Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Aldehyde dehydrogenase 2 rs671G>A polymorphism and ischemic stroke risk in Chinese population: a meta-analysis
Authors Xu YL, Hu YY , Li JW, Zhou L , Li L, Niu YM
Received 27 November 2018
Accepted for publication 3 March 2019
Published 23 April 2019 Volume 2019:15 Pages 1015—1029
DOI https://doi.org/10.2147/NDT.S196175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jun Chen
Yue-Long Xu,1 Yuan-Yuan Hu,2 Ji-Wei Li,1 Lan Zhou,3 Li Li,1 Yu-Ming Niu2
1Department of Neurology, Linyi Central Hospital, Linyi 276400, Shandong Province, People’s Republic of China; 2Department of Stomatology and Center for Evidence-Based Medicine and Clinical Research, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, People’s Republic of China; 3Department of Neurology, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, People’s Republic of China
Introduction: Recently, molecular epidemiological studies have suggested that aldehyde dehydrogenase 2 (ALDH2) rs671 G>A polymorphism may be a risk factor for ischemic stroke (IS). However, the results reported have not been consistent.
Methods: We conducted the meta-analysis to explore the precise association between ALDH2 rs671 G>A polymorphism and IS risk. Five online databases were searched and the relative studies were reviewed from inception to October 1, 2018. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated in each genetic model of the general and subgroup. Furthermore, the heterogeneity, accumulative analyses, sensitivity analyses and publication bias were calculated simultaneously.
Results: Overall, nine case-control studies involving 6,129 subjects were included in this meta-analysis. All studies were focused on the Chinese population and some significant associations were found between ALDH2 rs671 G>A polymorphism and IS risk (A vs G: OR=1.29, 95% CI=1.01–1.65, P=0.04, I2=78.2%; AA vs GG: OR=1.86, 95% CI=1.27–2.21, P<0.01, I2=11.3%; AA vs GG + GA: OR=1.67, 95% CI=1.27–2.19, P<0.01, I2=0%). Some significant and similar results were also observed in the subgroup analysis.
Conclusion: Our meta-analysis indicates that the ALDH2 rs671 G>A polymorphism may play an important role in the occurrence of IS by reducing the activity of ALDH2 and interfering with the metabolic processes involving acetaldehyde.
Keywords: aldehyde dehydrogenase 2, rs671, ischemic stroke, polymorphism, meta-analysis
Introduction
Stroke is the second most common cause of death globally and contributes to 9% of all deaths.1 According to recent epidemiological research, an estimated 6.2 million deaths occur per year due to stroke.2 Due to the aging of the global population, the prevalence of stroke is continuously increasing. Stroke cases and related deaths are projected to increase among people from China, India and other developing countries.3,4 Stroke and its sequela, such as post-stroke hemiplegia, depression and impairment of movement and cognition, were thought to be one of the five leading causes of reduced disability-adjusted life years in 2017. This ranking is expected to rise to the fourth place by the year 2030 in developed countries.5,6
Ischemic stroke (IS) accounts for more than 80% of diagnosed stroke cases in adults.7 High blood pressure, insufficient physical activity, apolipoprotein (Apo)B/ApoA1 ratio, unhealthy living habits and diabetes mellitus contribute to more than 90% of the population attributable risks for IS worldwide.8 However, differences in susceptibility to stroke in individuals of different ethnicities and regions have not been fully explained.
