Back to Journals » Clinical Interventions in Aging » Volume 9
Age as a risk factor for acute mountain sickness upon rapid ascent to 3,700 m among young adult Chinese men
Authors Tang X, Zhang J, Qin J, Gao X, Li Q, Yu J, Ding X, Huang L
Received 30 April 2014
Accepted for publication 6 June 2014
Published 6 August 2014 Volume 2014:9 Pages 1287—1294
DOI https://doi.org/10.2147/CIA.S67052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Xu-gang Tang,1 Ji-hang Zhang,1 Jun Qin,1 Xu-bin Gao,1 Qian-ning Li,2 Jie Yu,1 Xiao-han Ding,1 Lan Huang1
1Institute of Cardiovascular Diseases, 2Department of Neurology, Xinqiao Hospital, Third Military Medical University, Chongqing, People’s Republic of China
Background: The aim of this study was to explore the relationship between age and acute mountain sickness (AMS) when subjects are exposed suddenly to high altitude.
Methods: A total of 856 young adult men were recruited. Before and after acute altitude exposure, the Athens Insomnia Scale score (AISS) was used to evaluate the subjective sleep quality of subjects. AMS was assessed using the Lake Louise scoring system. Heart rate (HR) and arterial oxygen saturation (SaO2) were measured.
Results: Results showed that, at 500 m, AISS and insomnia prevalence were higher in older individuals. After acute exposure to altitude, the HR, AISS, and insomnia prevalence increased sharply, and the increase in older individuals was more marked. The opposite trend was observed for SaO2. At 3,700 m, the prevalence of AMS increased with age, as did severe AMS, and AMS symptoms (except gastrointestinal symptoms). Multivariate logistic regression analysis showed that age was a risk factor for AMS (adjusted odds ratio [OR] 1.07, 95% confidence interval [CI] 1.01–1.13, P<0.05), as well as AISS (adjusted OR 1.39, 95% CI 1.28–1.51, P<0.001).
Conclusion: The present study is the first to demonstrate that older age is an independent risk factor for AMS upon rapid ascent to high altitude among young adult Chinese men, and pre-existing poor subjective sleep quality may be a contributor to increased AMS prevalence in older subjects.
Keywords: acute mountain sickness, age, Athens Insomnia Scale, rapid ascent, sleep
Introduction
In recent years, the number of people ascending to high altitudes worldwide has clearly increased. However, acute mountain sickness (AMS) is a major obstacle faced by many people after arrival at high altitudes. AMS is a common syndrome encountered particularly by lowlanders after rapidly ascending to altitudes above 2,500 m.1 This syndrome is characterized by headache, dizziness or lightheadedness, gastrointestinal symptoms (anorexia, nausea, or vomiting), weakness or fatigue, and insomnia.2 Among these symptoms, headache is the most frequent and essential for final AMS diagnosis. Although self-limiting, AMS may develop into fatal high altitude pulmonary or cerebral edema in a small number of people.3 Therefore, prevention of AMS is particularly important. Research on risk factors for AMS (eg, female sex and younger age)4 is beneficial for the prevention of AMS, although further investigations are needed to establish these risk factors.5
With the development of transportation, rapid ascension to high altitudes is becoming common and easy. However, as yet, there has been no large-scale epidemiological investigation into the relationship between age and AMS upon rapid ascent to high altitudes. Thus, the relationship between age and AMS upon acute exposure to high altitudes remains unclear. Given that past literature6–8 indicated a tendency for older adults to be at greater risk of AMS than younger adults, we hypothesized that older age would increase the prevalence of AMS upon rapid ascent to high altitude. Therefore, this study uses a cross-sectional design aiming to explore the relationship between age and AMS in subjects suddenly exposed to high altitude.
Methods
Subjects and design
In the present study, 987 adult Chinese men aged ≥18 years were recruited in June 2012. All subjects were lowlanders, without preceding altitude exposure during the previous half year, and none suffered from cardiopulmonary disease or was currently taking any medicines (eg, vitamins, cold cure, etc). Prior to the investigation, all subjects provided written informed consent approved by the Ethics Review Board of Xinqiao Hospital, Second Affiliated Hospital of Third Military Medical University (decision number: 2012015). The study protocol was registered in the Chinese Clinical Trial Registry (http://www.chictr.org; registration no. ChiCTR-RCS-12002232). The investigation conforms with the principles outlined in the Declaration of Helsinki.
