Back to Journals » OncoTargets and Therapy » Volume 13
Adverse Effects of Immune-Checkpoint Inhibitors in Hepatocellular Carcinoma
Authors Cui TM, Liu Y , Wang JB, Liu LX
Received 6 September 2020
Accepted for publication 29 October 2020
Published 16 November 2020 Volume 2020:13 Pages 11725—11740
DOI https://doi.org/10.2147/OTT.S279858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Tian-ming Cui,1 Yao Liu,2 Jia-bei Wang,2 Lian-xin Liu2
1Department of Hepatic Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 2Department of Hepatobiliary Surgery, Anhui Province Key Laboratory of Hepatopancreatobiliary Surgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China
Correspondence: Lian-xin Liu
Department of Hepatobiliary Surgery, Anhui Province Key Laboratory of Hepatopancreatobiliary Surgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, 14th Floor, Building 3, South District, Anhui Provincial Hospital, Hefei City, Anhui Province, People’s Republic of China
Email [email protected]
Abstract: Immune-modulatory therapy, especially with immune-checkpoint inhibitors (ICIs), has reshaped cancer therapeutics. Immunotherapy is relatively a novel approach that can effectively delay the progression of aggressive tumors and inhibit tumor recurrence and metastasis in many different tumor types. In the past years, ICIs have shown a sustained response and promising long-term survival in patients with advanced hepatocellular carcinoma (HCC). Nevertheless, ICI therapy can unbalance the immune system and result in a wide range of immune-related adverse events (irAEs), which are generally manageable but occasionally lead to a fatal outcome. HCC generally develops in the context of liver cirrhosis which is typically caused by viral hepatitis and non-alcoholic steatohepatitis. These underlying diseases may cause symptoms that overlap with irAEs and lead to consequences such as late recognition, inadequate work-up, and inappropriate treatment. Owing to the growing use of immunotherapy in HCC, it is necessary for clinicians to strengthen their understanding of the frequency, clinical features, and management of irAEs. This review focuses on the common toxicities associated with ICI therapy in patients with HCC and summarizes therapeutic strategies that can be used to monitor and manage such toxicities.
Keywords: immune-checkpoint inhibitors, hepatocellular carcinoma, hepatotoxicity, cutaneous toxicity, gastrointestinal toxicity, therapeutic strategies
Introduction
Hepatocellular carcinoma (HCC) is the sixth-most common cancer and the fourth leading cause of cancer-related deaths worldwide. HCC is characterized by high malignancy and mortality, rapid progression, recurrence, and metastasis.1 There are several treatment strategies for HCC, including curative hepatectomy, ablation, embolization, chemotherapy, and liver transplantation. However, the prognosis is still rather poor, because the majority of patients are diagnosed at a terminal stage.2 To our best knowledge, tyrosine kinase inhibitors (TKIs) such as sorafenib3 and Lenvatinib4 as first-line treatment and regorafenib,5 cabozantinib,6 and ramucirumab7 as second-line treatment have led to clinically meaningful improvements in patients with advanced disease. However, the improvement in overall survival (OS) rate is still not positive. This is partly because of the liver’s drug-metabolizing properties and the increased levels of multidrug-resistance proteins expressed by HCC cells. In addition, the characteristics of adverse events associated with TKI use make it unsuitable for some patients. Thus, new treatment strategies are still needed for this intractable disease.
After decades of therapeutic stagnation, immunotherapy emerged as a promising therapy and has transformed the field of cancer therapeutics. Unlike other organs, the liver is considered a lymphatic organ. Hepatic immunity is associated with the induction of immunotolerance, which is one of the most formidable barriers to immune-based therapy for HCC. As the liver is constantly exposed to a large number of antigens and microorganisms contained in the diet, it requires complex immune tolerance in its environment to maintain immune homeostasis. During the development of HCC, tumor cells; negative immune regulatory cells (myeloid-derived suppressor cells, tumor-associated macrophages and T regulatory cells); and hepatic stromal cells (liver sinusoidal endothelial cell, dendritic cells, Kupffer cells, and hepatic stellate cells) can orchestrate a strong immunosuppressive milieu through the secretion of immunosuppressive cytokines and abnormal expression of antigens that can greatly modulate tumor growth by escaping the immunological surveillance system.8 The immunosuppressive properties of chronic hepatitis-B virus (HBV) or hepatitis-C virus (HCV) infection have also been well-documented.9 Therefore, compared with traditional chemotherapy and molecular-targeted therapy, immunotherapy utilizes the natural anti-tumor response of the immune system to amplify and prolong the immune response. The antitumor response that immunotherapy relies on can persist until the end of treatment.9 Because of impaired self-tolerance, a wide range of immunotherapy-related adverse events (irAEs) that involve a large spectrum of organs should be taken seriously. While most of them are generally manageable, some can be life-threatening if not managed properly. In this review, we have summarized the ICI-related adverse events in patients with HCC and discussed appropriate management methods.
