Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Adverse Events Induced by PD-1/PD-L1 Inhibitors: A Real-World Single-Centre Experience with a Management-Based Approach
Authors Grimaud F, Penaranda G , Stavris C, Retornaz F, Brunel V, Cailleres S, Pegliasco H, Le Treut J, Grisoni V, Coquet E , Chiche L , Rognon A
Received 24 February 2021
Accepted for publication 8 May 2021
Published 30 June 2021 Volume 2021:17 Pages 669—677
DOI https://doi.org/10.2147/TCRM.S308194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Fabien Grimaud,1 Guillaume Penaranda,2 Chloé Stavris,3 Frédérique Retornaz,3 Véronique Brunel,4 Sylvie Cailleres,4 Hervé Pegliasco,5 Jacques Le Treut,5 Vincent Grisoni,6 Emilie Coquet,1 Laurent Chiche,3 Amélie Rognon1
1Department of Pharmacy, Hôpital Européen, Marseille, France; 2Department of Biostatistics, Hôpital Européen, Marseille, France; 3Department of Internal Medicine, Hôpital Européen, Marseille, France; 4Department of Haemato-Oncology, Hôpital Européen, Marseille, France; 5Department of Pulmonology, Hôpital Européen, Marseille, France; 6Department of Urology, Hôpital Européen, Marseille, France
Correspondence: Amélie Rognon
Department of Pharmacy, Hôpital Européen, 6 Rue Désirée Clary, Marseille, 13003, France
Tel +33 4 13 42 73 00
Fax +33 4 13 42 76 80
Email [email protected]
Aim: To assess the efficacy and tolerance of programmed death-1 (PD-1) and PD-ligand 1 (PD-L1) inhibitors and the impact of a standardised management-based protocol in a real-world setting.
Patients and Methods: Data from patients who had received anti-PD-(L)1 were collected from our pharmacy database. Clinical response and toxicity were assessed using RECIST criteria and CTCAE version 5.0, respectively. Overall survival (OS) and progression-free survival (PFS) were estimated with the Kaplan–Meier method. Potential prognostic factors were identified using Cox’s model.
Results: A total of 196 patients and 201 lines of treatment were included (median age: 66 (range: 38– 89) years). Types of cancer included non-small cell lung cancer (73%), transitional cell carcinoma (10%), renal cell carcinoma (6%), small cell lung cancer (5%), head and neck squamous cell carcinoma (4%) and classical Hodgkin’s lymphoma (1%). Twenty-five (12%) patients had pre-existing autoimmune conditions. Our standardised management-based protocol included 129 (64%) patients. Objective response rate was 29%, median OS was 10 months (IQR: 7– 15) and median PFS was 5 months (IQR: 1– 22). Patients with an abnormal baseline complete blood count had a worse OS (HR=2.48 [95% CI: 1.24– 4.96]; p=0.0103). Thirty-three (16%) patients experienced severe (grade 3 or 4) immune-related adverse event (irAE). There were three (1%) irAE-related deaths. AEs resolved faster when patients were assessed by an internist before anti-PD-(L)1 initiation (p=0.0205).
Conclusion: PD-1 and PD-L1 inhibitors are effective and safe in a real-world setting. Implementation of a standardised management-based protocol with internal medicine specialists is an effective way to optimise irAE management.
Keywords: immunotherapy, elderly, PD-1 inhibitor, PD-L1 inhibitor, PDL-1 inhibitor, safety, immune-related adverse events, solid tumours, prognostic biomarkers
Introduction
Immune checkpoint inhibitors (ICIs) and especially programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) inhibitors have become the cornerstone of many cancer treatments.1 However, data on patients with pre-existing autoimmune conditions, numerous comorbidities and/or advanced age are lacking as these conditions often exclude such patients from clinical trials that are thus not representative of a real-world setting. In addition, the majority of clinical trials involving ICIs report efficacy data without considering concomitant drugs at the time of immunotherapy initiation that are now known to potentially impact ICI efficacy.2
Because of their mechanism of action, ICIs may cause side effects termed immune-related adverse events (irAEs) that can occur in all organs and can potentially be life-threatening and responsible for treatment discontinuation.3–6 Most of the time, corticosteroids are given and are sufficient to control irAEs but their impact on immunotherapy efficacy is still unclear.7 Although these complications are now better known, their management remains a diagnostic and therapeutic challenge.8,9
In our centre, oncologists can have their patients assessed by an internal medicine specialist (IMS) as part of a standardised management-based protocol before starting immunotherapy in order to identify risk factors for developing irAEs (ie, pre-existing or ongoing autoimmune conditions), for optimisation of co-prescriptions (ie, corticosteroids) and for therapeutic education on the management of the most common potential irAEs.10,11 In the case of development of severe and/or multiple irAEs, the IMS coordinates a multidisciplinary team including oncologists and other specialists depending on the complication site.
