Back to Journals » Journal of Inflammation Research » Volume 16
Albumin Corrected Anion Gap and the Risk of in-Hospital Mortality in Patients with Acute Pancreatitis: A Retrospective Cohort Study
Authors Li P, Shi L, Yan X, Wang L, Wan D , Zhang Z, He M
Received 11 April 2023
Accepted for publication 2 June 2023
Published 7 June 2023 Volume 2023:16 Pages 2415—2422
DOI https://doi.org/10.2147/JIR.S412860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Ping Li, Lvyuan Shi, Xin Yan, Lietao Wang, Dingyuan Wan, Zhongwei Zhang, Min He
Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Zhongwei Zhang; Min He, West China Hospital, Sichuan University, 37 Guoxue Lane, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86 13308203057 ; +86 18980600837, Email [email protected]; [email protected]
Purpose: To explore the prognostic value of albumin corrected anion gap (ACAG) within 24 hours of admission to the intensive care unit (ICU) for acute pancreatitis (AP).
Patients and Methods: This was a retrospective cohort study. Adult AP patients admitted to ICU from June 2016 to December 2019 were included in the study, who were divided into three groups according to initial serum ACAG within 24 hours upon ICU admission: ACAG ≤ 14.87 mmol/L, 14.87 < ACAG ≤ 19.03 mmol/L, and ACAG > 19.03 mmol/L. The primary study outcome indicator was in-hospital mortality. Age, sex, Glasgow Coma Scale score, and Acute Physiology and Chronic Health Evaluation II (APACHE II) score were matched through propensity score matching (PSM) method to balance the baseline between the survivors and non-survivors. Multivariate Cox regression was used to determine the relationship between ACAG and in-hospital mortality.
Results: A total of 344 patients (of them 81 non-survivors) were analyzed in this study. Patients with higher ACAG intended to present significantly higher in-hospital mortality, APACHE II score, creatine, lower albumin, and bicarbonate. Multivariate Cox regression analysis after matching demonstrated that white blood cell count, platelet count, and higher ACAG were independently associated with higher in-hospital mortality (ACAG ≤ 14.87 as a reference, 14.87 < ACAG ≤ 19.03 mmol/L with HR of 2.34 and 95% CI of 1.15– 4.76, ACAG > 19.03 with HR of 3.46 and 95% CI of 1.75– 6.84).
Conclusion: Higher ACAG was independently associated with higher in-hospital mortality in patients with AP after matching the baseline between the survivors and non-survivors.
Keywords: anion gap, albumin corrected anion gap, acute pancreatitis, mortality
Introduction
Acute pancreatitis (AP), the most common gastrointestinal disease with an annual incidence of 13–45 cases per 100,000 people worldwide,1,2 has been the fifth disease of in-hospital death in America with an annual cost exceeding 2.6 billion dollars.3 Exploring new predictors of AP prognosis may help in the early identification of high-risk patients with poor outcome and help physicians to select those patients who would benefit the most from close surveillance or aggressive intervention.
Anion gap (AG), reflecting the difference in serum unmeasured cation and anion concentrations, is one of the most commonly used biomarkers for diagnosing acid-base imbalance as well as identifying the reason for metabolic acidosis. AG consists of serum albumin, lactate, acetoacetate, sulfate, phosphate, and other anions. For patients with hypoproteinemia, the low level of albumin leads to the reduction of protein anions, which makes the false-negative result of AG and affects the judgment of results. Albumin corrected anion gap (ACAG) was proposed to avoid false-negative results. One study based on Medical Information Mart for Intensive Care database version III (MIMIC III) database presented that AG value was associated with all-cause mortality in patients with acute pancreatitis.4
Nevertheless, the association between the simple and inexpensive index, ACAG, and the prognosis of AP is not clarified. The objective of this study was to explore the prognostic value of ACAG for AP.