ICI Treatment
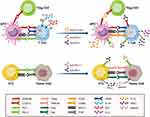
Lymphocyte co-stimulation is an essential process of T-cell function activation through molecules targeting its stimulatory receptors (signal 2); whereas, immune checkpoints including cytotoxic T-lymphocyte protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand PD-L1 are co-inhibitory molecules that play important roles in promoting the state of T-cell dysfunction known as exhaustion to avoid over-activation of T cells and collateral tissue damage10 (Figure 1). CTLA-4, which is expressed by activated T cells and Treg cells, can deliver inhibitory signals to the T cell during the initiation of an immune response that is opposite to the T-cell receptor (TCR) signal by binding its ligands CD80 and CD86 that are expressed on the surface of antigen-presenting cells (APCs). In tumors, CTLA-4 can induce Treg-cell activity and differentiation and upregulate indoleamine 2.3-dioxygenase (IDO) and interleukin-10 (IL-10) in dendritic cells (DCs) to further promote immunosuppression. PD-1, which is mainly expressed by CD8+ and CD4+ T cells, can prevent TCR signal transduction by binding its ligand PD-L1 and inhibit the activation of T cells and secretion of cytotoxic cytokines, thereby leading to exhaustion of T cells.11 Thus, reversing the anergy or exhaustion of T cells within the HCC microenvironment and promoting tumor cell death by blocking these inhibitory receptors with monoclonal antibodies (mAbs) makes immune checkpoints attractive therapeutic targets. Furthermore, the combination of ICIs with other therapies such as TKIs as well as dual inhibition of two immune checkpoint molecules also have reasonable theoretical basis. Inhibition of the B7-CTLA-4 pathway can increase the number of activated cytotoxic T lymphocytes (CTLs) infiltrating into tumor tissues.12 VEGF inhibitors can also facilitate the accumulation and infiltration of anticancer T cells within tumor tissues and upregulate PD-L1 expression on tumor cells, thereby enhancing the efficiency of the anti-PD-1/PD-L1 pathway and reversing the immunosuppressive microenvironment into an immunostimulatory microenvironment.13
The application of ICI-based immunotherapy in cancers—as either monotherapy or combination therapy—has made remarkable progress (Table 1). To date, nivolumab14 and pembrolizumab15 (anti-PD-1) have been approved by the US-FDA as second-line treatment for advanced HCC. Consistent with previous reports, monotherapy with nivolumab and pembrolizumab for advanced HCC showed clinically meaningful improvements in OS rate, Objective remission (ORR) rate, and complete response (CR) rates in the Checkmate 459 and Keynote-22416 Phase III clinical trials published in ECMO and ASCO, respectively; however, these results did not reach the predefined threshold of OS. Another large-scale trial for pembrolizumab (KEYNOTE-394, NCT03062358) is currently ongoing in the Asia-Pacific region. Recently, camrelizumab has been approved by NMPA as second-line treatment for Chinese patients with advanced HCC. According to the results published in CSCO, even in the case of poor baseline enrollment, camrelizumab achieved an objective response of 14.7% and a 6-month OS probability of 74.4%. Monotherapy with tislelizumab has showed some antitumor effect and fair tolerance in a phase Ia/Ib trial and phase III trial is underway.17 The combination of nivolumab plus ipilimumab has recently been approved by the US-FDA as second-line treatment for advanced HCC.18 Durvalumab combined with tremelimumab has also been proved effective and safe in advanced liver cancer. In the latest IMbrave150 clinical study, the combination of atezolizumab and bevacizumab has showed better OS and progression-free survival than sorafenib and have been approved as first-line treatment of unresectable or metastatic HCC.19 The result of camrelizumab plus apatinib20 and lenvatinib plus pembrolizumab are highly anticipated.21
 |
Table 1 Status of Current Immune Checkpoint Inhibitor Trials in Advanced HCC |
The gut commensal microbiota plays an important role in the formation of systemic immune responses by affecting local and systemic inflammatory responses. Therefore, manipulating the microbiota may become a promising adjuvant treatment that regulates cancer immunotherapy. According to vivo and vitro experiments results, commensal bifidobacterium,22 Faecalibacterium,23 and Akkermansia muciniphila can promote anti-tumor immunity and significantly improve the effect of anti-PD-1 therapy. Bacteroides fragilis24 and Faecalibacterium25 can play an important role in promoting anti-CTLA-4 immunotherapy. The effect of probiotics on HCC immunotherapy needs further verification. Besides, the richness and evenness of fecal bacterial community samples, the presence of microbiota-associated modules for bacterial polyamine transport system and the biosynthesis of thiamine, riboflavin and pantothenate were positively correlated to progression-free survival (PFS) time, and can be used as biomarkers to predict the risk of anti-CTLA-4 related colitis and screen sensitive patients.23
irAEs in ICIs Treatment
The precise rationale underlying irAEs is unknown, but given the fundamental function of maintaining the immune system activation within normal range, blocking inhibitory checkpoint molecules gives rise to wide-ranging irAEs that are mainly considered as an overaction of the immune system.26 The frequency of toxic reactions associated with ICI treatment is lower than that with TKIs and show prolonged duration and delayed onset, which tend to appear beyond week 8 of therapy.27 IrAEs can influence a broad range of organ systems. Most of them are typically reversible, but increasing awareness of fatal ICI-associated AEs is very important. The severity of irAEs is identified by the Common Terminology of Clinical Adverse Events (CTCAE) categorization. An accurate determination of the toxicity grade is of vital importance, because it determines immunotoxicity treatment and guides when ICIs therapy may be restarted.
irAEs in Patients with Cancer
In terms of the overall incidence of irAEs, CTLA-4 inhibitor-related irAEs tend to be dose-dependent. The incidence of all grades and ≥ grade 3 CTLA-4 inhibitor-related irAEs is 72% and 24%, respectively.28 IrAEs associated with PD-1 or PD-L1 inhibitors is not dose dependent, 66.0% of patients developed at least 1 irAE of any grade, and 14.0% of patients developed at least 1 irAE of grade 3 or higher severity.29 The median onset time of CTLA-4 inhibitors is generally shorter than that of PD-(L)1 inhibitors.30 These reports have shown that anti-CTLA-4 monotherapy is more likely to be related with higher rate of incidence and grade of irAEs than anti-PD-1/PD-L1 monotherapy.
When it comes to target organs, meta-analyses have indicated that the most common target organs with irAEs during anti-CTLA-4 monotherapy are the skin (44%), gastrointestinal tract (35%), endocrine glands (6%), and the liver (5%); rash and colitis appear to be more frequent. The involvement of other organ systems such as circulatory, ocular, and nervous and musculoskeletal systems was extremely low.28 Further, high-grade skin, endocrine, and hepatic events account for less than 5% in total, while gastrointestinal events account for 11%. In a recent meta-analysis, 6528 patients were treated with an ICI and 42 fatal irAEs (0.64%) were recorded. Among them, colitis was the most common cause of fatal irAEs.31
According to a large meta-analysis on patients who accepted anti-PD-L1 monotherapy, the most common targets for irAEs were the skin, gastrointestinal tract, and the thyroid gland. The most frequent fatal irAEs were pneumonitis, hepatitis, and neurotoxic effects.32 Hypothyroidism and hyperthyroidism were the most frequent all-grade endocrine irAEs. The liver was less involved and irAEs were mostly manifested as increased aspartate aminotransferase [AST] or alanine aminotransferase [ALT] levels. Less than 2% patients had grade 3 or higher irAEs. Interestingly, among patients who have pre-existing autoimmune diseases, exacerbation of autoimmune condition after anti-PD-(L)1 therapy is rarely reported, and the occurrence rate of irAEs is similar to that in other patients.33 The relevant mechanisms need further study.
