Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Adverse Drug Reaction and Its Predictors Among Psychiatric Patients Taking Psychotropic Medications at the Mizan-Tepi University Teaching Hospital
Authors Ejeta F , Aferu T , Feyisa D , Kebede O , Siraj J , Hammeso WW , Tadesse E , Tinishku A
Received 12 November 2021
Accepted for publication 23 December 2021
Published 29 December 2021 Volume 2021:17 Pages 3827—3835
DOI https://doi.org/10.2147/NDT.S349127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Fikadu Ejeta,1 Temesgen Aferu,1 Diriba Feyisa,1 Oliyad Kebede,1 Jafer Siraj,2 Workineh Woldeselassie Hammeso,2 Esayas Tadesse,3 Alemayehu Tinishku4
1Department of Pharmaceutics and Social Pharmacy, School of Pharmacy, Mizan-Tepi University, Mizan-Aman, Ethiopia; 2Department of Pharmacology and Pharmaceutical Chemistry, School of Pharmacy, Mizan-Tepi University, Mizan-Aman, Ethiopia; 3Department of Pharmacy, Ambo University, Ambo, Ethioipia; 4Department of Clinical Pharmacy and Pharmacy Practice, School of Pharmacy, Mizan-Tepi University, Mizan-Aman, Ethiopia
Correspondence: Fikadu Ejeta Email [email protected]
Introduction: Psychotropic medications are crucial in the treatment of a variety of psychiatric disorders. Use of second-generation antipsychotics (SGA) has been associated with many adverse events. Assessment and monitoring of ADRs is required to develop appropriate interventional strategies to manage, prevent and minimize the risks of undesirable effects and thus improve quality of life and adherence, avoid relapse, and reduce treatment costs.
Objective: The objectives of this study were to assess adverse drug reactions and its predictors in psychiatric patients taking psychotropic medications from the outpatient pharmacy of MTUTH.
Methods: A cross-sectional study design was conducted using a structured questionnaire interviewing a psychiatric patient. One way ANOVA and bivariate logistic regression was computed for all independent variables to identify variables that fit for multivariate logistic regression. A p-value less than 0.05 was considered significant.
Results and Discussions: A total of 101 patients (91.8%) experienced one or more types of ADR in the current study. There was a statistically significant relationship between ADRs management and age, marital status, educational status, occupation, and monthly income of the study participants. The odds of having experienced autonomic adverse drug reactions experience among respondents aged 30 years and older higher than those under 30 years. Participants taking multiple medications were more likely to face psychiatric adverse drug reactions compared to those taking a single medication. Patients taking multiple psychotropic medications were ten times more likely to develop autonomic adverse drug reactions compared to those taking a single drug.
Conclusion: The study revealed a moderate incidence of ADR in patients attending the psychiatric OPD. Age and number of antipsychotics were predictors of ADR (autonomic and psychiatric). This study revealed that there is a gap in the role of clinicians in the monitoring and reporting of ADRs.
Keywords: adverse drug reaction, pharmacovigilance, psychotropic drugs, predictors
Introduction
An adverse drug reaction (ADR) is a potentially hazardous or unpleasant reaction that occurs as a result of a procedure associated with the use of a medical substance. Adverse reactions often indicate a risk of harm from future administration, necessitating avoidance, particular therapy, adjustment of the dosing regimen adjustment, or product discontinuation.1 Antipsychotics are classes of medications that are used to treat psychiatric diseases, most notably schizophrenia, as well as bipolar disorder, delusional disorder, and, increasingly, non-psychotic disorders. Since the debut of antipsychotic drugs for the treatment of psychosis, a variety of medications have been created in this category. The “typical antipsychotics,” which were initially discovered in the 1950s, are the first generation of antipsychotic medications. It was quickly discovered that they generated extrapyramidal symptoms (EPS) in patients with Parkinsonism, tardive dyskinesia, akathisia, and other movement disorders.2
The severe adverse effects required the development of a new generation of these drugs that would be more patient-friendly. Following that, a second generation of antipsychotics known as atypical antipsychotics was developed, the first being clozapine, which was clinically introduced in the 1970s. Although a reduction in EPS has been observed, both generations of medications have been linked to a new list of worrying side effects, including weight gain and associated metabolic consequences, increased prolactin, associated sexual side effects, and prolongation of QTc. Although the link between antipsychotics, particularly second-generation antipsychotics (SGA), and weight gain in adults is widely recognized, children are particularly prone to SGA-induced CSE, and childhood CSE has a negative impact on cardiovascular outcomes in adult age cardiovascular outcomes via a number of long-term risk factors or accelerated pathways. Weight gain and sexual dysfunction among drug users can lead to nonadherence to treatment and, as a result, relapse.3,4