Materials and Methods
Diagnosis of Acute Pancreatitis
AP is diagnosed based on two of three criteria: (1) upper abdominal pain, (2) serum amylase or lipase (or both) of at least three times the upper limit of normal, or (3) findings on imaging consistent with AP.1
Patients and Data Collection
We retrospectively collected the patients who were diagnosed with AP at the West China Hospital of Sichuan University from June 2016 to December 2019. The inclusion criteria were as follows: (1) diagnosis of AP; (2) with age ≥18 years; (3) having been hospitalized in Intensive Care Unit (ICU). The pregnant patients were excluded.
This study complies with the Declaration of Helsinki and was approved by the biomedical ethics committee of West China Hospital of Sichuan University, and the approval ID was 2021 (1694). Considering its retrospective characteristic and no intervention implemented to the included patients, the study applied for the exemption from informed consent from these patients and the biomedical ethics committee of West China Hospital of Sichuan University passed the application. This work has been reported in compliance with the STROBE criteria.5
Patient demographics, serum data, Glasgow Coma Scale (GCS) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and in-hospital outcomes of the included patients were collected. Demographic information included sex and age. Serum data including White Blood Cell (WBC) count, Neutrophil count, Lymphocyte count, Hemoglobin (Hb), Platelet count (PLT), Total Bilirubin (TB), Direct Bilirubin (DB), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Creatine (Cr), Albumin (Alb), sodium, potassium, chlorine, bicarbonate were measured within 24 hours on admission to ICU. AG was calculated according to the formulae: 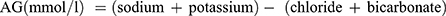 . ACAG was calculated according to the following equation:
. ACAG was calculated according to the following equation: 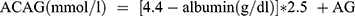 . GCS score was determined within 1 hour of being admitted to ICU. APACHE II score was calculated according to Knaus WA6 within 24 hours of being admitted to ICU. The primary outcome indicator was in-hospital mortality.
. GCS score was determined within 1 hour of being admitted to ICU. APACHE II score was calculated according to Knaus WA6 within 24 hours of being admitted to ICU. The primary outcome indicator was in-hospital mortality.
Statistical Analysis
Statistical analysis was performed by IBM SPSS software, version 23.0 (SPSS, Chicago, USA). Variables with normal distributions were presented as the means ± SD and compared with one way analysis of variance (ANOVA). Nonnormally distributed variables were expressed as medians and interquartile ranges (IQRs) and compared with the Kruskal–Wallis H-test. Categorical variables were described as count and percentages and compared by Chi-square test. Continuous variables were transformed into categorical variables based on their median value.
A propensity score matching (PSM) analysis model with a caliper of 0.2 was used to balance differences between the survivors and non-survivors, eliminate possible selection bias and increase the evidence level of the retrospective study. A 1:2 nearest neighbor matching was applied to select statistically matched pairs of AP patients according to age, sex, GCS, and APACHE II score. The standardized mean differences (SMDs) and p values were calculated to evaluate the effectiveness of the PSM. An SMD >0.1 is considered to be a significant difference between the two groups.
Multivariate Cox regression was applied to determine the relationship between ACAG and in-hospital mortality. Sensitivity analysis was performed to evaluate the stability of the results. The results were expressed as hazard ratio (HR) with 95% confidence interval (CI). A p-value < 0.05 was considered to indicate statistical significance.
Results
Population and Baseline Characteristics
A total of 350 patients were included according to the inclusion criteria. We excluded six pregnancy patients. In total, 344 patients were analyzed in this study, of them 81 were non-survivors and 263 survivors. Patient baseline characteristics are listed in Table 1. The tri-tile of ACAG was 14.87 and 19.03, according to which the included patients were divided into three groups: ACAG ≤ 14.87 mmol/L, 14.87 < ACAG ≤ 19.03 mmol/L, and ACAG > 19.03 mmol/L. Patients with higher ACAG intended to present significantly higher in-hospital mortality, APACHE II score, creatine, sodium, and lower albumin, chloride, and bicarbonate. Patients with lower ACAG showed to be younger. There were no differences in gender composition. The levels of GCS score, WBC count, neutrophil count, lymphocyte count, HB, PLT, TB, DB, and ALT were similar in the three groups.