Due to the distinct but complementary mechanism of anti-CTLA-4 and anti-PD-(L)1 therapy, the combination therapy is prone to appear incrementally toxicity. Compared with monotherapy, patients received combination immunotherapy had an increased risk of irAEs and more frequent multi-organ involvement. The incidence rates of any grade and grade 3 or higher irAEs were 88% and 41%, respectively.34 Myocarditis, myositis, and neurologic events are the most common fatal ICI toxic events in this combination strategy.32
irAEs in Patients with HCC
Because of the unique liver immunobiology and chronic inflammatory conditions of the liver such as cirrhosis and viral hepatitis, the diagnosis and management of irAEs in patients with HCC may present different challenges (Tables 2 and 3). In general, compared with other tumor types, the incidence of irAEs in HCC patients does not show significant difference. Overall, the use of PD-1 inhibitors, nivolumab and pembrolizumab, in patients with advanced HCC were associated with severe irAEs incidence of 10–20%, which was >30% in other tumors.35 According to a Phase I clinical trial evaluating the CTLA-4 checkpoint inhibitor, tremelimumab, in a small cohort of patients, few disabling AEs were reported. However, because of the limited experimental data, this result can not reflect the entire picture.36
 |
Table 2 The Incidence of irAE for Different Immunotherapy Methods for HCC |
 |
Table 3 Immune-Related Adverse Events in Patients with HCC |
Individually, the incidence of hepatic irAEs is higher in HCC patients. In total, 13.7% of HCC patients who accepted PD-1 inhibitors monotherapy experienced hepatitis enzymes elevation of any grade,37 while only 3% of patients experienced hepatitis enzymes elevation and 0.85% of patients experienced treatment-related hepatitis among other tumor types.29 Reactive cutaneous capillary endothelial proliferation (RCCEP) is a unique adverse effect related with camrelizumab treatment which is not reported in other tumor types. The safety profile of dual inhibition of two immune checkpoint molecules shows no significant difference from monotherapy. However, nivolumab and ipilimumab combination has higher rates of irAEs than anti-PD-1 monotherapy in HCC.
Considering that anti-VEGF therapies can facilitate the transport of tumor therapy drugs, combination therapy with immune-checkpoint inhibitors may reduce the dose of immune-checkpoint inhibitors, thereby reducing the risk of toxicity.13 According to the IMbrave150 clinical trial report, the incidence of all grades and high-grade irAEs in patients receiving atezolizumab–bevacizumab combination therapy is not significantly different from that of anti-PD-1 monotherapy and PD-1 plus CTLA-4 blockers combination therapy. Compared with the common irAEs in receiving anti-PD-1 monotherapy, the incidence of cutaneous irAEs decreased in patients receiving combination therapy (12% for rash, 19.5% for pruritus), the incidence of diarrhea was not significant difference, and less than 10% of patients reported abnormal thyroid function. Hypertension is the most common irAE (29.8%) in atezolizumab–bevacizumab combination therapy, which is consisted of the known safety profile of bevacizumab. A total of 20.1% and 10.3% of patients have proteinuria and epistaxis, which had rarely been reported in anti-PD-1 monotherapy and anti-PD-1 and CTLA-4 combination therapy. In total, 19.5% and 14% of patients had an increase in AST and ALT respectively, slightly lower than anti-PD-1 monotherapy and PD-1 plus CTLA-4 blockers combination therapy.
Chronic HBV/HCV infection is the common underlying disease in HCC patients. The reactivation of HBV during anti-tumor therapy such as conventional chemotherapy and some targeted therapies can deteriorate liver function, increase mortality rates and lead to therapy interruption.38 However, PD-1 work can as an important immunosuppressant to prevent severe liver damage that blocking the PD-1/PD-L1 axis may also cause the destruction of liver cells, release of the previously latent hepatitis virus into the circulation, and the reactivation of the virus. However, HBV/HCV infection is not a contraindication for ICIs treatment for HCC. The lack of antiviral prophylaxis is the only significant risk factor for HBV reactivation. Effective antiviral treatment before and during ICIs therapy and close monitoring of active viral hepatitis are recommended for HCC patients with HBV/HCV infection. According to a systematic review involving 186 HBV/HCV infected patients with various cancers, the safety and efficacy of ICIs treatment are not significantly affected by HBV/HCV infection. The incidence of hepatic transaminase elevating (HLE) among these patients after ICIs therapy is 22.0% and 10.8% of patients experienced grade 3 or 4 HLE which can be reversed by antivirus or using steroid without cease of ICIs.39 Consistent with the result of this systematic review, a retrospective study involves 60 HCC patients proved the safety of the patients whose HBV viral load are higher than 100 IU/mL when receive nucleos(t)ide analogs (NUCs) therapy during ICIs treatment. No HBV reactivation reported in the antiviral prophylaxis group. One out of six patients who did not receive NUCs treatment experienced HBV reactivation and was controlled by tenofovir treatment soon, no HBV-related liver failure occurred.40 Accordingly, HBV surface antigen (HBsAg), HBV core antibody (HBcAb), HBV surface antibody (HBsAb), and HCV antibody should be tested before ICI therapy. HBV DNA and quantitative HCV RNA levels and genotype should be obtained if HBsAg, HBcAb or HCV antibody are positive. The mechanism of virus reactivation induced by ICIs therapy is still need further investigation. HBV-specific CD8+ T cells can express PD-1 in chronic HBV infection, and can partially restore their antiviral function by blocking the effect of PD-1/PD-L1.
According to a recent retrospective study, ICI treatment is safe and have a certain effect on HCC patients with Child–Pugh class B (CPB) liver function, however, the prognosis remains poor and the rate of irAEs always higher than patients with Child-Pugh class A HCC, usage of ICI is likely to be safe.41,42 The treatment strategy for CPB HCC warrants further investigation. Here, we will describe the most common irAEs associated with ICC-treated HCC patients and discuss screening and monitoring strategies based on the general guidelines provided by SITC, ASCO, NCCN, and ESMO.