Adverse drug reactions (ADRs) are the leading source of morbidity and mortality in hospital and outpatient settings. In hospitalized patients, the total incidence of significant and fatal ADRs was reported to be 6.7% and 0.32%, respectively. In outpatient settings, the rate of ADRs ranges from 5% to 35%. ADRs have been identified as a substantial cause of hospital admissions, with rates ranging from 0.2% to 41.3%. Monitoring of ADR in a hospital context is a critical method for identifying patients who are at high risk for acquiring ADRs and to understand the nature and prevalence of ADRs in a given population. This activity helps develop appropriate interventional strategies to control, prevent, and decrease the risk of developing ADRs and reducing healthcare care costs. ADRs are commonly associated with psychotropic drugs and can occur even in standard doses used in the treatment of acute and chronic psychiatric problems. These ADRs can reduce quality of life, induce poor drug adherence, cause physical morbidity, generate shame, and, in the worst-case scenario, be lethal. Several studies have investigated the occurrence, nature and frequency of ADRs associated with various psychotropic medicines.4
According to research, antidepressants and antipsychotics are responsible for the majority of ADRs in the psychiatry department. Second-generation antipsychotics, particularly clozapine and olanzapine, are more likely to produce metabolic difficulties, whereas older first-generation antipsychotics are more likely to cause movement disorders. Other psychiatric and physical diseases that may mimic antipsychotic-related side effects must be carefully considered when identifying these adverse effects.
Polypharmacy with antipsychotics is becoming increasingly common; this practice is one of the main causes of ADRs in psychiatric patients.5 Early diagnosis of ADR in antipsychotic-treated patients is critical to reducing morbidity and economic burden on patients and the country. The ADRs associated with these medications should also be closely monitored because these medications are taken for months, years, or even a lifetime.
After a drug reaches the drug market, it is necessary to regularly evaluate its safety profile (post-marketing surveillance) and assess the ADRs associated with it because only the most prevalent ADRs are found during pre-marketing trials.6 As a result, developing strategies for quickly recognizing and monitor ADR and other post-marketing post-marketing surveillance activities is critical.7 The purpose of this study was to determine the prevalence of ADRs and associated factors among patients on psychotropic medications at Mizan Tepi University Teaching Hospital (MTUTH).
Methods
Study Area and Period
A facility-based cross-sectional study was conducted among psychiatric patients at MTUTH from March to April 2019. The hospital is located in the Bench Sheko Zone, 592 kilometers from Addis Ababa, the capital of Ethiopia. It is the only general hospital found in the zone and provides both inpatient and outpatient services for a population of nearly 2 million people in the catchment area. The hospital had four major wards with 121 beds, namely medical (32 beds), surgical (22 beds), pediatrics (37 beds) and gynecology/obstetrics (30 beds). A total of 326 health care professionals (4 specialists, 57 physicians, 19 pharmacy professionals (pharmacists and druggists), 30 midwives, 22 public health officers, 138 nurses, 5 anesthesiologists, 1 psychiatrist, 21 laboratory professionals, 5 radiologists, 1 dentist, and 23 other health professions in different disciplines were working in the hospital. The hospital manages various health problems, including chronic and acute psychotic problems. There were a total of 162 psychiatric patients on follow up/on treatment when this study was undertaken.8
Population
Source Population
All patients with psychiatric disorders receiving psychotropic medication from the hospital.
Study Population
All psychotic patients who received psychotropic medication from the hospital during the study period and met the inclusion criteria.
Eligibility Criteria
Inclusion Criteria
Patients taking psychotropic medication who can provide information on their own (can hear and speak).
Patients with 15 years and older and willing to participate in the study.
Exclusion Criteria
Clinically unstable patients.
Patients under 15 years of age, had hearing and speaking difficulties and those who were unwilling to participate in the study.