The aim of this study was to determine the efficacy and toxicity of PD-1/PD-L1 inhibitors in a real-world cohort. We also aimed to evaluate the impact of our standardised management-based protocol on the management of irAEs and patients’ outcomes.
Patients and Methods
Study Population
Patients followed-up at our institution (European Hospital of Marseille, France), aged ≥18 years, with malignant metastatic or non-metastatic solid tumours or with haematological malignancies who started treatment with ICIs (as a single agent or in combination with chemotherapy) were included. Their ICIs had to be a PD-1 inhibitor or a PD-L1 inhibitor and to be administered in standard clinical practice (ie, outside a therapeutic clinical trial) from the first dose at our institution.
Ethics and Regulations
This study, based on public interest, did not involve humans, but only the reuse of already recorded data. The data accessed complied with relevant data protection and privacy regulations. In accordance with the French regulation, this study required neither information nor non-opposition of the included individuals and the study was approved by the institutional and ethical review board of the European Hospital of Marseille.
Data Collection and Definitions
Data from patients who received ≥1 dose of ICI between August 2015 and February 2020 were collected from our pharmacy database. Efficacy and toxicity data were extracted from electronic medical records until May 31, 2020. Prescription software was used to obtain key dates for ICI administration (initiation, transient interruptions and final withdrawal). Tumour type, location of metastatic sites when existing and number of previous anti-cancer agents were obtained from multidisciplinary meeting reports. Baseline biological abnormalities before ICI initiation as well as data concerning comorbidities (especially pre-existing autoimmune conditions and smoking) and concomitant drugs (corticosteroids and PPIs at the time of immunotherapy initiation, antibiotics up to 1 month before ICI initiation) were collected (Supplementary Materials - Methods: Data Collected and Definitions Section).
As part of the management-based approach, consultations with an IMS before ICI initiation or for the management of potential irAEs were also documented systematically. Data about AE management (management start date, specialists involved, corticosteroids or other drugs used, efficacy of corticosteroids if used, hospitalisation due to AEs, outcomes and date of resolution) were also collected.
Clinical response was assessed using RECIST criteria (Response Evaluation Criteria in Solid Tumours) version 1.1, and toxicity was evaluated by study investigators according to Common Terminology Criteria for Adverse Events (CTCAE version 5.0).12,13 All AEs were analysed, including all potential irAEs, according to medical notes in electronic records and response to corticosteroids if used. A probability scale of being an irAE was used (unlikely, likely, very likely, almost confirmed) (Supplementary Materials, Methods: Data Collected and Definitions Section).
Statistical Analysis
Patient characteristics are reported as absolute frequency and percentage for categorical variables and as median and interquartile range (IQR: 25–75th) for continuous variables. Primary endpoint for treatment efficacy was overall survival (OS) and secondary endpoints were progression-free survival (PFS) and objective response rate (ORR). OS was defined as the time from ICI first dose to death from any cause. PFS was defined as the time from ICI first dose to progressive disease or death from any cause, whichever came first. ORR was defined as the proportion of patients showing a complete or partial response to anti-PD-(L)1. PFS and OS were estimated by the Kaplan–Meier method. Patients who were still alive at the last date of observation or who had not met a progression event were considered as censored for OS and PFS, respectively. For determining ICI treatment duration, patients who had not stopped immunotherapy on May 31, 2020, which was the last date of data collection, were considered as censored.
Univariate and multivariate Cox proportional hazards regression models were used to identify potential prognostic factors. Impact of age was assessed using the threshold of 70 years.21 Concerning treatment tolerance, primary endpoint was the presence of an “at least likely” irAE and grade ≥2, secondary endpoints were the presence of an “at least likely” irAE and grade ≥3 and presence of an “at least very likely” irAE and grade ≥2. Univariate analysis was performed to identify potential factors influencing AE frequency, considering severity and probability of AEs being irAEs using the Chi2 or Fisher test accordingly. A p-value <0.05 was considered to be statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.).