One week before ascending to 3,700 m, all subjects were interviewed and underwent a routine physical examination (echocardiogram, electrocardiogram, transcranial Doppler, chest X-ray, heart rate [HR], blood pressure [BP], and arterial oxygen saturation [SaO2]). Height and weight were measured with standard methods, and body mass index (BMI) was obtained by the formula (weight [kg] divided by height squared [m2]). In addition, sleep status of the subjects was investigated using the Athens Insomnia Scale score (AISS).9 Subjects’ demographic characteristics (age, race [Chinese Han, Miao, Man, Tibetan, etc], education, drinking10 and smoking11 habits, etc) were recorded in the questionnaire. In the morning, all subjects ascended by plane from 500 m to 3,700 m within 2 hours, in batches (with a 1-day interval between batches). After arrival at 3,700 m, all subjects undertook the same daily regimen and refrained from vigorous exercise. On the morning of the second day at 3,700 m, all subjects completed the Athens Insomnia Scale (AIS)9 and Lake Louise Score (LLS)2 questionnaires. HR and SaO2 were measured. All identifying characteristics (name, age, etc) were concealed before data analysis.
Acute mountain sickness assessment
In the present study, the LLS system2 was used for the diagnosis of AMS in subjects. The LLS questionnaire comprised five items: headache, dizziness or lightheadedness, gastrointestinal symptoms (anorexia, nausea, vomiting), fatigue and/or weakness, and insomnia. Each item was rated on a four-point Likert scale (0= not at all, 1= mild, 2= moderate, and 3= severe), with total scores varying from 0 to 15. Subjects completed their questionnaire after waking in the morning. AMS was determined when a subject reported a headache and one or more of the other symptoms as well as an LLS of ≥3. The AMS was then classified as mild (LLS 3 or 4) or serious (LLS ≥5).
Physiological parameter measurements
After completion of the LLS questionnaire, the HR, SaO2, and BP values of the subjects were measured after a 15-minute rest in a comfortable chair. HR and SaO2 values were obtained with finger-pulse oximetry (Onyx 9500; Nonin Medical, Inc., Plymouth, MN, USA). BP was measured with a sphygmomanometer (OMRON HEM-6200; Omron Health Care, Inc., Bannockburn, IL, USA). Three consecutive measurements for all parameters were obtained, and the mean value of the three measurements was used in the analysis.
Sleep assessment
Sleep status of the subjects in the past month was assessed using the AIS.9 The AIS includes eight items: the first five items assess sleep procedure (sleep induction, night awakening, awakening in the early morning, total sleep duration, and total quality of sleep), and the last three items estimate decreased sense and functioning, and sleepiness in the next day. Each item is rated from 0 to 3 using a four-point Likert scale (0= no event, 1= mild, 2= moderate, and 3= severe). Subjects with an AIS score ≥6 were considered to be suffering from insomnia.12 The AIS is considered an excellent instrument for assessing subjective sleep quality in both clinical and study settings as it has good reliability and validity9 and is easy to complete.13
Statistical analyses
Data are shown as mean ± standard deviation, median (interquartile range), or n (%), and were analyzed using SPSS version 13.0 (IBM Corporation, Armonk, NY, USA). All tests were two-tailed, and a P-value <0.05 was considered significant. The distribution normality of the parameters was tested using the Kolmogorov–Smirnov test. Mean values were compared using the Student’s t-test. The Chi-squared test was used to assess frequencies. The Mann–Whitney U-test was used for the comparison of non-normally distributed data. Relationship between LLS and age, BMI, and other variables, as well as between age and BMI, BP, and other variables, was determined using the Spearman correlation test. Before the multivariate analysis, an odds ratio (OR) with 95% confidence intervals (CIs) for AMS associated with risk factors was determined using logistic regression. On univariate analysis, factors with a P-value ≤0.10 were entered into multivariate logistic regression analysis. The backward stepwise likelihood ratio test was used to determine the independent factors associated with AMS.
