Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Age and Referral Route Impact the Access to Diagnosis for Women with Advanced Ovarian Cancer
Authors Norbeck A , Asp M , Carlsson T, Kannisto P, Malander S
Received 2 January 2023
Accepted for publication 17 March 2023
Published 3 May 2023 Volume 2023:16 Pages 1239—1248
DOI https://doi.org/10.2147/JMDH.S401601
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Anna Norbeck,1,2 Mihaela Asp,1,2 Tobias Carlsson,3 Päivi Kannisto,1,2 Susanne Malander4,5
1Department of Obstetrics and Gynecology, Skåne University Hospital, Lund, Sweden; 2Department of Clinical Sciences, Division of Obstetrics and Gynecology, Lund University, Lund, Sweden; 3Regional Cancer Centre South, Region Skåne, Lund, Sweden; 4Department of Oncology, Skåne University Hospital, Lund, Sweden; 5Department of Clinical Sciences, Division of Oncology, Lund University, Lund, Sweden
Correspondence: Anna Norbeck, Kvinnoklinken Skånes Universitetessjukhus, Klinikgatan 12, Lund, 221 85, Sweden, Tel +4646172106, Email [email protected]
Purpose: The majority of women with ovarian cancer are diagnosed in late stages. Most women do have symptoms prior to diagnosis, sometimes several months before the diagnosis. The aim of this study was to evaluate the timeline from the first presentation of symptoms to a physician until there is a reasonable suspicion of cancer, among women diagnosed with advanced stage ovarian cancer. We wanted to investigate which symptoms were the most common and whether there are other factors affecting the time interval before the suspicion of cancer was confirmed.
Patients and Methods: This was a retrospective population-based cohort study of women diagnosed with advanced ovarian cancer between January 1, 2017 and December 31, 2019 who were referred to Skane University Hospital Lund, Sweden. Data were collected from electronic medical records at Skane University Hospital. The time interval was recorded as the time from first physician consultation with predefined symptoms to the date when there was a reasonable suspicion of ovarian cancer. Data processing and statistical analysis were performed with the statistical software R.
Results: Among the 249 patients included in this study, the median time interval from the first consultation to the reasonable suspicion of cancer was 24 days. The first consultation in specialized care had a 70% decrease in delay compared to primary care. Emergency consultations had a 52.2% decrease in time delay compared to planned consultations. Older age was associated with an increase in the geometric mean by 54.7%, comparing the first to the third quartile. The most common symptom was abdominal pain.
Conclusion: The length of time interval from first presentation with symptoms relating to ovarian cancer to reasonable suspicion of cancer was associated with whether the consultation was in primary or specialized care, emergency or planned visit and the patient’s age.
Keywords: epithelial ovarian cancer, routes to diagnosis, cancer patient pathway, time to diagnosis
Introduction
Epithelial ovarian cancer is the seventh most common cancer in women worldwide and the most lethal gynecological malignancy, with an overall five-year survival rate of approximately 40%.1,2 The most important prognostic factor for the patient is the International Federation of Gynecology and Obstetrics (FIGO) stage at diagnosis, with a 70–90% five-year survival for early-stage disease (stage I and II). Unfortunately, the majority of women are diagnosed with advanced stage disease (stage III and IV), with a five-year survival of 20–40%.3 Epithelial ovarian cancer (EOC) consists of five histological subtypes, with high-grade serous carcinoma being the most common, accounting for 70%.4 EOC develops along two different tumorigenic pathways. Type I tumors are thought to arise from benign precursor lesions, and are often diagnosed at an early stage. Type II tumors include high-grade serous carcinoma and are more aggressive. These tumors develop from a precursor lesion in the fallopian tube and disseminate to the ovaries and peritoneum, initially giving vague symptoms and are often diagnosed at a late stage with poor survival.5 The most common genetic risk factors for EOC are BRCA1 and BRCA2 germline mutations, and are found in 15% of women with EOC.6