Results
Patient Characteristics at Baseline
Overall, 196 patients (71% males and 29% females) who received at least one dose of anti-PD-(L)1 during the study period were included, among which 191 received one anti-PD-(L)1, 175 as a single agent and 16 in combination with chemotherapy. The other five patients received two anti-PD-(L)1 as a single agent at different times; they have therefore each been considered as two patients making a total of 201 lines of treatment. The characteristics of the patients and their ICI are shown in Table 1. Median age at ICI initiation was 66 (range: 38–89) years and 74 (37%) were aged ≥70 years. Twenty-five (12%) patients had pre-existing autoimmune conditions including only a family history of autoimmune diseases in four (2%). Median ICI treatment duration was 4 months [95% CI: 3–5] (data not shown). One-hundred and twenty-nine (64%) patients (Table 1) could be assessed by an IMS as part of the standardised management-based protocol usually performed the day before ICI initiation (median time: 1 day (IQR: 0–5)).
 |
Table 1 Patient Characteristics According to Internal Medicine Consultation Before ICI Initiation |
Efficacy of PD-1 or PD-L1 Inhibitors
Median OS was 10 months [IQR: 7–15] and median PFS was 5 months [IQR: 1–22] (Table 1). The potential prognostic factors are analysed in Table S1. A better prognosis in terms of OS was observed in patients who experienced AEs (unadjusted p=0.0002, Figure 1A), an association confirmed after adjustment by multivariate analysis (HR=0.45 [95% CI: 0.25–0.82]; p=0.0083). The same observation was made for PFS in univariate analysis (unadjusted p<0.0001, Figure 1B) and multivariate analysis (HR=0.54 [95% CI: 0.31–0.93]; p=0.0277). Patients with transient treatment interruptions also had better OS and PFS (p<0.0001). The reasons for transient interruptions are listed in Table S2. A similar prognosis was observed for patients aged ≥70 years in terms of OS (unadjusted p=0.1176, Figure 1C) and PFS (unadjusted p=0.0859, Figure 1D). In contrast, a worse prognosis in terms of OS was observed for patients with abnormal CBC before ICI initiation in both univariate (unadjusted p<0.0001, Figure 1E) and multivariate analysis (HR=2.48 [95% CI: 1.24–4.96]; p=0.0103), which is in line with PFS (unadjusted p=0.0012, Figure 1F). Other clinical or biological factors did not appear to affect ICI efficacy. ORR was 29% as 58 cases had a complete or partial response to anti-PD-(L)1 (Table 1).
Tolerance of PD-1 or PD-L1 Inhibitors
Overall, treatment was well tolerated. One-hundred and sixty-five (82%) patients experienced 391 AEs with a median of two AEs per patient (Table 2). Most AEs were gastrointestinal (n=82, 21%), pulmonary (n=75, 19%) or dermatological (n=70, 18%). One-hundred and five (27%) AEs involved several sites. Thirty-three (16%) patients experienced severe (grade 3 or 4) toxicity, which was a very likely or almost confirmed irAE (Table S4). There were three (1%) treatment-related deaths very likely due to irAEs. IrAEs usually developed after a median time of 4 weeks (IQR: 2–9). The association between patient characteristics, AE severity and probability of being an irAE is explored in Table S3. Considering grade ≥3 AEs that are at least likely to be irAEs, patients with high serum creatinine before ICI initiation seemed to have a higher risk of experiencing an AE (p=0.0263). The same associations were observed for patients for whom autoantibodies were detected before the start of ICI (p=0.0277) and for patients with low TSH (p=0.0225). Overall, 53 (26%) and 39 (19%) patients received systemic corticosteroids to treat 71 grade ≥2 AEs and 46 grade ≥3 AEs, respectively. No other immunosuppressive/immunomodulatory agents were used, except hydroxychloroquine for managing two rheumatological AEs. Treatment was stopped because of AEs in 26 (13%) patients (Table S2).
 |
Table 2 Characteristics of irAEs |
Management of irAEs and Impact of Our Standardised Management-Based Protocol
Univariate analysis revealed an almost significant worse OS (p=0.0516) for patients who underwent an initial evaluation with an IMS before ICI initiation, but this was not confirmed by multivariate analysis (Table S1). AEs resolved faster when patients had been assessed by an IMS before ICI initiation, with a median time to resolution of 14 days (IQR: 7–28) vs 23 days (IQR: 8–56) (HR=1.50 [95% CI: 1.06–2.1]; p=0.0205). Management of AEs was usually started in the daytime and the specialists involved are listed in Table 3.