Sample Size Determination and Sampling Technique
The source population (ie, 162 patients taking psychotropic medication in the hospital) was too small to calculate the sample size and therefore the investigators used the consecutive sampling technique to include all psychotic patients who met the inclusion criteria and came to the OPD pharmacy to receive services during the study period. The investigators finally interviewed 110 patients using the stated sampling technique; the analysis was based on these interviewed patients. Care was taken not to re-interview patients who were approached on the previous contact but revisited the facility during data collection. We reviewed patients’ medical records for working diagnosis, previous allergies, and clinical and laboratory data The data on previous and current medications were obtained from the patient’s clinical notes, treatment sheets, drug administration charts, pill count validation, inspecting for left-over medicines, and through interviewing the patient/caregiver or ward staff. Patients were interviewed, and their information was recorded. Results of diagnostic and laboratory tests and pain scale were documented. Second, the rating of the causal relationship between an ADR and the suspected medication was done using the standard treatment guideline of Ethiopia ADR assessment scale as it was stated in an operational definition. We excluded all doubtful ADRs, whereas we considered those rated as possible, probable or definite for discussion and verification by the hospital staff. Therefore it is all definite ADR.
Data Collection Procedure, Processing, Analysis, and Quality Control
Data were collected using a semi-structured questionnaire which were developed according to WHO pharmacovigilance indicator, patients forward reports of suspected ADRs.9 The questionnaire was prepared in English and included all relevant variables according to the objectives of the study. After data collection, the data was coded and cleaned to ensure accuracy, consistency and completeness, entered to SPSS version 22 for analysis, and the result was presented using appropriate description. The pre-test was carried out on 10 patients on antipsychotic medication (excluded from actual data collection) in a similar setup to before beginning actual data collection. The tools and procedures were then revised in light of the pre-test performed and data was collected. The collected data was checked by the principal investigator daily for completeness and data quality.
The relationship between the dependent variable (type of ADR) and the independent variables (sex, age, marital status, educational status, occupation, and number of psychotropic medications taken) was examined using ANOVA Tukey post-hoc test tests to explore differences between multiple group means while controlling the experiment-wise error rate. Binary logistic regression was also performed. To do this, the dependent variable was made dichotomous (autonomic ADR (with anticholinergic ADR as one component) and psychiatric ADR (with extrapyramidal ADR as one element)). Participant patients were also categorized into two groups based on age (15–29 and over), marital status (not in marital union and married), educational status (illiterate and literate), occupation (governmental and private), monthly income (800 ETB and >800 ETB) and number of psychotropic medications taken (single and multiple). The rationale for categorizing the age of 30 as a criterion in this study was to compare the ADR in in young adults to old adults. First, bivariate logistic regression was calculated for all independent variables to identify variables that fit multivariate logistic regression at a p-value <0.05. Then a multivariate logistic regression analysis was performed to identify the independent predictors of types of ADR that occur among the patients. Variables with a p-value <0.05 in the multivariate logistic regression were considered statistically significant predictors of the types of ADRs that occurred.
Ethical Clearance
A formal letter was obtained from the School of Pharmacy, Faculty of Medicine and Health Sciences, Mizan Tepi University before data collection and given to the MTUTH administration to obtain permission before data collection in accordance with the Declaration of Helsinki. Moreover, written informed consent was also obtained from respondents before data collection. The confidentiality of the data collected and the privacy of the patients was maintained.
Operational Definition
ADR: noxious unwanted effects that occur at therapeutic doses
Mild ADR: where no intervention is required
Moderate ADR: where the switch to another drug is necessary
Severe ADR: where antidote should be employed to alleviate the situation
Results
Sociodemographic Profile of the Respondents
One hundred and ten of the total of 162 patients taking psychotropic medications participated in the study. Ninety-six (87.3%) respondents were male and 76 (69.1%) were 15 to 29 years old. The majority of participants were Bench in ethnicity, 63 (57.3%) and Orthodox Christians in religion, 49 (44.4%). Sixty-two participants (56.4%) were not in marital union. Ninety-nine respondents (90.0%) were literate while the occupation of 100 (90.9%) was of private type. Most of the study participants resided in rural areas, 87 (79.1%). The monthly income of the participants ranged from ETB 90 to ETB 6500 with a mean ETB 1893.6; 88 (80.0%) participants earned between 800 ETB per month (Table 1).