The gene for aldehyde dehydrogenase-2 (ALDH2) is located on chromosome 12q24.12 and contains 13 exons and 12 introns. It encodes a crucial mitochondrial enzyme that is distributed throughout in human cells. The enzyme is known to participate in aldehyde substrate metabolism and detoxification. ALDH2 is the most important enzyme for metabolizing short chain aliphatic aldehydes and promotes the conversion of acetaldehyde to acetate. ALDH2 is widely expressed in neurons in the brain and is believed to have protective effect.9
Single nucleotide polymorphisms (SNPs) are the most common heritable variation in the human genome. Such genetic modification can alter amino acid and their partial structure, influence the expression level and activities of target proteins, and contribute to the risk of various diseases.10 The rs671 polymorphism, which is located in exon 12 and comprises a base substitution from G to A, results in an amino acid change from glutamate to lysine. This is considered the most common locus for polymorphisms in ALDH2, which lead to significant decreases in its enzymatic activity.11 There are differences in the frequency of thers671 G>A polymorphism between individuals of different ethnic groups. The A-allele of the ALDH2 gene is rarely found in Caucasians, Africans and other ethnicities, but is highly prevalent in Asians (between 20% and 50%).12–14 In 2012, Sun conducted the first case-control study of the association between ALDH2 polymorphism and IS, and found that the GA/AA genotype was significantly associated with IS risk.15 Since then, some new studies have addressed the association between the rs671 G>A polymorphism and IS risk. These studies were mostly performed in Chinese populations, but have had inconsistent results. We performed a meta-analysis of all eligible case-control studies to precisely assess the association between the rs671 G>A polymorphism and IS risk.
Methods
This present meta-analysis was conducted with guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 All included data were collected from published studies, and no ethical issues were involved.
Search strategy
Five online databases (PubMed, Embase, Web of Science, CNKI and Wanfang) were searched for relevant studies on the association between the rs671 G>A polymorphism and IS risk from inception to October 1, 2018. Only studies written in English and Chinese were included. The bibliographies of the collected studies and relevant reviews were also reviewed to identify potential additional articles. The following search terms and strategy was adopted “Aldehyde dehydrogenase 2”, “Aldehyde Dehydrogenase-2”, “ALDH2”, “polymorphism”, “variant”, “mutation”, “stroke”, “ischemic stroke”, “cerebral infarction”.
Eligibility criteria
All studies included in this meta-analysis were required to meet the following criteria: 1) case-control studies focused on the relationship between rs671 G>A polymorphism and IS risk; 2) studies with sufficient data on the genotypes in the case and control groups to evaluate the crude odds ratios (ORs) and 95% confidence intervals (CIs); 3) studies published only in English or Chinese; and 4) only the largest or most recently updated sample data when some overlapping or duplicate publications were available on the same theme.
Data extraction and quality evaluation
Xu and Hu reviewed and extracted the following information from all included studies independently: name of the first author, publishing date, control design, genotyping method, sample sizes of the cases and controls, genotype frequency distribution, Hardy-Weinberg equilibrium (HWE) and minor allele frequency assessment in controls, and adjusted factors. Quality evaluation of the included studies was performed by the above authors using the modified Newcastle-Ottawa scale (NOS).17 The scores ranged from 0 (worst) to 11 points (best) (Table 1). Studies with a score of 8 or higher were classified as high quality.
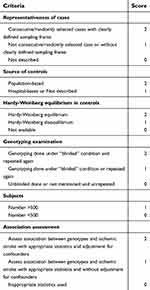 | Table 1 Scale for quality evaluation |
Statistical analysis
Crude ORs and 95% CIs were calculated to assess the statistical power of the association between the rs671 G>A polymorphism and IS risk. Five genetic models were examined, including the allele contrast model (A vs G), co-dominant models (GA vs GG and AA vs GG), dominant model (GA + AA vs GG) and recessive model (AA vs GG + GA). Heterogeneity among the included studies was calculated using Cochran’s Q test and the I2 test.18 The fixed-effect model was adopted when I2≤40%. Otherwise, a random-effects model was adopted.19,20 Subgroup analyses were conducted in accordance with the control design (population base and hospital base), subject number and NOS evaluation. Meta-regression was performed to identify the factors contributing to existing heterogeneity.21 Cumulative meta-analyses were performed to assess the statistical tendencies of the results. Sensitivity analyses were performed to examine the stability of the results by removing each study individually. Potential publication biases were assessed using Egger’s linear regression test and Begg’s funnel plots. All statistical analyses were performed with STATA version 14.0 (Stata Corporation, College Station, TX, USA). Two-sided P-values less than 0.05 were considered statistically significant.