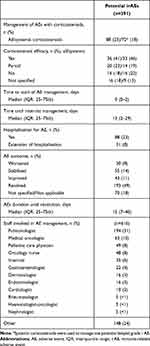 |
Table 3 Potential irAEs Management |
Discussion
The present study reports the long-term efficacy and tolerance of PD-1 and PD-L1 inhibitors among nearly 200 patients with various malignancies in real-world conditions. Importantly, our efficacy and tolerance results are in line with several meta-analyses of Phase II and Phase III clinical trials as well as recent real-world cohort studies.14–17
With regards to the efficacy outcomes, we confirmed interesting findings suggested by recent other studies. First, our results confirm that the occurrence of AEs is associated with anti-PD-(L)1 efficacy, suggesting that irAEs reflects a beneficial over-activation of the immune response.18,19 Second, although immunosenescence gives rise to concerns about response and tolerance to immunotherapy in older patients, we did not notice any significant negative impact in our older patients, a result that is similar to those of other recent real-world studies.20,21 Third, the usual concomitants drugs at the time of immunotherapy initiation such as corticosteroids, PPIs and antibiotics that are now known to potentially impact ICI efficacy did not seem to affect the overall ICI efficacy in our cohort.2,22,23 Finally, in contrast to other clinical or biological parameters, we observed that abnormal CBC at ICI initiation was significantly and strongly associated with a poorer outcome. Although this finding warrants further prospective validation, it is in line with the results observed for abnormal lymphocyte count and more generally, a recent effort to identify biomarkers for predicting response to ICIs using routinely available blood markers.24–26
With regards to safety outcomes, our cohort confirms the relatively good tolerance of anti-PD-(L)1 in a real-world setting. Although irAEs are frequent and may develop in any tissue and at any time after ICI initiation, grade 3 and 4 irAEs are not common and irAEs usually develop within the first 3 months.27 Most irAEs are easily managed or are sometimes responsible for transient ICI interruption and/or initiation of systemic corticosteroids for the most severe or persistent side-effects. Importantly, older age was not associated with poorer tolerance. Furthermore, our study seems to confirm that ICIs can be safely administered to patients with pre-existing autoimmune conditions, at least in a setting carrying out, as in our institution, an initial evaluation and regular follow-up to check for the quiescent status of underlying autoimmune conditions.28
Our management-based protocol was created by a multidisciplinary task force including IMS, oncologists and other specialists with close follow-up and we could observe a faster resolution of AEs in patients for which such protocol was initiated involving IMS assessment before ICI initiation. Due to the unbalanced ratio of the different cancer types between patients with or without an initial evaluation by an IMS, the results for the impact of cancer type on OS must be interpreted with caution. The paradoxical trend in univariate analysis could have been expected with regards to: the worst prognosis of lung cancer (versus other malignancies) and the overrepresentation of this type of cancer in the group of patients for which oncologists asked for an evaluation by an IMS before ICI initiation (98% vs 42%), the overrepresentation of RCC in the group without (17% vs 0%), and the overrepresentation of combined chemotherapy in the first group (12% vs 1%).29 Finally, the absence of significant differences for OS or PFS in multivariate analyses could reflect a relative protective effect of the intervention proposed to the most severe patients, as judged by the oncologist in charge at ICI initiation.
This study has several limitations. First, it was retrospective in nature and included different tumour types and different drugs targeting the PD-(L)1 axis, but overall, the study population is representative of a real-world setting. Second, conversely to prospective therapeutic trials, AE severity grade and probability of being an irAE was estimated using only medical records, which could limit the accuracy of these data, especially as we reported any AEs exhaustively. Finally, with regards to the possible impact of our standardised management-based protocol, we could not analyse separately the respective role of the various aspects of the intervention: the identification of risk factors for developing irAEs such as pre-existing autoimmune conditions, the optimisation of co-prescriptions such as corticosteroids, the coordination of irAE management with a multidisciplinary team including oncologists and other specialists, and therapeutic education on the management of the most common potential irAEs for which the delay to a patient reporting evocative symptoms was not recorded systematically.