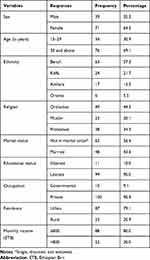 |
Table 1 Sociodemographic Characteristics of Patients Taking Antipsychotic Medications |
Schizophrenia, 79 (71.8%) was the most common psychiatric disorder recorded among study participants. Most of the disorders experienced were managed by a single medication, 87 (79.1%) tablets of amitriptyline being the most widely used, 76 (69.1%). Sixty-eight (61.8%) patients received counseling services on ADRs experienced (Table 2).
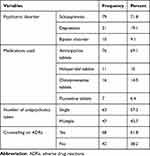 |
Table 2 Clinical Characteristics of Patients Taking Antipsychotic Medications |
A total of 101 patients (91.8%) experienced one or more types of adverse drug reactions in the current study. Sedation and constipation were the most common autonomic ADRs reported among the patients, accounting for 36 (32.7%) each. Thirty-two patients (29.1%) experienced anxiety as psychiatric adverse drug reactions, while 44 (40%) patients faced restlessness (Table 3).
 |
Table 3 Type of Adverse Drug Reactions Faced by Patients Taking Antipsychotic Medications |
Regarding the treatment of ADRs, nothing was done for 33 (32.7%) patients who experienced the same while 31 (30.7%) were treated for the experienced ADR and made to continue the already started medication. A dose and regimen change was made for 20 (19.8%) and the culprit medication was discontinued among 17 (16.8%) patients (Table 4).
 |
Table 4 Response to Adverse Drug Reactions Experienced Among Antipsychotic Medication Patients on Antipsychotic Medications |
ANOVA of independent variables on ADR management showed significance for age, marital status, educational status, occupation and monthly income as shown in Table 5.
 |
Table 5 Analysis of Variance of Independent Variables on Adverse Drug Reactions Management |
Factors Associated with ADR Experience
The residence and income of the participants showed values less than the expected minimum expected (<5) each in one cell while cross-tabulating with ADR types and therefore were not involved in binary logistic regression. Educational status also showed the same value while cross-tabulating it with autonomic adverse drug reactions experience and hence excluded from the analysis. The sex of the patients showed a p-value greater than 0.25 in the bivariate analysis for psychiatric adverse drug reactions while marital status and occupation showed the same in the bivariate analysis for both autonomic and psychiatric adverse drug reactions and therefore were excluded from the multivariate analysis. Multivariate logistic regression analysis revealed that the age and number of psychotropic medications taken by the participants had a significant association with the experience of adverse drug reactions.
The odds of having experienced autonomic adverse drug reactions experience among respondents aged 30 years and older were 5.94 times higher than those under 30 years [CI = 1.91–18.46, P-value = 0.002]. Patients taking multiple psychotropic medications were 10.80 times more likely to develop autonomic adverse drug reactions compared to those taking a single drug [CI = 3.93–39.64, P-value = 0.000]. Participants taking multiple medications were 4.88 times more likely to face psychiatric adverse drug reactions compared to those taking a single medication [CI=1.94–12.29, P-value=0.001] (Tables 6 and 7).
 |
Table 6 Experienced Autonomic Adverse Drug Reaction and Its Associated Factors |
 |
Table 7 Experienced Psychiatric Adverse Drug Reaction and Its Associated Factors |
Discussion
In addition to the ADR spontaneously reported by patients, the current study to assess psychotropic ADR was based on active surveillance via questionnaire. First-generation antipsychotic drugs (chlorpromazine and haloperidol) are still the first line of treatment at the Mizan Tepi University Teaching Hospital, and this study found that 91.8% of patients had adverse drug reactions. This is similar to outpatient management of ADRs at Malawi’s Zomba hospital,10 Kenya’s Mathari hospital,11 Uganda’s Butabika National Referral Mental Hospital,12 and Ethiopia’s Amanuel Mental Specialized Hospital.13 However, in a psychiatric facility in Kashmir, India, atypical antipsychotic medications were first-line antipsychotic medications.14 This may be due to the difference in study area.