Results
Study characteristics
In total, 116 studies were found in the first step of the systematic literature search. Figure 1 illustrates the inclusion procedures used to select the related studies. Following analysis of the titles and abstracts and full article screenings, 107 articles were excluded and nine studies involving 3,236 patients and 2,893 controls were included in our meta-analysis.15,22–29 These nine studies focused on Chinese populations, and the genotype distributions in the control groups in all nine studies satisfied Hardy-Weinberg equilibrium (HWE). Five studies used polymerase chain reaction (PCR)-sequencing, one study used PCR-restriction fragment length polymorphism, one study used PCR-amplified product length polymorphism and another study used the MassARRAY method. All of the information from the collected studies included in our analysis is listed in Table 2.
 | Table 2 Characteristics of case-control studies on ALDH2 rs671G>A polymorphism and ischemic stroke risk included in the meta-analysis |
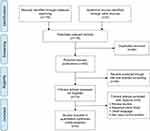 | Figure 1 Flow diagram of the study selection process. |
Quantitative and subgroup analyses
Overall, the pooled results revealed an increased IS risk associated with the rs671 G>A polymorphism in the allele contrast (A vs G: OR=1.29, 95% CI=1.01–1.65, P=0.04, I2=78.2%); homozygote model (AA vs GG: OR=1.68, 95% CI=1.27–2.21, P<0.01, I2=11.3%, Figure 2) and recessive model (AA vs GG+GA: OR=1.67, 95% CI=1.27–2.19, P<0.01, I2=0%) (Figure S1 for other models). In the subsequent analysis, which was based on control design, there were also significant associations between rs671 G>A polymorphism and IS risk, for example in the subgroups of hospital-based controls (A vs G: OR=1.34, 95% CI=1.03–1.73, P=0.03, I2=74.0%; AA vs GG: OR=1.71, 95% CI=1.25–2.34, P<0.01, I2=18.9%; AA vs GG + GA: OR=1.68, 95% CI=1.24–2.29, P<0.01, I2=0%). In addition, elevated IS risk was observed in the subgroups of subject number and NOS evaluation analysis (Table 3). Heterogeneity was observed in the allele contrast, heterozygote and dominant models. Meta-regression analysis was conducted with the aforementioned three stratified factors, but no apparent factor was found to contribute to the existent heterogeneity. (eg, A vs G model: P=0.99 for control design, P=0.38 for subject number and P=0.34 for NOS evaluation).
 | Table 3 Summary ORs and 95% CI of ALDH2 rs671G>A polymorphism and ischemic stroke risk |
 | Figure 2 OR and 95% CIs of the associations between ALDH2 rs671G>A polymorphism and ischemic stroke risk in AA vs GG model. |
Accumulative analysis was also conducted and revealed an apparently increased risk from 2014 when the report by Zhou et al. was included (Figure 3 for AA vs GG model) (Figure S2 for other models). Sensitivity analyses were conducted by removing each included study sequentially based on the published date, which indicated that only the study by Xu et al. influenced the corresponding result in the allele contrast, heterozygote and dominant models (Figure 4 for AA vs GG model) (Figure S3 for other models).
 | Figure 3 Cumulative meta-analyses according to publication year in AA vs GG model of ALDH2 rs671G>A polymorphism. |
 | Figure 4 Sensitivity analysis involving deletion of each study to reflect the influence of the individual dataset to the pooled ORs in AA vs GG model of ALDH2 rs671G>A polymorphism. |
Publication bias was evaluated, and the examination did not reveal any significant asymmetry in any of the funnel plots (Figure 5 for AA vs GG model) (Figure S4 for other models). The results of the Egger’s test were also evaluated (A vs G, P=0.33; GA vs GG: P=0.24; AA vs GG, P=0.31; GAh + AA vs GG, P=0. 28; AA vs GG + GA, P=0.39).