In the present study, the dependent variable was AMS and recorded as 0= no and 1= yes. The independent variables were age, BMI, race, education, smoking status, drinking status, physiological parameters, and AISS. We recorded the age value as 0 (≤20 years), 1 (≤25 years), 2 (≤30 years), and 3 (≤35 years).
Results
Subjects
We enrolled 987 participants at 500 m, 79 of whom were excluded for incomplete data or preceding exposure to high altitude. In addition, 22 Tibetan subjects were removed from the study because of the small sample size. After arrival at 3,700 m, one subject withdrew from the study because of high altitude pulmonary edema, and a further 29 subjects withdrew due to severe headache, diarrhea, or other unknown reasons. Thus, we analyzed data obtained from the remaining 856 subjects. Of these, 195 subjects were aged 18–20 years, 314 were aged 21–25 years, 238 were aged 26–30 years, and 109 were aged 31–35 years. The mean age in the groups aged ≤20, 21–25, 26–30, and 31–35 years was 19, 22, 27, and 33 years, respectively. Increase in BMI was consistent with increase in age (21.1±2.4, 22.0±2.3, 22.3±2.5, and 23.6±2.8 kg/m2 in the four age groups, respectively). Except for age (P<0.001) and BMI (P<0.001), no statistically significant differences in smoking and drinking status, race, and education were observed between the four age groups.
Acute mountain sickness
At an altitude of 3,700 m, the LLS in the age groups ≤20, 21–25, 26–30, and 31–35 years was 3.4±2.1, 3.3±2.1, 3.9±2.3, and 4.6±2.1, respectively. Differences in LLS between the four groups (P<0.05 or P<0.01) were significant, except for between the age groups ≤20 years and 21–25 years (P=0.68).
Accordingly, the increased prevalence of AMS was consistent with the increase in LLS (54.36%, 59.55%, 68.07%, and 73.39% in the four groups, respectively). The prevalence of AMS in the ≤20 and 21–25 years age groups was significantly lower than in the 26–30 and 31–35 years age groups (P<0.05 or P<0.01). Notably, the prevalence of severe AMS increased with increasing age (Table 1). In addition, the overall prevalence of AMS at the 3,700 m altitude was 62.38%.
In terms of AMS symptoms, the most common were headache (74.88%), followed by weakness or fatigue (73.36%), dizziness or lightheadedness (73.23%), difficulty sleeping (66.59%), and the prevalence of gastrointestinal symptoms is relatively lower than the prevalence of other AMS symptoms (22.43%). Moreover, the prevalence of AMS symptoms clearly increased with age (Figure 1), and the difference in the prevalence of headache, insomnia, and weakness or fatigue reached statistical significance (P<0.001, P<0.001, and P<0.01, respectively).
Physiological parameters
At 500 m, the HR and SaO2 for the whole group were 68±9 beats/minute and 98.1%±0.9%, respectively. No significant differences between the four age groups were observed in both HR and SaO2 (both P>0.05) (Figure 2).
  | Figure 2 Heart rate (A) and arterial oxygen saturation (B) of the four age groups at 500 m. |
After arrival at 3,700 m, the mean HR for the whole group increased to 84±12 beats/minute. Conversely, the mean SaO2 decreased to 88.8%±2.9%. This tendency was observed in all four age groups. Furthermore, significant differences between these groups were observed in HR (Figure 3), and there was a tendency for SaO2 to decrease with increasing age.
Athens Insomnia Scale score
The AISS and prevalence of insomnia in each group are shown in Table 2. AISS increased with increasing age at both 500 m and 3,700 m, and the AISS obtained at 3,700 m was significantly higher than that obtained at 500 m (each P<0.001). The same tendency was observed in insomnia prevalence. Upon rapid ascent from 500 m to 3,700 m, the increase in insomnia prevalence in the group aged 31–35 years (2.79 times) was a little more than in the other three groups (2.74, 2.58, and 2.69 times in groups aged ≤20, 21–25, and 26–30 years, respectively). Consequently, the prevalence of insomnia was significantly higher in the group aged 31–35 years than in the other three groups (each P<0.001, Table 2). Insomnia prevalence in the whole group at 500 m and 3,700 m was 18.57% and 49.88%, respectively.