Cutaneous Adverse Events
Cutaneous toxicity is the first common AE among patients receiving ICI therapy and generally occurs within 2 weeks of beginning treatment. Among them, rash and pruritus are the most common clinical features. Incidence rates of ≥grade 3 skin toxicities were less than 1% and 4% in monotherapies and combination therapies, respectively. According to the single-agent nivolumab clinical trial, the occurrence of rash and pruritus was not dose-dependent. The incidence of rash and pruritus in HCC patients treated with nivolumab and pembrolizumab monotherapy was 11–23% and 10–11.5%, and 13–19% and 12–18.3%, respectively. In the phase I clinical trial of combination treatment with durvalumab and tremelimumab and Phase II clinical trial of combination of nivolumab and ipilimumab, the incidence of pruritus and rash was 22.5% and 12.5%, and 30–45% and 17–29%, respectively. Among patients received atezolizumab–bevacizumab combination therapy, 12% of them reported rash and 19.5% of them reported pruritus. However, no grade 3 or 4 skin toxicity was reported. Interestingly, 67% of patients with HCC who accepted camrelizumab monotherapy experienced RCCEP. All of them had grade 1–2 toxicity that tended to appear after 4 weeks of therapy.43 Data on tremelimumab are limited. According to the small phase II trial, the occurrence of a grade 1 or 2 rash was 65% and that of a grade 3 rash was 5%.36
When diagnosing and treating skin complications, the causes of other skin-related symptoms, such as infections, effects of other drugs (eg, TKI), autoimmune diseases, and skin conditions related to chronic liver dysfunction should be carefully excluded. Therefore, it is necessary to record a comprehensive assessment of cutaneous involvement and systemic effects, as well as professional dermatologist intervention to assist with diagnosis and treatment. Patients with mild grade 1 or 2 reactions can continue ICI therapy and relieve symptoms by topical emollients, topical mild-strength corticosteroids (triamcinolone [0.1%]) and oral antihistamines. For more symptomatic grade 2 or 3 reactions, in addition to local treatment, corticosteroids may be administered systemically at a dose of 0.5–1 mg/kg depending on the severity of the symptoms. Biopsy should be considered and ICI treatment must be discontinued until the severity of AEs drop to grade 1. For higher grade or life-threatening toxic skin reactions, ICI should be permanently discontinued and patients should be admitted immediately under the close supervision of a dermatologist. Treatment includes intravenous injection of methylprednisolone 1–2 mg/kg with gradual tapering of dose according to drug response.44,45
Digestive System AEs
Diarrhea is a common adverse reaction of the digestive system during ICI therapy. Diarrhea occurred in 11% of HCC patients during pembrolizumab treatment, 10% during nivolumab treatment, 12–24% during nivolumab and ipilimumab combination treatment, 12% during durvalumab and tremelimumab combination treatment and 18.8% during atezolizumab–bevacizumab combination therapy. Tremelimumab use showed a higher incidence, with 30% having grade 1–2 diarrhea and 5% having ≥ grade 3 diarrhea.36 In HCC patients receiving PD-L1 inhibitors and combination therapy, the rates of ≥ grade 3 complications were 1% and 2–4%, respectively.
Colitis is another serious irAE. Severe colitis can lead to fatal colonic perforation and peritonitis.46 As for the overall incidence, colitis was reported in 10–12% of patients receiving anti-CTLA-4 treatment, 1% of patients receiving anti-PD-L1 treatment, and 14% of patients receiving combination therapies. It has been reported that 7–8% of patients with CTLA-4 block, 0.5–1.5% of patients with PD-L1 block, and 8–9% of patients with dual block developed ≥ grade 3 complications. In patients with HCC, colitis is mostly of grade 3 and occurs in 1% of those receiving PD-L1 inhibitor and 2.6% of those receiving anti-CTLA-4 and anti-PD- L1 combination therapy. Less than 10% of patients reported colitis during atezolizumab–bevacizumab combination therapy.
The gastrointestinal condition should be comprehensively evaluated before receiving ICI, an accurate baseline should be established, and the patient’s diarrhea and colitis symptoms should be evaluated regularly during treatment for effective management. When patients report new onset diarrhea or worsening of existing symptoms, care should be taken to exclude gastrointestinal infections, tumor progression, diarrhea that may be caused by other drugs, and non-colitis immune-mediated toxicity such as hyperthyroidism. Blood tests and stool routine should be performed to identify thyroid abnormalities, bacterial or parasitic infections, and autoimmune disease. Biopsy samples are also necessary to define the pathological characteristics and for molecular analyses. In case of colitis, endoscopy is the gold standard for diagnosis and can help to assess the degree of illness and prognosis.47
For mild diarrhea, ICI treatment can be continued on the basis of maintaining homeostasis of the internal environment. For persistent grade 2 and higher degree of diarrhea or colitis, clinicians should consider permanently discontinuing CTLA-4 agents and provisionally withholding PD-1 and PD-L1 agents. Systemic corticosteroid treatment (1–2 mg/kg/d, intravenous) should be administered immediately; g PD-1 and PD-L1 agents can be restarted if the patient can recover to grade 1 or less diarrhea and/or colitis. If the patients have an intravenous intolerance for 3–5 days, the drug should be taken orally and reduced gradually after 8–12 weeks. If symptoms do not improve in 2–5 days, clinicians should consider immunosuppressive therapy with infliximab (5 mg/kg).48 For grade 4 diarrhea or colitis, in addition to the above treatment, clinicians should permanently withhold ICI treatment. If colon perforation occurs during treatment, emergency surgery should be performed. In a recently published retrospective review, early introduction of selective immunosuppressive treatment (infliximab) was shown to improve the prognosis of patients with ICI treatment-related colitis.49 Recent studies have shown that vedolizumab can work as an alternative therapy for infliximab in immune-associated enterocolitis.50 In one case report, cytomegalovirus reactivation was found in a patient with refractory colitis after ICI treatment, which may provide evidence for future treatment.51
The basic composition of the gut microbiota may be an important determinant of immune-related colitis after anti-CTLA-4 treatment. Patients with overrepresented faecalibacterium and firmicutes have an increased risk of therapeutic colitis compared with patients with intestinal flora dominated by bacteroides.25 The recolonization of B fragilis and burkholderia cepacia could alleviate treatment-induced colitis.24 Therefore, the baseline microbiota composition of patients is recommended to be tested before treatment to screen the beneficiaries, increase the abundance of anti-cancer probiotics, enhance effect, and overcome resistance in immunotherapy.