 | Figure 5 Funnel plot analysis to detect publication bias for AA vs GG model of ALDH2 rs671G>A polymorphism. Circles represent the weight of the studies. |
Discussion
Based on the data from the Sino-MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Beijing project from 1984 to 2004, the incidence of IS in China increased along with the fast-growing economy. This observation indicates that IS continues to account for most cases of stroke.30 IS is a complex disease and is pathologically based on atherosclerosis which can affect the interactions between genomic abnormalities and various environmental factors, and has become a major public health burden in China.31
Previous epidemiological studies have suggested that alcohol consumption exerts dual effects on the incidence of IS due to ethnic diversity, sex differences and difference in amounts of alcohol consumed.32–34 ALDH2 plays a critical role during the metabolism of ethanol and aldehyde detoxification. ALDH2 is an enzyme responsible for the rapid conversion of acetaldehyde to acetic acid in liver mitochondria. The rs671 G>A polymorphism in ALDH2 results in an inactive A-allele and a dramatic reduction in the enzyme’s activity. This remarkable decrease in activity subsequently leads to dysfunctional acetaldehyde metabolism.35,36 Compared with the wild-type homozygous GG genotype, the heterozygous GA genotype and homozygous AA genotype lead to forms of the enzyme with only approximately 16% of its original effectiveness. This may lead to high acetaldehyde concentrations. Individuals with the latter two genotypes have been shown to be more susceptible to various diseases, including digestive system cancer, diabetes and cardio-cerebrovascular disease.37
Since 2012, several case-control studies have been published regarding ALDH2 rs671 G>A polymorphism and IS risk. However, the results of these studies have been inconsistent. Sun firstly reported an increased risk with the A-allele in Shandong Province, China. Similar increases in IS risk were reported by Wang et al.24 and Zhou.25 In contrast, Xu et al. found that the prevalence of the G-allele of ALDH2 in the rs671 G>A polymorphism was higher in controls than in patients with IS, and stated that the A-allele and the GA genotype exerted protective effects against IS (A vs G, OR=0.37, 95% CI=0.19–0.72; GA vs GG, OR=0.30, 95% CI=0.14–0.65).22 Other studies, such as those by Qu et al.29 and Sun et al.,28 did not find any significant relationship between the ALDH2 rs671 G>A polymorphism and IS risk.
By reviewing the above-collected studies, we found that the included sample sizes ranged from hundreds to thousands of subjects. In molecular epidemiological studies, small sample sizes may lead to false results and the drawing of inaccurate conclusions. Thus, we conducted this meta-analysis using nine published case-control studies to enlarge the sample size and investigate the association between the ALDH2 rs671 G>A polymorphism and IS risk. To our knowledge, this study is the first meta-analysis of this particular association. Our results strongly suggest that the polymorphic locus of ALDH2 rs671 G>A may be an independent risk factor for IS. Moreover, the quantitative increase in risk can be observed in all subgroup analyses based on control design, subject number and NOS evaluation groups. Some studies have shown that heterozygous and homozygous mutants of ALDH2 rs671 G>A polymorphism block aldehyde metabolism and result in acetaldehyde accumulation, which in turn leads to significant impairments in cardiovascular vasorelaxation.38 Animal studies indicate that ALDH2-knockout mice would progressively develop age-related heart dysfunction and show reduction in life span, which strongly suggests that ALDH2 ablation leads to cardiac aging.39 In ALDH2 (-/-) mice, some exhibitions of endothelial dysfunction, increased amyloid-beta in cerebral microvessels and atrophy were observed in the brain.40 These evidences suggested that the decreased activity of ALDH2 would aggravate oxidative stress responses and aging of the cerebrovascular system, and increase the risk of IS. Other studies have reported that the proportion of GA and AA genotypes were higher in subjects with carotid atherosclerosis than in control subjects. This observation indicates that the rs671 G>A polymorphism is associated with the severity and thickness of carotid atherosclerosis in Asians, and that carotid atherosclerosis is the main pathological mechanism underlying IS.41–43 All these observations imply that the rs671 G>A polymorphism in ALDH2 may play an indirect role in IS development.
This analysis integrated all published case-control studies on ALDH2 rs671 G>A polymorphism and IS risk with both a large sample size and the use of rigorous statistical methods. However, some limitations remain due to the inherent deficiencies of meta-analyses. First, the subjects in all of the included studies were mainly Chinese. Therefore, the results of this analysis may not represent those obtained in all ethnic populations. A recently published genome-wide association study in Caucasian populations had reported that the ALDH2 rs10744777 polymorphism but not the rs671polymorphism was associated with IS risk.44 So, further studies are needed to verify the potential correlation between ALDH2 rs671 G>A polymorphism and IS risk in Caucasians. Second, only one SNP locus (ALDH2 rs671 G>A polymorphism) was examined, and the investigation was not adjusted for interfering gene–gene and gene–environment factors. The absence of such adjustments may have led to bias in the general conclusions. Finally, some heterogeneity emerged in the included studies. Meta-regression was performed but failed to identify any factor that contributed to the heterogeneity.