In this study, improvement of the underlying mental disorder, such as depression, could lead to head ache, sedation, constipation, loss of appetite, and weight gain. Weight gain and headache were the most frequently associated ADRs in the present study. Treatment of these ADRs was achieved by treating the ADR and continuing the current medication. This is in line with the study conducted by Wubshet et al at Amanuel Mental Specialized Hospital.13
Management of ADR is a crucial part of mental disorder treatment. In this study, one third of the patients experienced mild, moderate, and severe ADR, respectively. This study is in line with the study conducted in United States.15
Adverse effects on the CNS, such as sedation, are common with psychotropic medications because these drugs act on the CNS. Most of the suspected ADRs were possible in nature. However, some other studies have reported a higher number of ADRs, which are mild in nature. In contrast, several studies have reported serious or fatal ADRs for a different class of psychotropic medications, such as antidepressants and atypical antipsychotic medications. A recent study has reported that even ADR, which is “not severe” in nature, can have a significant impact on patients with psychiatric illness. Therefore, managing and preventing ADR in patients with psychiatric illness is vital.
As reported in the literature, the appearance of ADR can lead to nonadherence to medications. In the present study, we observed five patients out of 8, who had stopped their medication on their own at home after the development of ADR. This observation supports the fact that adverse drug reactions might lead to non-adherence or discontinuation of therapy. This study is similar to the study conducted by Katusiime et al in Uganda.12
On the other hand, there was a statistically significant relationship between ADRs management and age (p=0.000) or marital status (p=0.000), secondary stage educational status (p=0.002), occupation (p=0.031), and monthly income (p=0.000) except for those who gained 100–800 ETB (p=0.163) of the study population as shown in Table 5. This is in line with the study conducted at the Neuro-Psychiatric Referral Hospital in Eritrea.16
Association of Socio-Demographic and with ADRs
In the current study, higher ADRs were found in patients older than 30 years of age compared to patients between 15 and 29 years of age. The prevalence of adverse drug reactions increases with age, with twice as many patients 65 years and older hospitalized for ADR-related problems than their younger counterparts.17 For drugs associated with a high number of ADRs, it is important to explain the possibility of ADRs to a patient and parents at the time of drug administration and provide information on how to deal with ADRs. As types of medication Amitriptyline (p=0.04) had a likelihood of causing psychiatric ADRs and Haloperidol (p=0.003) on autonomic ADR. There was no association between ADR development and comorbidities.
Polypharmacy is a risk factor for the development of ADR in outpatients and hospitalized patients.18 It is not realistic to establish the use of two or more drugs as the cut-off point to avoid polypharmacy-inducing adverse drug reactions (ADRs), according to Receiver Operating Curves (ROC). The prevalence of ADR was significantly higher among patients who used 4–5 or 6 drugs rather than just one drug.
Limitation of the Study
The sample size of the study was small and the study period was too short; this created a limitation for the inferential analysis of the total population in this study.
Conclusion
Generally, all patients in this study had at least one adverse drug reactions. Adverse drug reactions are a frequent occurrence in patients with schizophrenia, which are mild in most cases. The incidence of ADRs can be reduced, and compliance and patient quality of life can be improved by early detection and management. The present study offers a represented profile of ADRs that can be expected in the outpatient psychiatry department at MTUTH. The study revealed a moderate incidence of ADR in patients attending the psychiatric OPD. Most of the adverse reactions reported during the study were mild in nature and could not be prevented. Statistically significant predictors of ADR (autonomic and psychiatric) were older in age and number of psychotropic medications than in polypharmacy. Many of the ADRs were recovered after the drug was stopped or the dose was changed. The study promotes the role of clinicians in the monitoring and reporting of ADRs.
Recommendation
The association of psychotropic medications with ADRs is common and can occur even in the normal doses used in the management of acute and maintenance phases of psychiatric disorders.
- Health professionals should be aware of the risks and benefits of psychotropic medications.
- Caregivers, physicians, pharmacists, nurses, and health counselors should pay more attention to keep this promising trend going.
- More research must be conducted considering its associated factors with a better study design and a sufficient sample size.