Most women with advanced ovarian cancer (AOC) do have symptoms prior to diagnosis, such as abdominal pain, bloating, urinary urgency, loss of appetite, increased abdominal size and change in bowel habits, sometimes several months before the diagnosis. The most common symptoms bringing women to the physician are abdominal pain, increased abdominal size, urinary urgency and loss of appetite. These symptoms are usually nonspecific and have a low positive predictive value.7,8 If the symptoms are of recent onset, more frequent or severe, they are more likely to be caused by an ovarian tumor. Symptoms can occur at all stages of the disease and the majority of patients first present with symptoms to a general practitioner.8 However, a study from Denmark demonstrated that in only approximately 50% of cancer cases seen by a general practitioner were the patients’ symptoms interpreted as alarm symptoms. Therefore, symptoms not interpreted as alarm symptoms result in a longer diagnostic interval for the patient.9
Efforts have been made to speed up the diagnosis of ovarian cancer with screening programs using ultrasonography in combination with biomarkers in asymptomatic patients but without affecting the mortality rate in ovarian cancer.10 Over the last decade, several studies have investigated the cost effectiveness of screening programs for early detection and prevention of ovarian cancer, with mixed results.11 Cancer patient pathways (CPPs) for AOC have been introduced in several European countries. The intention is to create a fast-track pathway for patients with suspected malignancy and to not take more time than necessary for diagnosis and treatment, and it is most often initiated in primary care. All CPPs act with criteria-based suspicion and fixed time duration for treatment.12,13
Some studies have shown that reducing the time to diagnosis when the disease is already symptomatic does not affect overall survival in AOC or stage at diagnosis.14,15 On the other hand, time intervals greater than 80 days have been shown to be associated with decreased survival.16 A study by Rai et al showed that guideline implementation with symptom triggered-testing, including CA125 and pelvic ultrasound, followed by urgent referral to rapid access clinics resulted in increased detection of ovarian cancers, but no stage shift was observed.17 Earlier diagnosis did not lead to a down shift in stage but could result in reduced cancer burden and facilitate complete surgical resection of the tumor.18 Since complete surgical resection is a major prognostic survival factor, it is important to speed up the process for each cancer patient.19,20
In Sweden, CPP for ovarian cancer was implemented in 2016. The national guidelines include predefined alarm symptoms created by experts in the field. For ovarian cancer, the alarm symptoms were as follows: abdominal or pelvic pain, increased abdominal size or bloating, loss of appetite, frequent need to urinate, venous thromboembolism, abnormal or postmenopausal bleeding, respiratory problems or coughing, change in bowel habits or debut of inflammatory bowel syndrome.21
In an ideal flow, as shown in Figure 1, when a patient presents with alarm symptoms, the physician suspects ovarian cancer, and the patient is referred to a gynecologist for filter function. Both general practitioners, private gynecologists and other specialists refer patients to the filter function. The consultation includes a gynecological examination, vaginal ultrasonography using the International Ovarian Tumor Analysis group (IOTA) simple rules, CA125 level, and risk of malignancy index (RMI).22,23 If the suspicion of ovarian cancer is rejected by the gynecological specialist, the CPP for ovarian cancer is closed. If reasonable suspicion of ovarian cancer remains, further investigation is performed, including computed tomography of the thorax, abdomen and pelvis and, optionally, a guided biopsy of the tumor, if it is a carcinoma of unknown origin and if primary surgery is not an option. The patient is then referred to a tertiary cancer center for a multidisciplinary cancer conference (MCC) to determine the final preferred mode of treatment. The guidelines in Sweden recommend all patients with AOC to be referred to a MCC at initial diagnosis. According to the Swedish national guidelines for ovarian cancer, the lead time in the CPP from suspicion of cancer by a gynecology specialist to the start of treatment is 24 days for upfront debulking surgery and 22 days for chemotherapy.21
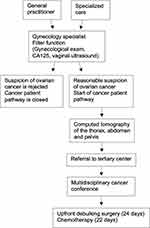 |
Figure 1 Overview of the cancer patient pathway (CPP) for ovarian cancer in Sweden. Abbreviation: CA125, cancer antigen 125. |
Aims
The aim of this study was to evaluate the timeline from the first presentation of symptoms to a physician until there is a reasonable suspicion of cancer among women diagnosed with advanced stage ovarian cancer. In addition, the most common symptoms for patients with AOC are documented. The possible role of age, mode of consultation, mode of appointment, CA125 level at time of diagnosis, histological subtype and place of residence were examined.