Conclusion
The data from all the included studies suggest that the ALDH2 rs671 G>A polymorphism may be a risk factor for IS development. Additional case-control studies including gene–environment interactions in diverse populations are needed to explore the underlying mechanisms of this potential association.
Acknowledgments
This study was supported by the Foundations of the Science and Technology Department of Hubei Province (No. 2016CFB567), and the Hubei Province health and family planning scientific research project (No. WJ2017F069). The funders had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflcits of interest in this work.
References
1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–1623. doi:10.1016/S0140-6736(08)60694-7
2. Engels T, Baglione Q, Audibert M, et al. Socioeconomic status and stroke prevalence in Morocco: results from the Rabat-Casablanca study. PLoS One. 2014;9(2):e89271. doi:10.1371/journal.pone.0089271
3. Venketasubramanian N, Yoon BW, Pandian J, Navarro JC. Stroke epidemiology in South, East, and South-East Asia: a review. J Stroke. 2017;19(3):286–294. doi:10.5853/jos.2017.00234
4. Kalkonde YV, Sahane V, Deshmukh MD, et al. High prevalence of stroke in Rural Gadchiroli, India: a community-based Study. Neuroepidemiology. 2016;46(4):235–239. doi:10.1159/000444487
5. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi:10.1016/S0140-6736(06)68770-9
6. DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi:10.1016/S0140-6736(18)32335-3
7. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi:10.1161/CIR.0000000000000485
8. O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. doi:10.1016/S0140-6736(16)30506-2
9. Chen CH, Joshi AU, Mochly-Rosen D. The role of mitochondrial aldehyde dehydrogenase 2 (ALDH2) in neuropathology and neurodegeneration. Acta Neurol Taiwan. 2016;25(4):111–123.
10. Sachidanandam R, Weissman D, Schmidt SC, et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409(6822):928–933. doi:10.1038/35057149
11. Kitagawa K, Kawamoto T, Kunugita N, et al. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; in vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. FEBS Lett. 2000;476(3):306–311.
12. Cichoz-Lach H, Partycka J, Nesina I, et al. Alcohol dehydrogenase and aldehyde dehydrogenase gene polymorphism in alcohol liver cirrhosis and alcohol chronic pancreatitis among Polish individuals. Scand J Gastroenterol. 2007;42(4):493–498. doi:10.1080/00365520600965723
13. Zhong Z, Hou J, Li B, et al. Genetic polymorphisms of the mitochondrial aldehyde dehydrogenase ALDH2 gene in a large ethnic Hakka population in Southern China. Med Sci Monit. 2018;24:2038–2044.
14. Kang TS, Woo SW, Park HJ, Lee Y, Roh J. Comparison of genetic polymorphisms of CYP2E1, ADH2, and ALDH2 genes involved in alcohol metabolism in Koreans and four other ethnic groups. J Clin Pharm Ther. 2009;34(2):225–230. doi:10.1111/j.1365-2710.2008.00986.x
15. Sun HL. Relationship between ALDH2 polymorphism and the formation of carotid atherosclerotic plaques in cerebral infarction patients (Chinese). J Shandong Univ. 2012;50(7):65–67,73.
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2651
17. Niu YM, Weng H, Zhang C, et al. Systematic review by multivariate meta-analyses on the possible role of tumor necrosis factor-alpha gene polymorphisms in association with ischemic stroke. Neuromolecular Med. 2015;17(4):373–384. doi:10.1007/s12017-015-8365-7
18. Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160. doi:10.1093/ije/dyn204
19. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748.
20. DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med. 1996;15(12):
21. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi:10.1002/sim.1187
22.
23. Zhang CY, Dai YP, Li J, et al. Association between acetaldehyde dehydrogenase 2 gene polymorphism and acute cerebral infarction in Han nationality of Heilongjiang Province (Chinese). Chin J Sch Doctor. 2014;28(5):334–335.
