J Bone Joint Surg Am. 2013;95(2):132–137. | ||
Lucchinetti E, Awad AE, Rahman M, et al. Antiproliferative effects of local anesthetics on mesenchymal stem cells: potential implications for tumor spreading and wound healing. Anesthesiology. 2012;116(4):841–856. | ||
Barminko J, Kim JH, Otsuka S, et al. Encapsulated mesenchymal stromal cells for in vivo transplantation. Biotechnol Bioeng. 2011;108(11):2747–2758. | ||
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. | ||
Meirelles LS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5–6):419–427. | ||
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. | ||
Hegyi B, Sági B, Kovács J, et al. Identical, similar or different? learning about immunomodulatory function of mesenchymal stem cells isolated from various mouse tissues: bone marrow, spleen, thymus and aorta wall. Int Immunol. 2010;22(7):551–559. | ||
Gray A, Maguire T, Schloss R, Yarmush ML. Identification of IL-1β and LPS as optimal activators of monolayer and alginate-encapsulated mesenchymal stromal cell immunomodulation using design of experiments and statistical methods. Biotechnol Prog. 2015;31(4):1058–1070. | ||
Barminko JA, Nativ NI, Schloss R, Yarmush ML. Fractional factorial design to investigate stromal cell regulation of macrophage plasticity. Biotechnol Bioeng. 2014;111(11):2239–2251. | ||
Gray A, Marrero-Berrios I, Weinberg J, et al. The effect of local anesthetic on pro-inflammatory macrophage modulation by mesenchymal stromal cells. Int Immunopharmacol. 2016;33:48–54. | ||
Gray A, Marrero-Berrios I, Ghodbane M, et al. Effect of local anesthetics on human mesenchymal stromal cell secretion. Nano Life. 2015;5(2):1550001–1550014. | ||
Ohri R, Wang JC, Blaskovich PD, et al. Inhibition by local bupivacaine-releasing microspheres of acute postoperative pain from hairy skin incision. Anesth Analg. 2013;117(3):717–730. | ||
Santamaria CM, Woodruff A, Yang R, Kohane DS. Drug delivery systems for prolonged duration local anesthesia. Mater Today. 2017;20(1):22–31. | ||
Shikanov A, Domb AJ, Weiniger CF. Long acting local anesthetic–polymer formulation to prolong the effect of analgesia. J Contr Release. 2007;117(1):97–103. | ||
Sokolsky-Papkov M, Golovanevski L, Domb AJ, Weiniger CF. Prolonged local anesthetic action through slow release from poly(lactic acid co castor oil). Pharmaceut Res. 2009;26(1):32–39. | ||
Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25(19):4797–4804. | ||
Kim T, Seol DR, Hahm S-C, et al. Analgesic effect of intra-articular injection of temperature-responsive hydrogel containing bupivacaine on osteoarthritic pain in rats. Bio Med Res Int. 2015;2015(9):1–9. | ||
Cohen S. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–572. | ||
Lewis AN. Liposoman Bupivacaine (Exparel). 2013. Available from: https://www.pharmacytimes.com/publications/health-system-edition/2013/january2013/liposomal-bupivacaine-exparel. Accessed December 5, 2017 | ||
Butz DR, Shenaq DS, Rundell VL, et al. Postoperative pain and length of stay lowered by use of Exparel in immediate, implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2015;3(5):e391. | ||
Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530–536. | ||
Mont MA, Beaver WB, Dysart SH, Barrington JW, del Gaizo DJ. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90–96. | ||
Dasta J, Ramamoorthy S, Patou G, Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012;28(10):1609–1615. | ||
Gorfine S R, Onel E, Patou G, Krivokapic Z V. Bupivacaine extended release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial; Dis Colon Rectum. 2011;54(12):1552–1559. | ||
Maguire T, Davis M, Marrero-Berrios I, et al. Control release anesthetics to enable an integrated anesthetic: mesenchymal stromal cell therapeutic. Int J Anesthesiol Pain Med. 2016;2(1):3. | ||
Davis MS, Marrero-Berrios I, Perez XI, et al. Alginate-liposomal construct for bupivacaine delivery and MSC function regulation. Drug Deliv Transl Res. 2018;8(1):226–238. | ||
Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18(7–8):806–815. | ||
FDA. Bupivacaine hydrochloride injection, USP. 2012. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018692s015lbl.pdf | ||
Brounéus F, Karami K, Beronius P, Sundelöf L. Diffusive transport properties of some local anesthetics applicable for iontophoretic formulation of the drugs. Int J Pharm. 2001;218(1–2):57–62. | ||
Li RH, Altreuter DH, Gentile FT. Transport characterization of hydrogel matrices for cell encapsulation. Biotechnol Bioeng. 1996;50(4):365–373. | ||
Sargent A, Miller RH. MSC therapeutics in chronic inflammation. Curr Stem Cell Rep. 2016;2(2):168–173. | ||
Shin TH, Kim HS, Choi S, Kang KS. Mesenchymal stem cell therapy for inflammatory skin diseases: clinical potential and mode of action. Int J Mol Sci. 2017;18(2):244. | ||
Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–227. | ||
Gray A, Schloss RS, Yarmush M. Donor variability among anti-inflammatory pre-activated mesenchymal stromal cells. Technology. 2016;4(3):201–215. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.