Materials and Methods
The health-care system in Sweden is publicly funded. Patients suspected of malignancy in primary care or regional hospitals are referred to a regional tertiary center. Skane University Hospital Lund Sweden is a tertiary center for all gynecological cancers and has a referral area for advanced ovarian cancer of approximately 1.9 million people.
This retrospective cohort study included patients diagnosed with AOC (FIGO stages III and IV) between January 1, 2017 and December 31, 2019 who were referred to Skane University Hospital Lund. All patients had a primary diagnosis with an International Statistical Classification of Diseases and Related Health Problems (ICD-10) code of C56.9 malignant neoplasm of unspecified ovary, C57.0 malignant neoplasm of fallopian tube, C76.2 malignant neoplasm of abdomen or C49.5 malignant neoplasm of connective and soft tissue of pelvis. Patients with relapse of AOC were not included in this study.
Data were collected from electronic medical records of Skane University Hospital (Melior.220-9.3.0.400–20210909.3, Cerner Corporation, Kansas City, USA). The following variables were recorded: age at diagnosis, time in days from first presentation with predefined symptoms to reasonable suspicion of cancer, first consultation in primary care or specialized care, first appointment as emergency or planned consultation, S-CA125 level at time before start of treatment, date of diagnosis, kind of symptoms for first consultation, place of patient residence, histological subtype and mode of treatment.
The group specialized care included both consultants in the hospital, regardless of specialty, and gynecologists in private health care. If a patient was referred from a general practitioner in primary care to the emergency department the same day as she first presented with symptoms, primary care was recorded as the first place of consultation. The diagnostic time interval was recorded as the time from the first physician consultation with predefined symptoms associated with ovarian cancer or incidental findings to the date when there was a reasonable suspicion of ovarian cancer.
The primary symptoms were classified as abdominal or/and pelvic pain, bloating or/and increased abdominal size, loss of appetite, urinary urgency, change in bowel habits, abnormal or/and postmenopausal bleeding, respiratory problems or/and coughing, venous thromboembolism and incidental findings. If more than a single symptom was presented, the symptom that led the patient to seek medical care was recorded as the main symptom. Only symptoms that were new within the last 13 months were included.
Patients’ residency at the time of diagnosis was recorded. According to the Swedish Association of Local Authorities and Regions, urban areas are classified as large cities and municipalities near large cities, with a population of at least 200,000 inhabitants. The urban area also includes medium-sized towns and municipalities with a population of at least 50,000 inhabitants and commuting municipalities near medium-sized towns. All other places are classified as rural areas.
Statistical Analyses
For descriptive statistics, continuous variables are presented as medians and first and third quartiles, and categorical variables are presented as frequencies and proportions (%).
Since the data were skewed, they were log transformed for the statistical analysis. A multivariable log-linear regression model was used to assess the relationship between time in days from first presentation with predefined symptoms to reasonable suspicion of cancer; one day was added as log (0) is undefined. The primary independent variable of interest was whether patients’ first level of consultation occurred in specialized care or in primary care. Other variables were age, type of consultation, mode of treatment, S-CA125 level and place of residence. All estimates were adjusted and presented as continuous variables with corresponding 95% confidence intervals (CIs), and presented in terms of interquartile ranges. The geometric mean was calculated for the time interval between the first presentation with symptoms and reasonable suspicion of cancer.
A simple nonparametric test as a complementary analysis was used when the distribution of the type of consultation was compared to the other variables, and an unpaired two-samples Wilcoxon test and the chi-square test of independence with Monte Carlo simulation were used. Data processing and statistical analysis were performed with the statistical software R.24
Ethical Approval
The study complies with the Declaration of Helsinki and it was approved by the Swedish Ethical Review Authority (Dnr 2019-04450). Date of approval 2019-10-26. Informed consent was not required in this retrospective study. Our study is a non-intervention study with no direct medical implication on the patient’s health. Regarding GDPR and integrity questions, we secured the patients’ data, and the individual identification of the patient was disabled. All patients have a study number and all patient data is under locked deposition.