 |
Table 1 Baseline Characteristics Comparison Among the Albumin Corrected Anion Gap Groups Before Propensity Score Matching |
Association Between ACAG and Mortality Before PSM
Univariate Cox regression before PSM was performed to explore the relationship between the mortality in AP and the included variables (Table 2). And the variates with p < 0.05 in the Univariate Cox regression were included in the Multivariate Cox regression, and the results are shown in Table 2. Multivariate Cox regression found that WBC count, PLT count, and higher ACAG were independently associated with higher in-hospital mortality. Figure 1 presents the Kaplan–Meier survival curves before PSM.
 |
Table 2 Cox Regression of the Patients with Acute Pancreatitis Before Propensity Score Matching |
 |
Figure 1 Kaplan-Meier survival curves of in-hospital survival in acute pancreatitis patients with three ACAG groups before propensity score matching. Abbreviation: ACAG, Albumin Corrected Anion Gap. |
Association Between ACAG and Mortality After PSM
To balance the baseline between the survivors and non-survivors, the included patients were matched by age, gender, GCS, and APACHE II score. After matching, there were 75 non-survivors and 130 survivors. The PSM results are displayed in Supplementary Table 1. All SMDs of the matched variables were <0.1 after PSM.
Univariate Cox regression analysis showed that high WBC, PLT count, high Neutrophil count, AG >14.50 mmol/L, and ACAG group were all associated with poor outcome of AP with p value <0.05 (Table 3). Multivariate Cox regression analysis demonstrated that WBC count, PLT count, and higher ACAG were independently associated with worse in-hospital outcomes (Table 3, Figure 2).
 |
Table 3 Cox Regression of the Patients with Acute Pancreatitis After Propensity Score Matching |
 |
Figure 2 Kaplan-Meier survival curves of in-hospital survival in acute pancreatitis patients with three ACAG groups after propensity score matching. Abbreviation: ACAG, Albumin Corrected Anion Gap. |
Sensitivity analysis was performed by dividing ACAG into two groups based on its median value: ACAG ≤ 17.14 mmol/L, ACAG > 17.14 mmol/L. Multivariate Cox regression after PSM showed similar results presented in Supplementary Table 2 and Supplementary Figure.
Discussion
After balancing the basal information of the non-survivors and the survivors, this study found that higher initial serum ACAG within the first 24 hours after ICU admission was independently associated with higher in-hospital mortality in AP patients. The study presented that we could predict the in-hospital mortality for the patients with AP with the help of ACAG, which can be calculated easily from the inexpensive and convenient examine.
AG, as a biomarker for assessing the acid–base of the biotic internal environment, is associated with higher in-hospital mortality in patients presenting with cardiac arrest and sepsis;7,8 with higher ICU and in-hospital mortality in critically ill patients.9 An elevated level of AG increases all-cause mortality in unselected adults.10 Moreover, AG can predict short-time prognosis for cerebral infarction patients.11 Although the specific mechanisms were not well understood and explained. Additionally, the change in AG also indicates bromide intoxication, lithium intoxication, iodide intoxication,12 and pesticide poisoning.13
Fang Gong et al4 have studied the relationship between AG and mortality in AP based on 279 cases extracted from the MIMIC III. The results showed that serum AG was related to 90-day all-cause mortality in patients with AP after adjusting potential confounders. However, hypoalbuminemia occurred in 19–39% of patients with AP and was one of the risk factors for mortality.14,15 In the present study, the level of albumin was 32.69±6.76. Kraut JA indicated that every 1 g/dl change in serum albumin resulted in 2.3 to 2.5 mmol/L change in the serum anion gap.12 Hypoalbuminemia may contribute to the false-negative value of AG and influence the interpretation of the results. Therefore, we focused to explore the relationship between ACAG and in-hospital mortality of AP.