Liver-Related Complications
Liver-related AEs tend to appear at 4–12 weeks after treatment initiation. Delayed hepatotoxicity can also occur after discontinuation of immunotherapy or after treatment of hepatotoxicity. The severity of hepatotoxicity is determined by the degree of abnormalities in biochemical hepatic indicators in patient sera, classification by multiple degrees of the upper limit of normal (ULN). The diagnosis of immune-related hepatitis requires exclusion of all causes of hepatitis, including hepatitis flare, obstruction of biliary tract, bacterial infection, tumor progression, and use of hepatotoxic drugs. Even in severe cases, hepatotoxicity tends to be asymptomatic; thus, regular examination of liver function is warranted and a liver function baseline should be measured before each treatment cycle. Generally, compared with anti-CTLA-4 mAbs, the onset of irAEs caused by anti-PD(L)1 mAbs is delayed.52 Although acute hepatitis resulting from ICI treatment is rare, cases of acute hepatic failure have been reported after administration of CTLA-4 and PD-1 inhibitors.53,54
Liver histology and liver function tests are essential to distinguish between immune-related hepatitis from autoimmune hepatitis, make a definite diagnosis, and assess the severity of hepatotoxicity. The predominant histological pattern of ICI-induced hepatitis demonstrated pan-lobular hepatitis and bile duct injury including fibrin ring granulomas, central vein endotheliitis, prominent sinusoidal lymphohistiocytic infiltrates, and endothelialitis involving central veins.55 CTLA-4 blockade can cause elevation of several biochemical indicators in liver tests, including alkaline phosphatase, AST/ALT, and bilirubin. Histology related to anti-CTLA-4 mAbs use showed granulomatous hepatitis with fibrin deposition and central vein endotheliitis. PD-(L)1 inhibitors generally just cause an elevation of AST/ALT, and the histological features associated with anti-PD-(L)1 mAbs is lobular non-granulomatous hepatitis.52 In addition, compared with autoimmune hepatitis and drug-induced liver injury, the number of CD3+ and CD8+ lymphocytes in ICIs treatment-related hepatitis increased, and the number of CD20+ B cells and CD4+ T cells decreased.55 Moreover, lobular hepatitis with necrosis caused by anti-CTLA-4 mAbs is either spotty or confluent because of the inflammatory infiltration that is generated by CD8 lymphocytes,56 while anti-PD-(L)1 mAbs can cause both CD4 and CD8 lymphocytes infiltration. Furthermore, autoimmune hepatitis has characteristics of plasma cell infiltration, severe interface hepatitis, piecemeal necrosis, and rosette formation, which immune-related hepatitis lacks.
Generally, hepatotoxicity is categorized as grade 1 (AST/ALT ≤3× ULN), grade 2 (AST/ALT 3–5× ULN), grade 3 (ALT/AST 5–20× ULN) and grade 4 (ALT/AST>20× ULN) according to CTCAE version 4.03. Considering that the basic liver function of HCC patients may be abnormal, hepatotoxicity also can be categorized as grade 1 (AST/ALT ≤5× ULN), grade 2 (AST/ALT 5–8× ULN), grade 3 (AST/ALT 8–20× ULN) and grade 4 (ALT/AST>20× ULN).35 Among patients received ICI therapy, AST and ALT elevation of all levels and ≥ grade 3 has been reported in 21% and 12%, and 15% and 6% patients, respectively, among those receiving nivolumab; 13–22.6% and 7–13.3%, and 9–17.6% and, 4–6.1% among those receiving pembrolizumab; and 21% and 5%, and 22% and 1% among those receiving camrelizumab; 19.5% and 14%, and 7% and 3.6% among those receiving atezolizumab–bevacizumab combination therapy. In a phase III clinical trial of nivolumab monotherapy, 17% of patients experienced hepatic AEs and 10% of them were ≥ grade 3. In total, 17.5% of patients showed AST elevation when administered a combination of durvalumab and tremelimumab. Chronic hepatitis infection and nonalcoholic steatohepatitis of HCC patients may make the diagnosis of treatment-related hepatitis more difficult. A total of 10–20% of patients with HCC experienced any level of AST elevation when receiving a placebo.5,57,58 Therefore, to accurately grasp the appropriate treatment timing of ICI-induced hepatotoxicity, it is essential to clarify the classification and treatment principles of liver AE.
It is critical for patients to accept routine medical assessments for early detection and treatment of ICI-induced hepatotoxicity. When immune-mediated hepatitis is suspected, drugs that can cause latent liver toxicity should be ruled out, and levels of alkaline phosphatase, GGT, bilirubin, albumin, and thrombin should be checked. If the condition worsens, ultrasound or computed tomography examination should be performed to exclude ascites, biliary tract obstruction, and/or tumor progression. Tissue biopsy can be used to differentiate ICI-induced hepatotoxicity from autoimmune liver disease. The viral load of patients infected with HBV and HCV should be monitored carefully. A recent study reported that cytomegalovirus reactivation may also be the reason for immune-related hepatitis which is worth further investigation.59
Steroid therapy is the most common treatment in most ICI-induced hepatotoxicity. For grade 2 hepatotoxicity, ICI treatment should be suspended and can be resumed when hepatitis improves to grade 1. Routine use of corticosteroids is not recommended. For grade 3 or higher, ICI treatment should be hold and consider resuming ICI when hepatitis improves to grade 2. If grade 3 hepatotoxicity does not improve while suspend ICI, oral prednisone 0.5–1 mg/kg/day is recommended. Intravenous injection methylprednisone 1–2 mg/kg/day is recommended for grade 4 hepatotoxicity and need to discontinue ICI treatment permanently. Reintroduction of ICI treatment may not necessarily cause relapse of hepatitis.60 Mycophenolate mofetil, azathioprine, budesonide, cyclosporine, tacrolimus, ursodeoxycholic acid (UDCA), anti-thymocyte globulin (ATG), tocilizumab (IL-6 receptor antagonist), and plasma exchange could be used as an additional immunosuppressant to treat steroid refractory hepatotoxicity.60 Because of the concern for anti-TNF induced liver injury, most guidelines do not support infliximab as a treatment for steroid-refractory toxicity, however, no such cases have been reported.