Results
A total of 252 patients were identified, and three patients were excluded from the cohort due to a lack of essential data (date of first consultation with predefined symptoms); thus, 249 patients were included in the study. The median age of the patients was 72 years. No differences were seen in median age in the patient group that first visited a general practitioner compared to those who visited a physician in specialized care.
Descriptive statistics are presented in Table 1. The majority of patients, 65.9% first presented with symptoms in primary care, whereas 34.1% had their first consultation in specialized care. Among the 249 patients included in this study, 53.4% were planned and 46.6% were emergency consultations. The median time from the first consultation with a physician to reasonable suspicion of cancer was 24 days, as shown in Table 2.
 |
Table 1 Descriptive Statistics, Days from First Consultation to Reasonable Suspicion of Cancer per Patient Characteristics |
 |
Table 2 Patient Characteristics, Stratified by Level of Consultation |
Figure 2 shows how different variables affect the time interval from first consultation to reasonable suspicion of cancer. Patient age was related to a longer delay to reasonable suspicion of cancer by 54.7% (16.6–105.3) 95% CI when comparing the first to the third quartile. When the first visit was in specialized care, a decrease in delay by 70% (−78.8 to −57.4) (95% CI) was observed compared to primary care. Emergency consultations were linked to a decrease in time delay by 52.2% (−65.9 to −32.9) (95% CI) compared to planned consultations. No significant difference in the time interval was found when comparing the different symptoms.
Discussion
In this population-based retrospective study from Southern Sweden, approximately 66% of the patients diagnosed with AOC first went to a general practitioner to get their diagnosis. Nevertheless, the results showed that the median time interval from the first consultation to reasonable suspicion of cancer was significantly shorter when the first physician’s visit took place in specialized care compared with primary care, given other factors constant. Some patients might have presented directly to specialized health care as emergency visits if the waiting time to primary care was related to their symptoms. This study did not assess the grade of the symptoms at the first consultation, and there were no objective measurements of the symptoms. The overall time interval from the first consultation to reasonable suspicion of cancer was a median of 24 days, which is comparable to what has previously been reported for diagnostic time intervals for AOC.16 It has also been shown that long diagnostic delay among women with gynecological cancer could be caused by lack of cancer suspicion by referrals or a suspicion of other diseases.25 This might be related to the relatively rare incidence of gynecologic cancer patients in general practitioners’ clinics. Compared to related cancers such as colorectal cancer, AOC is an uncommon disease and therefore not easily recognizable in everyday practice.26
When the first consultation was an emergency visit compared to a planned physician’s appointment, the time interval to reasonable suspicion of cancer was significantly shorter. The reason for this could be that these patients had more severe symptoms, such as vomiting, severe abdominal pain or difficulty breathing, at the time of presentation, indicating a need for fast-track care. Moreover, others have shown that if the first consultation is at the emergency department, it is associated with a worse prognosis, which could be due to the patients having more advanced disease.16 It has also been shown that patients with poor survival had a shorter time to diagnosis.15 Similarly, in our study, the patients treated with the best supportive care had a rather short time to treatment, indicating that a poor performance score is a natural reason for faster referral.