Collectively, our study adds to recent findings confirming the efficacy and safety of PD-1 and PD-L1 inhibitors in a real-world setting. Importantly, older and younger patients treated with PD-1 and PD-L1 inhibitors had similar long-term oncological outcomes and a similar risk of irAEs. Better identification of patients at risk of poorer outcomes is still a challenge. Although AE occurrence is associated with anti-PD-(L)1 efficacy, early management for the diagnosis and treatment of AEs is the key to a successful outcome. Our study suggests that the implementation of a standardised management-based protocol with an IMS and a multidisciplinary approach is an effective way to prevent and treat irAEs faster and should be extended to all patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. doi:10.1038/s41467-020-17670-y
2. Rossi G, Pezzuto A, Sini C, et al. Concomitant medications during immune checkpoint blockage in cancer patients: novel insights in this emerging clinical scenario. Crit Rev Oncol Hematol. 2019;142:26–34. doi:10.1016/j.critrevonc.2019.07.005
3. Postow MA, Sidlow R, Hellmann MD, Longo DL. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481
4. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi:10.1038/nrclinonc.2016.58
5. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385
6. Stein C, Burtey S, Mancini J, et al. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: a real-life study in a single-centre cohort. Nephrol Dial Transplant. 2020:gfaa137. doi:10.1093/ndt/gfaa137.
7. Garant A, Guilbault C, Ekmekjian T, et al. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017;120:86–92. doi:10.1016/j.critrevonc.2017.10.009
8. Naidoo J, Zhang J, Lipson EJ, et al. A multidisciplinary toxicity team for cancer immunotherapy–related adverse events. J Natl Compr Canc Netw. 2019;17(6):712–720. doi:10.6004/jnccn.2018.7268
9. Kostine M, Chiche L, Lazaro E, et al. Opportunistic autoimmunity secondary to cancer immunotherapy (OASI): an emerging challenge. Rev Méd Interne. 2017;38(8):513–525. doi:10.1016/j.revmed.2017.01.004
10. Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a Nationwide, Multicenter Cohort Study. Arthritis Rheum. 2019;71(12):2100–2111. doi:10.1002/art.41068
11. Kostine M, Stavris C, Chiche L. Opportunistic autoimmunity secondary to immunotherapy and melanoma: back to ABCDE? Eur J Cancer. 2017;81:240–241. doi:10.1016/j.ejca.2017.03.017
12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
13. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). version 5.0 [Internet]; 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
14. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6(1):26. doi:10.1186/s40425-018-0336-8
15. Zhao B, Zhao H, Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. 2020;12. doi:10.1177/1758835920937612
16. Alguilar EJ, Gainor J, Kravets S, et al. MA04.05 outcomes in NSCLC patients treated with first-line pembrolizumab and a PD-L1 TPS of 50–74% vs 75–100% or 50–89% vs 90–100%. J Thorac Oncol. 2018;13(10):S367–8. doi:10.1016/j.jtho.2018.08.343
17. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016
18. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018:4. doi:10.1001/jamaoncol.2017.2925.
19. von Pawel J, Syrigos K, Mazieres J, et al. Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol. 2017;28:v469. doi:10.1093/annonc/mdx380.017
20. Pawelec G, Derhovanessian E, Larbi A. Immunosenescence and cancer. J Geriatr Oncol. 2010;1(1):20–26. doi:10.1016/j.jgo.2010.04.002
21. Corbaux P, Maillet D, Boespflug A, et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. Eur J Cancer. 2019;121:192–201. doi:10.1016/j.ejca.2019.08.027
22. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J Clin Oncol. 2018:36. doi:10.1200/JCO.2018.79.0006.
23. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi:10.1126/science.aan4236
24. Ménétrier-Caux C, Ray-Coquard I, Blay J-Y, et al. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7(1):85. doi:10.1186/s40425-019-0549-5
25. Diehl A, Yarchoan M, Hopkins A, et al. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8(69):114268–114280. doi:10.18632/oncotarget.23217
26. Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117(7):913–920. doi:10.1038/bjc.2017.274
27. Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl4):iv264–6. doi:10.1093/annonc/mdy162
28. Donia M, Pedersen M, Svane IM. Cancer immunotherapy in patients with preexisting autoimmune disorders. Semin Immunopathol. 2017;39(3):333–337. doi:10.1007/s00281-016-0595-8
29. Sukari A, Nagasaka M, Alhasan R, et al. Cancer site and adverse events induced by immune checkpoint inhibitors: a retrospective analysis of real-life experience at a single institution. Anticancer Res. 2019;39(2):781–790. doi:10.21873/anticanres.13175
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