Thyroid Dysfunction
Thyroid dysfunction is the most common endocrine irAE in ICI treatment. The rationale of immune-related thyroid dysfunction is not completely known, but mainly involves that anti-PD-(L)1 therapy can enhance preexisting antithyroid antibodies through modulating humoral immunity, thereby causes silent inflammatory thyroiditis.61 Immune-related thyroid dysfunction usually manifests as hyperthyroidism and hypothyroidism, which can generally be diagnosed by detecting serum TSH level and comparing with the level before treatment. Among them, hypothyroidism is more common, usually manifested as fatigue, weight gain, bradycardia and slow transit. Hyperthyroidism usually manifests as fatigue, nervousness, weight loss, and palpitations.
In patients receiving pembrolizumab and camrelizumab monotherapy, the incidence of hypothyroidism was 5–6% and 9%, respectively, and almost no high-level events reported. In total, 3.2% of patients had hyperthyroidism in pembrolizumab monotherapy, and there were no reports of grade 3 irAE. In the atezolizumab plus bevacizumab combination therapy, less than 10% of patients with abnormal thyroid function and the specific proportion did not reflect in the report. Thyroid dysfunction has a higher incidence in the combined treatment of nivolumab and ipilimumab, with hypothyroidism in 8–20% of patients and hyperthyroidism in 6–10% of patients, but no high-level events occurred. Since the liver is an important place for the conversion of thyroxine T4 to T3, the free T4 level and free T3 level of patients with cirrhosis are higher than normal. When the liver function of patients with liver cirrhosis damaged severely, a decrease in T3 level will also be found. Moreover, the clinical manifestations of abnormal thyroid function are usually masked by the clinical manifestations of tumors. Therefore, before receiving ICI treatment, screening for existing thyroid dysfunction through TSH assay and establishing a baseline of T4 and TSH levels are particularly important for the diagnosis of treatment-related thyroid dysfunction. When abnormal TSH and T4 levels are found, it is necessary to ensure that there is no iodine saturation associated with the injection of contrast media, and to exclude drug interference that easily affects the hypothalamic-pituitary-thyroid axis (such as glucocorticoids), and TSH concentration caused by non-thyroid diseases Decrease (such as low T3/T4 syndrome) and other primary diseases that cause hyperthyroidism (such as primary hyperthyroidism). Generally, elevated TSH indicates hypothyroidism, and elevated T4 level indicates thyrotoxicosis.
Generally, there is no need to stop immunotherapy when immune-related thyroid function abnormalities occur. When symptomatic hyperthyroidism occurs, β-blocker therapy can be used to control the heart rate. Treatment should be carried out according to EUGOGO group guidelines when Graves disease reported. If TSH> 10mIU/L or symptomatic hypothyroidism occurs and TSH 5–10 mIU/L is tested twice in a row, levothyroxine should be considered. Early intervention of endocrinologists to develop individualized treatment plans is recommended.
Rare Immune-Related Toxicities
The incidence of irAEs was relatively low in the other target organs involved. For PD-1 inhibitors, the incidence of pneumonia in HCC patients treated with nivolumab was approximately 3%, and there were few patients with ≥grade 3 severity. The incidence of anemia was 8%, and about 2% of patients developed grade 3 complications. In addition, only one case of AKI was reported out of all 262 patients. In the phase II pembrolizumab clinical trial, 6% and 1% of HCC patients reported grade 1 or 2 and grade 3 myalgia, respectively, and no cases were found in phase III clinical trial. Only 4% of patients experienced anemia, while 0.5–3% of patients experienced adrenal insufficiency. However, the number of patients involved is not enough to exclude the potential risk of irAEs. It is worth noting that irAEs may appear within a few months after the interruption of ICI.62 Additionally, in other ICIs trials, only one of the 104 patients developed grade 3 heart failure, and there were no other heart-related complications.
In clinical trials of tremelimumab for HCC, only three out of 15 patients developed acute renal failure, and three out of every 20 patients developed ≥grade 3 encephalopathy, yet these complications are thought to be more associated with underlying cirrhosis than treatment with ICIs.
For the combination therapy of anti-CTLA-4 and anti-PD-1, 10% of patients developed pneumonia when treated with a combination of nivolumab and high-dose ipilimumab, while 2.5% of patients developed pneumonia when treated with combination of durvalumab and tremelimumab.
Despite the low incidence, if left untreated, complications related to the respiratory, circulatory, and nervous systems can prove fatal. For pneumonia, early diagnosis and classification are critical to improving prognosis. When immune-related pneumonia is suspected, chest radiographies and lung CT scans should be carried out, and respiratory and/or infectious disease specialists should be recommended for early multidisciplinary input to exclude infectious diseases, tumor progression, and pulmonary embolism. Unless there is a diagnostic problem, no biopsy of the affected lung tissue is required. For grade 1 pneumonitis, consideration should be given to discontinuing ICI treatment and monitoring every 2–3 days. For grade 2 pneumonitis, patients with mild-to-moderate symptoms should be given oral prednisolone (1 mg/kg/day) and antibiotics. Once the pneumonia resolves to ≤grade 1 and the patient is no longer on steroids, ICI treatment can be resumed. In terms of grade 3 or 4 disease, clinicians should permanently discontinue ICI treatment and immediately start intravenous methylprednisolone at a rate of 1–2 mg/kg/day. Further respiratory support treatment should be considered. If there is no improvement after 48 h, infliximab (5 mg/kg) or MMF (1 g, b.i.d.) should be considered. Infliximab can be reused after 2 weeks depending on the patient’s condition.
For neurological complications, it is important to rule out other causes of neurological symptoms and consult a neurologist as soon as possible. Further, serum ammonia levels should be measured to rule out hepatic encephalopathy due to cirrhosis. Brain MRI can rule out tumor metastasis. Wernicke’s encephalopathy can be excluded by inquiring about alcohol and dietary history, and electroencephalography can also be used to rule out subclinical epileptic activity. Nerve conduction studies, electromyography, and cerebrospinal fluid tests can be used to exclude peripheral sensory or motor neuropathy. Hepatitis C virus RNA, liver tests, and serum cryoglobulin can identify autonomic neurological diseases caused by chronic HCV infection. Examination of paraneoplastic autoantibodies can be used to exclude autoimmune encephalitis and paraneoplastic syndrome.63,64 For Guillain–Barre syndrome and myasthenia gravis, ICI treatment should be stopped immediately, and steroids, intravenous immunoglobulin, and plasma exchange therapy should be considered. Although corticosteroids are not recommended for idiopathic Guillain–Barre syndrome, they are applicable when it comes to ICI-related forms. For aseptic meningitis and encephalitis, steroids should be used as soon as possible; if herpes simplex virus or bacterial infection are also present, patients should be treated with antiviral drugs and antibiotics, respectively. For neurological complications, extra care should be taken before resuming ICI treatment and discussed in detail with the patient.