Our results show that older patients had a significantly longer diagnostic time interval. This could be a result from misinterpretation of the patient’s status and attributing cancer symptoms to the normal aging process, which is understandable due to the uncommon diagnosis.8 Interestingly, the dominant symptom for women with AOC was abdominal or pelvic pain, which was present in almost half of the patients studied. On the other hand, many women presenting to primary care have vague symptoms that could be associated with harmless transient conditions but not with ovarian cancer.8 A recent study by Chan et al showed that more than 70% of patients with high-risk early-stage ovarian cancer have symptoms.27
There is evidence that the way the physician interprets the patients’ symptoms, as vague or alarming, affects the lead time to diagnosis. If the physician suspects ovarian cancer, they are more often urgently referred to CPP and have a shorter diagnostic time interval. Patients with symptoms that are nonspecific or have comorbidities have longer diagnostic time intervals. A recently published study from Denmark showed that only one-third of patients with ovarian cancer were referred urgently to CPP.28
In our study, patients with high CA125 values had a shorter time interval for CPP. This finding is, however, close to zero and therefore probably not important clinically. A recent study by Funston et al examined the performance of assessing CA125 levels in primary care and found that the test is useful for detecting ovarian cancer, particularly in women >50 years. Furthermore, an interesting finding was that nonovarian cancers could also be detected by the test, indicating that the CA125 test could help in decision-making for further investigation and urgent referral to a specialist.29
When the place of residence was in a rural area, the median time interval was 22.5 days, and for urban areas, it was 28 days. There was no difference in the two groups, rural area or urban area, when comparing whether the first consultation was in primary care or specialized care. In our study, 68.7% of the women underwent surgery and received chemotherapy, which is the recommended mode of treatment.30
A limitation of this retrospective study is that it was not possible to determine the severity of symptoms before the first consultation. We also do not know the date of symptom onset, which could have been months before the patient first presented to a physician. Patients might also have had their first contact with a nurse before their first visit to a physician. Another limitation is the small number of patients included in the study. Confounders such as performance status and comorbidity might have affected the results and thus the time from first consultation to reasonable suspicion of cancer. A strength of our study is that it is a population-based study and it includes all patients diagnosed with AOC in the population of Southern Sweden over three years in recent past.
Conclusion
In conclusion, we found that the time interval from first presentation with symptoms to reasonable suspicion of ovarian cancer was associated with whether the consultation was in primary care or specialized care, emergency or planned visit and the patient’s age. Improved awareness of ovarian cancer symptoms in primary care is of vital importance so that patients are referred urgently to gynecologists to reduce the time to diagnosis. Although no difference in overall survival has been shown for a longer time to diagnosis, it could be important at the individual level if more patients receive the recommended treatment of both surgery and chemotherapy. Educational programs for rapid admittance from health centers to gynecologists are warranted. Further research is needed to investigate whether other factors are associated with a longer time to diagnosis and whether confounders affect the results.
Abbreviations
FIGO, International Federation of Gynecology and Obstetrics; EOC, epithelial ovarian cancer; AOC, advanced ovarian cancer; CPP, cancer patient pathway; CA125, cancer antigen 125; MCC, multidisciplinary cancer conference; IQR, interquartile range.
Acknowledgments
The authors would like to acknowledge Malin Crusensvärd at Region Skane, Dept Obstetrics and Gynecology for skillful help in finalizing the list of patients included in the cancer patient pathway.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Regional Cancer Centre in Southern Sweden, grants from Region Skane.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15(1):23–34. doi:10.1016/S1470-2045(13)70546-1
3. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
4. Kossaï M, Leary A, Scoazec JY, Genestie C. Ovarian cancer: a heterogeneous disease. Pathobiology. 2018;85(1–2):41–49. doi:10.1159/000479006