Correlational analysis
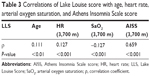
On simple univariate analysis, age, HR, and AISS were positively correlated with LLS, whereas SaO2 was negatively correlated with LLS (Table 3). No significant correlations were found between LLS and other variables (BMI, race, etc). Additionally, age was significantly positively correlated with BMI, HR, and AISS, but significantly negatively correlated with SaO2 (data not shown).
Logistic regression analysis
On univariate logistic regression analysis, AMS was associated with age (OR 1.035, 95% CI 1.003–1.068, P<0.05), smoking (OR 0.633, 95% CI 0.462–0.868, P<0.01), SaO2 (OR 0.933, 95% CI 0.890–0.978, P<0.01), and AISS (OR 1.411, 95% CI 1.333–1.495, P<0.001), but not with BMI and other variables (race, education, drinking, etc).
A multivariate analysis that considered previous age, smoking, SaO2, and AISS showed that all variables except smoking and SaO2 were significantly associated with AMS (Table 4).
Discussion
To our knowledge, this is the first large-scale epidemiological investigation of the relationship between age and AMS upon rapid ascent to high altitude. The main finding of the present study is the significantly higher prevalence of AMS in the older, young adults than in the younger, young adults after rapid ascent from 500 m to 3,700 m. Furthermore, older age, one of the risk factors for AMS, was identified by univariate and multivariable analyses in the present study. Additionally, pre-existing subjective poor sleep quality was an important contributor to the increase of the prevalence of AMS in the older, young adults.
Prevalence of AMS was higher in the present study than in two other similar altitude studies,7,8 but lower than in the studies by Ren et al14 and Murdoch.6 Differences between our study and others in terms of duration of hypoxic exposure, ascent rate, and age of subjects may account for this result. The prevalence of AMS in the study by Li et al8 was 40%; perceptibly lower than our result (54.36% in the group aged ≤20 years). The first clear difference between our study and that of Li et al8 is the duration of hypoxic exposure, which was 24 hours in our study and 48 hours in that of Li et al.8 It is known that AMS is present within 6–12 hours after exposure to altitudes above 2,500 m, and that AMS symptoms gradually resolve within 48 hours if 1 day of rest or other interventions are undertaken.4 Thus, the prevalence of AMS would be substantially affected by the duration of hypoxic exposure. In our study, the mean age in the groups aged ≤20, 21–25, 26–30, and 31–35 years was 19, 22, 27, and 33 years, respectively; whereas that of Li et al8 was 18 years. Notably, with the same ascent rate and altitude, as well as a big sample size, there is good comparability and strength between our study and that of Li et al.8 In the study by Moraga et al7 the prevalence of AMS was 56%, which was a little lower than in the group aged 21–25 years (59.55%) in our study. The discrepancy may be partially because of the slower ascent rate (approximately 435 m/hour) in Moraga et al.7 Moreover, in other research with a large sample size, Murdoch6 reported an AMS prevalence of 84% at 3,860 m, wherein subjects flew directly to 3,740 m and slept at 3,860 m. Age of the subjects in Murdoch’s study6 was clearly higher (mean age 26 versus 45 years) than in our study, which may at least partly explain the difference in prevalence of AMS between the two studies. At the same time, we also noted one other similar altitude and ascent rate study (Ren et al).14 With an average subject age of 18 years, the AMS prevalence in the study by Ren et al14 was 57.2%, which was higher than that for the group aged ≤20 years (54.36%) in the present study. The discrepancy may be mainly due to the different scoring system for the assessment of AMS used in our study and that of Ren et al.14 Through comparison of the studies by Li et al8 and Ren et al14 we can clearly see that the prevalence of AMS diagnosed by the Chinese criteria is higher than the Lake Louise criteria. In the present study, we found that higher age was a risk factor for AMS (adjusted OR 1.07, 95% CI 1.01–1.13, P=0.03), adjusted for smoking, SaO2, and AISS. However, this is in contrast to a previous study.5 After careful assessment, we think the difference in ascent profile between our study and that of Wu et al5 may mainly account for the result. On the other hand, after arrival at high altitude, the subjects in the present study mainly rested in their rooms. The subjects in the study by Wu et al5 did not rest, and the authors thought younger people were at higher risk of AMS than older people because of vigorous physical activity after arrival at high altitude. Notably, another two recent studies15,16 have suggested that older subjects may experience higher AMS prevalence upon rapid ascent to high altitudes.