According to the Atlanta classification system revised in 2012,16 AP was classified into three types: mild, moderate, and severe. Severe acute pancreatitis (SAP) is characterized as persistent (>48 hours) either single or multiple organ failure. The majority of AP patients present with a mild form,17 however approximately 20% progressed to SAP with higher in-hospital mortality ranging from 36% to 50%.16,18–20 The APACHE-II system has been verified to discriminate mild pancreatitis and SAP,21,22 and to predict the prognosis of AP.22 In this study, APACHE II score was matched between the non-survivors and the survivors through PSM method, which could exclude the effect of disease severity on the mortality of AP and contribute to the more credible conclusion. The present study found that higher ACAG showed higher in-hospital mortality. Furthermore, the sensitivity analysis presented similar results.
The main causes of elevated level of serum AG include excessive synthesis of organic acids, such as lactate, and/or decreased excretion of anions. SAP is usually accompanied by hypovolemic shock and infectious shock, presenting with microcirculatory dysfunction and the increase of lactate. Elevated arterial lactate was independently related to poor outcomes for AP23 and could predict SAP.24 There is controversy on the relevance between AG and lactate. Some research found AG was related to lactate,7,9 while some insisted that AG cannot predict hyperlactatemia25 and the use of AG as an alternative to lactate cannot be recommended.26 The possible reason results from the various baseline AG levels, albumin levels, clinical setting among individuals, and differences between arterial, venous, and capillary lactate.12,25,26 Studies have demonstrated that ACAG increased the sensitivity of identifying hyperlactatemia by 36–37%.25,27,28 Additionally, SAP is characterized as multiple organ dysfunction syndrome (MODS) including acute liver insufficiency, disseminated intravascular coagulation (DIC), acute kidney injury (AKI), and so on. Patients with renal dysfunction presented with decreased excretion of anions, which contributed to an increase in AG. Meanwhile, AKI, particularly if kidney replacement therapy was required, increased the mortality rates to 75%.29
Our study has some limitations. Firstly, the included serum AG was measured within 24 hours upon ICU admission without follow-up data, because the objective of this study was to investigate the prognostic value of early serum AG on the outcome of AP. Further studies on the relationship between the dynamic variation of serum AG and the prognosis of AP are expected to provide a novel idea. Secondly, the change in serum AG indicated not only acidosis, but also intoxication, laboratory error, and other disorders.11 Potential covariates may exist even after comprehensive adjustment for measurable variables. Finally, the conclusions found in this study were constricted by the single-center retrospective study.
Conclusion
Higher ACAG was independently associated with higher in-hospital mortality in patients with AP after matching the baseline between the survivors and non-survivors.
Ethics Approval and Informed Consent
This study was approved by the biomedical ethics committee of West China Hospital of Sichuan University, and the approval ID was 2021 (1694).
Consent for Publication
We have confirmed that the details included in this study can be published.
Acknowledgments
We would like to thank all the volunteers who took part in this study and all the participants for their contribution to data collection and analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Project funded by China Postdoctoral Science Foundation (2021M692298).
Disclosure
The authors declare that they have no competing interests, and confirm that there are no financial or personal relationships with other people or organizations.
References
1. Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–734. doi:10.1016/S0140-6736(20)31310-6
2. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi:10.1136/bmj.l6227
3. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology. 2022;162(2):621–644. doi:10.1053/j.gastro.2021.10.017
4. Gong F, Zhou Q, Gui C, et al. The relationship between the serum anion gap and all-cause mortality in acute pancreatitis: an analysis of the MIMIC-III database. Int J Gen Med. 2021;14:531–538. doi:10.2147/IJGM.S293340
5. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies [published correction appears in Ann Intern Med. 2008 Jan 15;148(2):168]. Ann Intern Med. 2007;147(8):573–577. doi:10.7326/0003-4819-147-8-200710160-00010
6. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
7. Chen J, Dai C, Yang Y, et al. The association between anion gap and in-hospital mortality of post-cardiac arrest patients: a retrospective study. Sci Rep. 2022;12(1):7405. doi:10.1038/s41598-022-11081-3
8. Hu T, Zhang Z, Jiang Y. Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: a retrospective propensity score matching analysis. Clin Chim Acta. 2021;521:272–277. doi:10.1016/j.cca.2021.07.021
9. Li R, Jin X, Ren J, et al. Relationship of admission serum anion gap and prognosis of critically ill patients: a large multicenter cohort study. Dis Markers. 2022;2022:5926049. doi:10.1155/2022/5926049
10. Ji X, Peng S. The association between serum anion gap and all-cause mortality of unselected adult patients: a retrospective cohort study of >20,000 patients. J Clin Lab Anal. 2023;37(1):e24818. doi:10.1002/jcla.24818
11. Liu X, Feng Y, Zhu X, et al. Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: evidence from the MIMIC-III database. Biomarkers. 2020;25(8):725–732. doi:10.1080/1354750X.2020.1842497
12. Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2(1):162–174. doi:10.2215/CJN.03020906
13. Lee SB, Kim DH, Kim T, et al. Anion gap and base deficit are predictors of mortality in acute pesticide poisoning. Hum Exp Toxicol. 2019;38(2):185–192. doi:10.1177/0960327118788146
14. Hong W, Lin S, Zippi M, et al. Serum albumin is independently associated with persistent organ failure in acute pancreatitis. Can J Gastroenterol Hepatol. 2017;2017:5297143. doi:10.1155/2017/5297143
15. Ocskay K, Vinko Z, Nemeth D, et al. Hypoalbuminemia affects one third of acute pancreatitis patients and is independently associated with severity and mortality. Sci Rep. 2021;11(1):24158. doi:10.1038/s41598-021-03449-8
16. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
17. Baron TH, Dimaio CJ, Wang AY, et al. American gastroenterological association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(1):67–75 e1. doi:10.1053/j.gastro.2019.07.064
18. Silva-Vaz P, Abrantes AM, Castelo-Branco M, et al. Multifactorial scores and biomarkers of prognosis of acute pancreatitis: applications to research and practice. Int J Mol Sci. 2020;21(1):338. doi:10.3390/ijms21010338
19. Silva-Vaz P, Abrantes AM, Castelo-Branco M, et al. Murine models of acute pancreatitis: a critical appraisal of clinical relevance. Int J Mol Sci. 2019;20(11):2794. doi:10.3390/ijms20112794
20. Parniczky A, Kui B, Szentesi A, et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS One. 2016;11(10):e0165309. doi:10.1371/journal.pone.0165309
21. Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2(8656):201–205. doi:10.1016/s0140-6736(89)90381-4
22. Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta classification. Gastroenterol Rep. 2018;6(2):127–131. doi:10.1093/gastro/gox029
23. Shu W, Wan J, Chen J, et al. Initially elevated arterial lactate as an independent predictor of poor outcomes in severe acute pancreatitis. BMC Gastroenterol. 2020;20(1):116. doi:10.1186/s12876-020-01268-1
24. Valverde-Lopez F, Matas-Cobos AM, Alegria-Motte C, et al. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32(9):1649–1656. doi:10.1111/jgh.13763
25. Chawla LS, Jagasia D, Abell, LM et al. Anion gap, anion gap corrected for albumin, and base deficit fail to accurately diagnose clinically significant hyperlactatemia in critically ill patients. J Intensive Care Med. 2008;23(2):122–127. doi:10.1177/0885066607312985
26. Glasmacher SA, Stones W. A systematic review and diagnostic test accuracy meta-analysis of the validity of anion gap as a screening tool for hyperlactatemia. BMC Res Notes. 2017;10(1):556. doi:10.1186/s13104-017-2853-9
27. Kraut JA, Nagami T, et al. The serum anion gap in the evaluation of acid-base disorders: what are its limitations and can its effectiveness be improved?. Clin J Am Soc Nephrol. 2013;8(11):2018–2024. doi:10.2215/CJN.04040413
28. Dinh CH, Ng R, Grandinetti A, et al. Correcting the anion gap for hypoalbuminaemia does not improve detection of hyperlactataemia. Emerg Med J. 2006;23(8):627–629. doi:10.1136/emj.2005.031898
29. Nassar TI, Qunibi WY. AKI associated with acute pancreatitis. Clin J Am Soc Nephrol. 2019;14(7):1106–1115. doi:10.2215/CJN.13191118
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