5. Kurman RJ, Shih IM. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747. doi:10.1016/j.ajpath.2015.11.011
6. Hodgson A, Turashvili G. Pathology of hereditary breast and ovarian cancer. Front Oncol. 2020;10:531790. doi:10.3389/fonc.2020.531790
7. Hamilton W, Peters TJ, Bankhead C, Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339(aug25 2):b2998. doi:10.1136/bmj.b2998
8. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291(22):2705–2712. doi:10.1001/jama.291.22.2705
9. Jensen H, Tørring ML, Olesen F, Overgaard J, Vedsted P. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14(1):636. doi:10.1186/1471-2407-14-636
10. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–2303. doi:10.1001/jama.2011.766
11. Ghose A, Bolina A, Mahajan I, et al. Hereditary ovarian cancer: towards a cost-effective prevention strategy. Int J Environ Res Public Health. 2022;19(19):12057. doi:10.3390/ijerph191912057
12. Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101(Suppl S2):S5–S8. doi:10.1038/sj.bjc.6605383
13. Prades J, Espinàs JA, Font R, Argimon JM, Borràs JM. Implementing a cancer fast-track programme between primary and specialised care in Catalonia (Spain): a mixed methods study. Br J Cancer. 2011;105(6):753–759. doi:10.1038/bjc.2011.308
14. Dilley J, Burnell M, Gentry-Maharaj A, et al. Ovarian cancer symptoms, routes to diagnosis and survival - population cohort study in the ‘no screen’ arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Gynecol Oncol. 2020;158(2):316–322. doi:10.1016/j.ygyno.2020.05.002
15. Nagle CM, Francis JE, Nelson AE, et al. Reducing time to diagnosis does not improve outcomes for women with symptomatic ovarian cancer: a report from the Australian ovarian cancer study group. J Clin Oncol. 2011;29(16):2253–2258. doi:10.1200/JCO.2010.32.2164
16. Altman AD, Lambert P, Love AJ, et al. Examining the effects of time to diagnosis, income, symptoms, and incidental detection on overall survival in epithelial ovarian cancer: Manitoba Ovarian Cancer Outcomes (MOCO) study group. Int J Gynecol Cancer. 2017;27(8):1637–1644. doi:10.1097/IGC.0000000000001074
17. Rai N, Nevin J, Downey G, et al. Outcomes following implementation of symptom triggered diagnostic testing for ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2015;187:64–69. doi:10.1016/j.ejogrb.2015.02.011
18. Gilbert L, Basso O, Sampalis J, et al. Assessment of symptomatic women for early diagnosis of ovarian cancer: results from the prospective DOvE pilot project. Lancet Oncol. 2012;13(3):285–291. doi:10.1016/S1470-2045(11)70333-3
19. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized Phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) [German working group for gynecological oncology, study group for ovarian carcinoma (AGO-OVAR) and the French group of National Investigators for Ovarian Cancer Studies (GINECO)]. Cancer. 2009;115(6):1234–1244. doi:10.1002/cncr.24149
20. Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103(2):559–564. doi:10.1016/j.ygyno.2006.03.051
21. Confederation of Regional Cancer Centers. Standardiserat vårdförlopp ovarialcancer, epitelial [Cancer patient pathway for epithelial ovarian cancer]. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/aggstockscancer-epitelial/vardforlopp/.
22. Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;16(5):500–505. doi:10.1046/j.1469-0705.2000.00287.x
23. Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990;97(10):922–929. doi:10.1111/j.1471-0528.1990.tb02448.x
24. Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing V; 2017.
25. Vandborg MP, Edwards K, Kragstrup J, Vedsted P, Hansen DG, Mogensen O. A new method for analyzing diagnostic delay in gynecological cancer. Int J Gynecol Cancer. 2012;22(5):712–717. doi:10.1097/IGC.0b013e31824c6d0e
26. Ingeman ML, Christensen MB, Bro F, Knudsen ST, Vedsted P. The Danish cancer pathway for patients with serious non-specific symptoms and signs of cancer-a cross-sectional study of patient characteristics and cancer probability. BMC Cancer. 2015;15:421. doi:10.1186/s12885-015-1424-5
27. Chan JK, Tian C, Kesterson JP, et al. Symptoms of women with high-risk early-stage ovarian cancer. Obstet Gynecol. 2022;139(2):157–162. doi:10.1097/AOG.0000000000004642
28. Baun ML, Jensen H, Falborg AZ, Heje HN, Petersen LK, Vedsted P. Ovarian cancer suspicion, urgent referral and time to diagnosis in Danish general practice: a population-based study. Fam Pract. 2019;36(6):751–757. doi:10.1093/fampra/cmz013
29. Funston G, Hamilton W, Abel G, Crosbie EJ, Rous B, Walter FM. The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: a population-based cohort study. PLoS Med. 2020;17(10):e1003295. doi:10.1371/journal.pmed.1003295
30. Colombo N, Sessa C, Bois AD, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;29(4):728–760. doi:10.1136/ijgc-2019-000308
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