A significant increase in HR was observed in the whole group after arrival at altitude, and, furthermore, a more marked increase was observed in older subjects. This is supported by the literature.17,18 Conversely, SaO2 decreased in each group, and a slightly larger decrease was observed in older subjects. At the same time, correlational analysis showed that there was a weak correlation between age and HR (r 0.064, P=0.048), as well as SaO2 (r −0.072, P=0.029). The lower SaO2 values may mean that the older subjects experience more severe hypoxia. Therefore, the HR increased more markedly in the older subjects in response to the more severe hypoxia.
Poor subjective sleep in the general population is very common in modern societies, and can be caused by diversiform factors (eg, aging, physical and mental illness, etc).19 In terms of different definitions, the prevalence of insomnia ranges from 5% to 50%,20 and the symptoms of insomnia increase with age in men.21 However, whether the sleep status of the subjects prior to the ascent would affect the prevalence of AMS is unclear, and furthermore, the sleep status of the subjects cannot be completely presented by the Lake Louise diagnostic criteria for AMS. Therefore, the AIS was used in our study to assess the sleep status of the subjects at both 500 m and 3,700 m. Consistent with previous studies,21–23 we found that AISS and prevalence of insomnia increased with age among young adult Chinese men at 500 m (Table 3), and the prevalence of insomnia was within the reported range (5%–50%). After high altitude exposure, the prevalence of insomnia markedly increased in each age group, which is consistent with a previous study conducted with the AIS.24 Furthermore, increase in insomnia in the older age group was more marked (2.79 versus 2.74, 2.58, and 2.69 times) (Table 3). High altitude hypoxia is one important reason for sleep impairment;25,26 however, existing poor subjective sleep status before the rapid ascent may be another important contributor to the increase in insomnia in the present study. Moreover, the AISS was positively correlated with age and LLS, and was a risk factor for AMS (adjusted OR 1.39, 95% CI 1.28–1.51, P<0.001). In combination, our data showed that poor subjective sleep quality was one important reason why the older, young adult Chinese men were at high risk of AMS upon rapid ascent to high altitude.
In the present study, we also observed that smoking and high SaO2 were significant protective factors against AMS by univariate analysis; however, the significance disappeared when adjusted for other significant predictors in the multivariate analysis. These results may reveal that there remain correlations between AMS and smoking status and SaO2; however, further work is needed to explore whether smoking status and SaO2 level are sensitive predictors for AMS.
The strengths of the present study are the large sample sizes, the identical ascent profile, and controlled physical activity. However, the present study has some limitations. First, the participants were all male, which could probably introduce a sex bias. Second, no participants were older than 35 years, which, therefore, makes it hard to extend our results to other age groups. Therefore, further research involving females and older subjects should be conducted to confirm these findings.
Conclusion
The present study suggests that ‘older’ age is an independent risk factor for AMS upon acute exposure to high altitude among young adult Chinese men, and sustained poor subjective sleep prior to ascent may be an important contributor to an increased prevalence of AMS in the older subjects. With the development of transportation in our modern society, it is realistic to expect that more and more people could rapidly ascend to high altitudes. A better understanding of the relationship between age and AMS could lead to highly effective prevention of AMS. Thus, the present study may have implications for the prevention of AMS among older populations upon rapid ascent to high altitudes. Further research involving females and subjects older than 36 years should be conducted to confirm the present results.
Acknowledgments
This study was supported by a grant from the Special Health Research Project, Ministry of Health, People’s Republic of China (No. 201002012). We would like to thank all the subjects as well as all the investigators whose participation enabled the study to be conducted.
Disclosure
The authors declare they have no actual or potential competing financial interests.