Electrocardiography, chest radiography, echocardiography, and blood levels of troponin and brain natriuretic peptide should be assessed as soon as possible if any level of cardiac AEs occur. In this case, ICI treatment should be permanently discontinued and prednisolone (1–2 mg/kg) treatment initiated, along with close monitoring. If the steroid is ineffective, MMF, tacrolimus, or anti-thymocyte globulin therapy should be considered. Patients with moderate-to-severe heart failure should discontinue high-dose infliximab.45
Regular examination of adrenal tests and serum ion levels in patients receiving ICIs should be carried out to screen for hypophysitis and adrenal insufficiency at an early stage. If there are abnormalities, a further ACTH stimulation test, pituitary MRI, and consultation with an endocrinologist should be advised. Any increase in creatinine levels during ICI treatment, excluding other causes of renal failure, should be considered potential irAEs. Renal consultation and biopsy should be performed as soon as possible.
Future Directions
Presently, studies of immunotherapy on new inhibitory targets (LAG-3, TIM-3, BTLA, TIGIT, VISTA); stimulation checkpoints (OX40, ICOS, GITR, 4–1bb, CD40); tumor microenvironmental component targets (IDO, TLR); vaccines; GPC3-targeted CAR-T therapy;65 and JX-594 (Pexa-Vec) oncolytic virus66 are under development. However, taking into account the complexity of hepatocarcinogenesis and heterogeneity of HCC, a combination strategy seems to provide the most efficient immunotherapy. Besides the combination of ICIs and multi-kinase inhibitors mentioned earlier, other combination strategies such as with locoregional therapies, vaccines, and oncolytic viruses may also provide synergistic effects and improve the therapeutic efficacy. AFP-derived vaccines can boost the immune system by generating effective CD8+ T-cell response to specific antigenic AFP peptides. Preclinical data has proven that local oncolytic virus injection can cause inflammatory immune infiltration of distant tumors, rendering tumors susceptible to checkpoint blockade.67 Locoregional treatments involving transarterial chemoembolization and ablation can result in the release of neoantigens, thereby activating antigen presentation and stimulating peripheral immune response that can be potentially amplified by immune-modulating agents.68 Nevertheless, effective management of potential irAEs is important to safely provide these combinational treatments.
The efficacy of immunotherapy is affected by factors from comprehensive involving tumor genomics, germline genetics, the composition of host intestinal microbiome and others. Therefore, effective biomarker-based patient selection should be paid more attention. Further studies are required to identify biomarkers that can predict therapeutic response and the onset of potential irAEs in HCC, which could help to personalize patient immunotherapy. The high expression of PD-L1 in HCC is associated with poor prognosis, and it has been shown to be an important biomarker for patient selection for ICI therapy. However, clinical responses to atezolizumab and bevacizumab were observed irrespective of PD-L1 status.15 The presence of tumor-infiltrating lymphocytes (TILs), tumor mutation burden (TMB), T cell-inflamed gene expression profile (GEP), immune gene expression signatures, and characterization of the microbiome can also influence prognosis and prediction in many cancer types; however, these need to be further verified in HCC.69
Immunotherapy can also be utilized as neoadjuvant therapy in the future to reduce the size of the primary lesion, lower the clinical stage, increase the chance of radical surgery, and preserve organ and tissue function. Furthermore, it can also control and eliminate clinical or subclinical small metastases to reduce the iatrogenic spread of cancer cells during surgery and postoperative recurrence and metastasis.
Conclusion
Immunotherapy is widely used in the treatment of liver cancer. The safety and efficacy of drugs are equally important in clinical application. Clinicians must strive to gain more knowledge about immunology, carry out corresponding organ evaluation before treatment, and be familiar with the manifestations of adverse events associated with immunotherapy. Early multidisciplinary intervention and personalized treatment for irAEs are recommended. Efforts should also be made to tailor treatment for individual patients based on predictive biomarkers and etiology that could reshape many previous treatment regimens and guidelines and carry new hope for patients with advanced disease.
Contribution to the Field Statement
HCC is one of the most common cancers worldwide and is characterized by high malignancy and mortality, rapid progression, recurrence, and metastasis. Despite the many treatment strategies for HCC, its prognosis remains poor. Recently, the field of cancer immune-checkpoint inhibitors (ICIs) immunotherapy has made promising advancements. However, ICI immunotherapy can lead to a wide range of immune-related adverse events including hepatotoxicity, cutaneous toxicity, and gastrointestinal toxicity, which are usually manageable but occasionally lead to fatal outcomes. This review focuses on the common toxicities of ICIs in patients with HCC and summarizes the therapeutic strategies that can be used to monitor and manage such toxicities to improve prognosis, reshape previous treatment guidelines, and present new hope for those with advanced cancer.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 81772588, 81972307, U19A2008, and 81773194).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi:10.1038/s41575-019-0186-y
2. Chacko S, Samanta S. Hepatocellular carcinoma: a life-threatening disease. Biomed Pharmacother. 2016;84:1679–1688. doi:10.1016/j.biopha.2016.10.078
3. Keating GM. Sorafenib: a review in hepatocellular carcinoma. Target Oncol. 2017;12:243–253. doi:10.1007/s11523-017-0484-7
4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet Lond Engl. 2018;391:1163–1173. doi:10.1016/S0140-6736(18)30207-1
5. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2017;389:56–66. doi:10.1016/S0140-6736(16)32453-9
6. Personeni N, Rimassa L, Pressiani T, Smiroldo V, Santoro A. Cabozantinib for the treatment of hepatocellular carcinoma. Expert Rev Anticancer Ther. 2019;19:847–855. doi:10.1080/14737140.2019.1674141
7. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi:10.1016/S1470-2045(18)30937-9
8. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi:10.1038/ni.2691
9. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Clin Oncol. n.d.;19.
10. Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46. doi:10.1093/intimm/dxu095
11. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma 23.57. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi:10.1038/nrgastro.2015.173
12. Kudo M. Combination cancer immunotherapy in hepatocellular carcinoma. Liver Cancer. 2018;7:20–27. doi:10.1159/000486487
13. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi:10.1038/nrclinonc.2018.29
14. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi:10.1016/S0140-6736(17)31046-2
15. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19:940–952. doi:10.1016/S1470-2045(18)30351-6
16. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi:10.1200/JCO.19.01307
17. Qin S, Finn RS, Kudo M, et al. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811–1822. doi:10.2217/fon-2019-0097
18. Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020. doi:10.1001/jamaoncol.2020.4564
19. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745
20. Zhang Z, Zhou Y, Hu K, Li Z, Wang Z, Huang Y. Complete response of early stage hepatocellular carcinoma in a patient treated with combination therapy of camrelizumab (SHR-1210) and apatinib. Dig Liver Dis. 2019;51:1488–1490. doi:10.1016/j.dld.2019.07.005
21. Kudo M. Immuno-oncology therapy for hepatocellular carcinoma: current status and ongoing trials. Liver Cancer. 2019;8:221–238. doi:10.1159/000501501
22. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi:10.1126/science.aac4255
23. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi:10.1126/science.aan4236
24. Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi:10.1126/science.aad1329
25. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–1379. doi:10.1093/annonc/mdx108
26. Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. [Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment]. Bull Cancer (Paris). 2018;105:1033–1041. French. doi:10.1016/j.bulcan.2018.07.005
27. Pennock GK, Chow LQM. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist. 2015;20:812–822. doi:10.1634/theoncologist.2014-0422
28. Bertrand A, Kostine M, Barnetche T, Truchetet M-E, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi:10.1186/s12916-015-0455-8
29. Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008. doi:10.1001/jamaoncol.2019.0393
30. Hao C, Tian J, Liu H, Li F, Niu H, Zhu B. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e7325. doi:10.1097/MD.0000000000007325
31. De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–318. doi:10.1158/2326-6066.CIR-16-0237
32. Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721. doi:10.1001/jamaoncol.2018.3923
33. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death–1 pathway inhibitors among patients with non–small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36:1905–1912. doi:10.1200/JCO.2017.77.0305
34. Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: a comprehensive meta-analysis of randomized controlled trials. Int Immunopharmacol. 2018;63:292–298. doi:10.1016/j.intimp.2018.08.014
35. Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma 14. J Hepatol. 2020;72:320–341. doi:10.1016/j.jhep.2019.10.021
36. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi:10.1016/j.jhep.2013.02.022
37. Voutsadakis IA. PD-1 inhibitors monotherapy in hepatocellular carcinoma: meta-analysis and systematic review. Hepatobiliary Pancreat Dis Int. 2019;18:505–510. doi:10.1016/j.hbpd.2019.09.007
38. Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–220. doi:10.1002/hep.21051
39. Shah NJ. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;8.
40. Lee P-C, Chao Y, Chen M-H, et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2020;8:e001072. doi:10.1136/jitc-2020-001072
41. Kambhampati S, Bauer KE, Bracci PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child‐Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019;125:3234–3241. doi:10.1002/cncr.32206
42. Ng KYY, Wong LWJ, Ang AJS, et al. Real‐world efficacy and safety of immune checkpoint inhibitors in advanced hepatocellular carcinoma: experience of a tertiary Asian Center. Asia Pac J Clin Oncol. 2020;
43. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi:10.1016/S1470-2045(20)30011-5
44. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. doi:10.1093/annonc/mdx225
45. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi:10.1200/JCO.2017.77.6385
46. Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47:781–785. doi:10.1097/MCG.0b013e31828f1d51
47. Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6:95. doi:10.1186/s40425-018-0411-1
48. Cheng R, Cooper A, Kench J, et al. Ipilimumab-induced toxicities and the gastroenterologist: ipilimumab-induced toxicities. J Gastroenterol Hepatol. 2015;30:657–666. doi:10.1111/jgh.12888
49. Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor–induced colitis. J Immunother Cancer. 2019;7:93. doi:10.1186/s40425-019-0577-1
50. Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581–592. doi:10.1007/s00262-017-1962-6
51. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. OncoImmunology. 2016;5(6):e1128611. doi:10.1080/2162402X.2015.1128611
52. De Martin E, Michot J-M, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. doi:10.1016/j.jhep.2018.01.033
53. Chmiel KD, Suan D, Liddle C, et al. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol. 2011;29:e237–40. doi:10.1200/JCO.2010.32.2206
54. Wu Z, Lai L, Li M, Zhang L, Zhang W. Acute liver failure caused by pembrolizumab in a patient with pulmonary metastatic liver cancer: a case report. Medicine (Baltimore). 2017;96:e9431. doi:10.1097/MD.0000000000009431
55. Tian Y, Abu-Sbeih H, Wang Y. Immune Checkpoint Inhibitors-Induced Hepatitis. In: Naing A, Hajjar J, editors. Immunotherapy. Vol. 995. Cham: Springer International Publishing; 2018: 159–164. doi:10.1007/978-3-030-02505-2_8.
56. Johncilla M, Misdraji J, Pratt DS, et al. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015;39:1075–1084. doi:10.1097/PAS.0000000000000453
57. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi:10.1056/NEJMoa1717002
58. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi:10.1016/S1470-2045(15)00050-9
59. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. Autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38:212–215. doi:10.1097/CJI.0000000000000081
60. Peeraphatdit T, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72:315–329. doi:10.1002/hep.31227
61. Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583–589. doi:10.1093/annonc/mdw640
62. Spänkuch I, Gassenmaier M, Tampouri I, et al. Severe hepatitis under combined immunotherapy: resolution under corticosteroids plus anti-thymocyte immunoglobulins. Eur J Cancer. 2017;81:203–205. doi:10.1016/j.ejca.2017.05.018
63. Williams TJ, Benavides DR, Patrice K-A, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73:928. doi:10.1001/jamaneurol.2016.1399
64. Feng S, Coward J, McCaffrey E, Coucher J, Kalokerinos P, O’Byrne K. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol. 2017;12:1626–1635. doi:10.1016/j.jtho.2017.08.007
65. Nishida T, Kataoka H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers. 2019;11:1339. doi:10.3390/cancers11091339
66. Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi:10.1038/nm.3089
67. Cheng H, Sun G, Chen H, et al. Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res. 2009;10.
68. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi:10.1016/j.jhep.2016.10.029
69. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi:10.1093/annonc/mdy495
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.