Abbreviations
ADRs, Adverse drug reactions; EPS, Extrapyramidal symptoms; OPD, Outpatient Department; CSE, Cardio-metabolic Side Effects; SGA, Second-Generation Antipsychotics; SSRIS, Selective Serotonin Reuptake Inhibitors; CNS, Central Nervous System; ECG, Electrocardiogram; MTUTH, Mizan Tepi University Teaching Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28(10):851–870. doi:10.2165/00002018-200528100-00003
2. Pakpoor J, Agius M. A review of the adverse side effects associated with antipsychotics as related to their efficacy. Psychiatr Danub. 2014;26(Suppl 1):273–284.
3. Patil H, Goswami M, Patil R, Gupta S. Comparison of adverse drug reactions with typical and atypical antipsychotic drugs. Innov Pharm Pharmacother. 2013;1(2):99–102.
4. Sridhar SB, Al-Thamer SSF, Jabbar R. Monitoring of adverse drug reactions in psychiatry outpatient department of a Secondary Care Hospital of Ras Al Khaimah, UAE. J Basic Clin Pharm. 2016;7(3):80–86. doi:10.4103/0976-0105.183263
5. Solanke B, Mahatme M, Dakhale G, Hiware S, Shrivastava M, Waradkar P. Adverse drug reaction profile at psychiatry out-patient department of a tertiary referral centre in Central India. Int J Basic Clin Pharmacol. 2013;2(3):341. doi:10.5455/2319-2003.ijbcp20130623
6. Herrera Comoglio R. Undergraduate and postgraduate pharmacovigilance education: a proposal for appropriate curriculum content. Br J Clin Pharmacol. 2020;86(4):779–790. doi:10.1111/bcp.14179
7. DACA. Standard treatment guideline for general hospital. Drug Administration and Control Authority of Ethiopia; 2010.
8. Meleko K. Adane A. Assessment of health care waste generation rate and evaluation of its management system in Mizan-Tepi University Teaching Hospital, Bench Maji Zone, Southwest Ethiopia, Mizan-Tepi University; 2018 (Unpublished research).
9. World Health Organization. WHO Pharmacovigilance Indicators Assessment: a practical manual for the Systems, of pharmacovigilance; 2015.
10. Chikowe I, Domingo M, Mwakaswaya V, Parveen S, Mafuta C, Kampira E. Adverse drug reactions experienced by out-patients taking chlorpromazine or haloperidol at Zomba Mental Hospital, Malawi. BMC Res Notes. 2019;12(1):1–6. doi:10.1186/s13104-019-4398-6.
11. Okinda KE. Impact of side effects of antipsychotics on attitude and adherence to treatment among adult psychiatric outpatients at Mathari Hospital in Kenya; 2014.
12. Katusiime B, Semakula D, Lubinga SJ. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. Afr Health Sci. 2015;15(4):1308–1317. doi:10.4314/ahs.v15i4.34
13. Wubeshet YS, Mohammed OS, Desse TA. Prevalence and management practice of first generation antipsychotics induced side effects among schizophrenic patients at Amanuel Mental Specialized Hospital, central Ethiopia: cross-sectional study. BMC Psychiatry. 2019;19(1):1–8. doi:10.1186/s12888-018-1999-x
14. Piparva KG, Buch JG, Chandrani KV. Analysis of adverse drug reactions of atypical antipsychotic drugs in psychiatry OPD. Indian J Psychol Med. 2011;33(2):153–157. doi:10.4103/0253-7176.92067
15. Hale GM, Kane-Gill SL, Groetzinger L, Smithburger PL. An evaluation of adverse drug reactions associated with antipsychotic use for the treatment of Delirium in the Intensive Care Unit. J Pharm Pract. 2016;29(4):355–360. doi:10.1177/0897190014566313
16. Bahta M, Berhe T, Russom M, Tesfamariam EH, Ogbaghebriel A. Magnitude, nature, and risk factors of adverse drug reactions associated with first generation antipsychotics in outpatients with Schizophrenia: a cross-sectional study. Integr Pharm Res Pract. 2020;9:205–217. doi:10.2147/IPRP.S271814
17. Beijer HJM, De Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24(2):46–54. doi:10.1023/A:1015570104121
18. Sugioka M, Tachi T, Mizui T, et al. Effects of the number of drugs used on the prevalence of adverse drug reactions in children. Sci Rep. 2020;10(1):1–8. doi:10.1038/s41598-020-78358-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