References
Maggiorini M, Bühler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss alps. BMJ. 1990;301(6756):853–855. | ||
Roach RC, Bartsch P, Hackett PH, Oelz O. The Lake Louise acute mountain sickness scoring system. In: Sutton JR, Houston CS, Coates G, editors. Hypoxia and Molecular Medicine. Burlington, VT: Queen City Printers; 1993:272–274. | ||
Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. New insights in the pathogenesis of high-altitude pulmonary edema. Prog Cardiovasc Dis. 2010;52(6):485–492. | ||
Bärtsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294–2302. | ||
Wu TY, Ding SQ, Liu JL, Jia JH, Chai ZC, Dai RC. Who are more at risk for acute mountain sickness: a prospective study in Qinghai-Tibet railroad construction workers on Mt. Tanggula. Chin Med J (Engl). 2012;125(8):1393–1400. | ||
Murdoch DR. Altitude illness among tourists flying to 3740 meters elevation in the Nepal Himalayas. J Travel Med. 1995;2(4):255–256. | ||
Moraga FA, Flores A, Serra J, Esnaola E, Barriento C. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollagüe (3696 m) in northern Chile. Wilderness Environ Med. 2007; 18(4):251–257. | ||
Li FX, Ji FY, Zheng SZ, Yao W, Xiao ZL, Qian GS. MtDNA haplogroups M7 and B in southwestern Han Chinese at risk for acute mountain sickness. Mitochondrion. 2011;11(4):553–558. | ||
Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. | ||
Caetano R, Mills B, Vaeth PA. Alcohol consumption and binge drinking among U.S.-Mexico border and non-border Mexican Americans. Alcohol Clin Exp Res. 2012;36(4):677–685. | ||
Houston TK, Person SD, Pletcher MJ, Liu K, Iribarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ. 2006;332(7549):1064–1069. | ||
Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55(3):263–267. | ||
Gómez-Benito J, Ruiz C, Guilera G. A Spanish version of the Athens Insomnia Scale. Qual Life Res. 2011;20(6):931–937. | ||
Ren YS, Fu ZM, Shen WM, et al. Incidence of high altitude illnesses among unacclimatized persons who acutely ascended to Tibet. High Alt Med Biol. 2010;11(1):39–42. | ||
Kriemler S, Bürgi F, Wick C, et al. Prevalence of acute mountain sickness at 3500 m within and between families: a prospective cohort study. High Alt Med Biol. 2014;15(1):28–38. | ||
MacInnis MJ, Carter EA, Freeman MG, et al. A prospective epidemiological study of acute mountain sickness in Nepalese pilgrims ascending to high altitude (4380 m). PLoS One. 2013;8(10):e75644. | ||
Siqués P, Brito J, Banegas JR, et al. Blood pressure responses in young adults first exposed to high altitude for 12 months at 3550 m. High Alt Med Biol. 2009;10(4):329–335. | ||
Hainsworth R, Drinkhill MJ, Rivera-Chira M. The autonomic nervous system at high altitude. Clin Auton Res. 2007;17(1):13–19. | ||
Vitiello MV. Sleep disorders and aging: understanding the causes. J Gerontol A Biol Sci Med Sci. 1997;52(4):M189–M191. | ||
Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. | ||
Liu X, Uchiyama M, Kim K, et al. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93(1): 1–11. | ||
Chiu HF, Xiang YT, Dai J, et al. The prevalence of sleep problems and their socio-demographic and clinical correlates in young Chinese rural residents. Psychiatry Res. 2012;200(2–3):789–794. | ||
Xiang YT, Ma X, Cai ZJ, et al. The prevalence of insomnia, its sociodemographic and clinical correlates, and treatment in rural and urban regions of Beijing, China: a general population-based survey. Sleep. 2008;31(12):1655–1662. | ||
Szymczak RK, Sitek EJ, Sławek JW, Basiński A, Siemiński M, Wieczorek D. Subjective sleep quality alterations at high altitude. Wilderness Environ Med. 2009;20(4):305–310. | ||
de Aquino Lemos V, Antunes HK, dos Santos RV, Lira FS, Tufik S, de Mello MT. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology. 2012;49(9):1298–1306. | ||
Nussbaumer-Ochsner Y, Ursprung J, Siebenmann C, Maggiorini M, Bloch KE. Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. Sleep. 2012;35(3):419–423.
|






 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at