24. Wang GY, Chen NY, Xu YJ. The relationship between genetic polymorphism of aldehyde dehydrogenase-2 and cerebral infarction (Chinese). Chin J Coal Ind Med. 2014;19(1):29–31.
25.
26. Li QY, Zhao NM, Ma JJ, et al. ALDH2*2 Allele is a negative risk factor for cerebral infarction in Chinese women. Biochem Genet. 2015;53(9–10):260–267. doi:10.1007/s10528-015-9686-9
27. Sung YF, Lu CC, Lee JT, et al. Homozygous ALDH2*2 is an independent risk factor for ischemic stroke in Taiwanese men. Stroke. 2016;47(9):2174–2179. doi:10.1161/STROKEAHA.116.013204
28. Sun S, He J, Zhang Y, et al. Genetic polymorphisms in the ALDH2 gene and the risk of ischemic stroke in a Chinese han population. Oncotarget. 2017;8(60):101936–101943. doi:10.18632/oncotarget.21803
29. Qu Y, Zhang HL, Li HY, Yu LM, Sun Y. ALDH2*2 polymorphism is associated with an increased risk of extra cranial vascular stenosis and poor collateral vessels in ischemic stroke in Han Chinese. Int J Clin Exp Med. 2016;9(10):19944–19952.
30. Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39(6):1668–1674. doi:10.1161/STROKEAHA.107.502807
31. Jia Q, Liu LP, Wang YJ. Stroke in China. Clin Exp Pharmacol Physiol. 2010;37(2):259–264. doi:10.1111/j.1440-1681.2009.05290.x
32. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi:10.1136/bmj.d671
33. Sacco RL. Alcohol and stroke risk: an elusive dose-response relationship. Ann Neurol. 2007;62(6):551–552. doi:10.1002/ana.21298
34. Wassertheil-Smoller S, Kaplan RC, Salazar CR. Stroke findings in the women’s health initiative. Semin Reprod Med. 2014;32(6):438–446. doi:10.1055/s-0034-1384627
35. Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30(1):5–13.
36. Zhang R, Wang J, Xue M, Xu F, Chen Y. ALDH2 – the genetic polymorphism and enzymatic activity regulation: their epidemiologic and clinical implications. Curr Drug Targets. 2017;18(15):1810–1816. doi:10.2174/1389450116666150727115118
37. Lai CL, Yao CT, Chau GY, et al. Dominance of the inactive Asian variant over activity and protein contents of mitochondrial aldehyde dehydrogenase 2 in human liver. Alcohol Clin Exp Res. 2014;38(1):44–50. doi:10.1111/acer.12215
38. Szocs K, Lassegue B, Wenzel P, et al. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol. 2007;42(6):1111–1118. doi:10.1016/j.yjmcc.2007.03.904
39. Wu B, Yu L, Wang Y, et al. Aldehyde dehydrogenase 2 activation in aged heart improves the autophagy by reducing the carbonyl modification on SIRT1. Oncotarget. 2016;7(3):2175–2188. doi:10.18632/oncotarget.6814
40. D’Souza Y, Elharram A, Soon-Shiong R, Andrew RD, Bennett BM. Characterization of Aldh2 (-/-) mice as an age-related model of cognitive impairment and Alzheimer’s disease. Mol Brain. 2015;8:27. doi:10.1186/s13041-015-0117-y
41. Narita M, Kitagawa K, Nagai Y, et al. Effects of aldehyde dehydrogenase genotypes on carotid atherosclerosis. Ultrasound Med Biol. 2003;29(10):1415–1419.
42. Ma XX, Zheng SZ, Shu Y, Wang Y, Chen XP. Association between carotid intima-media thickness and aldehyde dehydrogenase 2 Glu504Lys polymorphism in Chinese Han with essential hypertension. Chin Med J (Engl). 2016;129(12):1413–1418. doi:10.4103/0366-6999.183413
43. Howard DP, van Lammeren GW, Rothwell PM, et al. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46(1):182–189. doi:10.1161/STROKEAHA.114.007221
44.
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




