Back to Journals » OncoTargets and Therapy » Volume 12
Advances Of Chimeric Antigen Receptor T Cell Therapy In Ovarian Cancer
Received 30 January 2019
Accepted for publication 10 September 2019
Published 30 September 2019 Volume 2019:12 Pages 8015—8022
DOI https://doi.org/10.2147/OTT.S203550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Wenying Yan,1 Hongmei Hu,2 Biao Tang2
1Department of Gynecology, Wangjiang Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Department of Gynecology, Sichuan Maternal and Child Health Hospital, Chengdu, Sichuan Province, People’s Republic of China
Correspondence: Biao Tang
Department of Gynecology, Sichuan Maternal and Child Health Hospital, No. 290 Shayan West Second Street, Jinyang Road, Chengdu City, Sichuan Province 610041, People’s Republic of China
Tel +86 28 8546 3088
Email [email protected]
Abstract: Ovarian cancer, as a common gynecological tumor, is currently recognized as the most lethal gynecological malignancy. In addition to conventional treatment methods such as surgery, radiotherapy and chemotherapy, adoptive immunotherapy represented by modified immune cells also shows good curative effects and is becoming an important method in the treatment of ovarian cancer. Studies have shown that most cancer cells can avoid the recognition of the immune system, thus limiting the anticancer effect of immunotherapy. Chimeric antigen receptor T (CAR-T) cell technology has emerged and has good targeting, killing, proliferation and persistence. A large number of clinical trials also have shown that this technology has achieved great success in improving the quality of life and prolonging the survival time of patients with malignant hematological tumors. CAR-T cell technology has become a research hotspot for immunotherapy. This article mainly reviews various CAR-T cell treatments and their specific mechanisms in the field of ovarian cancer treatment to provide new ideas for the treatment of ovarian cancer.
Keywords: immunotherapy, T cell, ovarian cancer, chimeric antigen receptor, tumor antigens
Introduction
Ovarian cancer is one of the most lethal gynecological malignant tumors. In the United States, about 22,530 new ovarian cancer patients are diagnosed each year, resulting in about 13,980 deaths.1 Patients diagnosed with stage III or IV ovarian cancer have a 5-year survival rate of less than 25%, even after aggressive surgical resection and first-line chemotherapy drugs. Although more than 80% of the patients diagnosed had significant remission in the early stage of treatment, most of them relapsed and eventually developed anti-chemotherapeutic diseases. At the same time, because of the occult onset of ovarian cancer and the nonspecificity of screening tools and clinical manifestations, the early detection rate of ovarian cancer is very low. The fact that patients show obvious symptoms only when the cancer has spread to the pelvic cavity is also one of the reasons for the high mortality rate of ovarian cancer.2 The standard treatment for ovarian cancer is surgical operation; where necessary, a combination of platinum- and taxane-based chemotherapy should be used.3 Recent clinical trials have shown that the overall survival rate and quality of life of ovarian cancer patients were not significantly improved after bevacizumab was used as conventional chemotherapy. Therefore, new therapeutic strategies need to be explored urgently.
In recent years, T cell adoptive immunotherapy, as a new strategy in cancer therapy, has been developing rapidly.4 Meanwhile, T cell transplantation experiments in ovarian cancer patients have shown that it has significant therapeutic effects. Chimeric antigen receptor T (CAR-T) cell therapy, as a representative of adoptive T cell immunotherapy, plays an important role in cancer treatment and is currently the focus of researchers. CAR-T cell immunotherapy mainly uses gene-editing technology to modify the patient’s autologous T cells to express chimeric antigen receptors and then mediates the direct killing of cancer cells by cytotoxic T cells.5 Previous studies have confirmed that CAR-T cell therapy has a significant effect on a variety of solid tumors and hematological malignancies,6,7 and early results in ovarian cancer have also shown a good effect; however, it is undeniable that this therapy also has certain toxic and side effects.6,7 In this review, we will review the molecular biological basis, antigen modification targets, preparation and clinical application of CAR-T cell therapy in ovarian cancer.
Current Status Of Ovarian Cancer
Globally, ovarian cancer is one of the most common gynecological malignancies, accounting for the seventh leading cause of death in women. Currently, the initial treatment for ovarian cancer patients can be divided into three stages: surgical treatment to remove the tumor as much as possible, first-line platinum-based adjuvant chemotherapy after surgery to eliminate incomplete microsurgery, and second-line drug maintenance therapy to delay the progress and recurrence of tumors as much as possible.8,9 Early stage ovarian cancer is difficult to diagnose due to the lack of clinical symptoms, and patients do not have the awareness of the need for regular physical examination so diagnosis often occurs at an advanced tumor stage. However, advanced ovarian cancer relies on traditional treatment methods with low curative effects and high recurrence rates, which can easily cause serious toxic and side effects, such as gastrointestinal reactions, hematopoietic suppression or impaired liver and kidney functions. Overall, the current treatment for ovarian cancer patients is not ideal.
Currently, the treatment methods for advanced ovarian cancer mainly include operation, radiotherapy and chemotherapy, but these methods cannot effectively control the recurrence and progression of cancer, so it is urgent to find new, safe and effective treatment methods.10,11
Effect Of Ovarian Cancer On The Host Immune System
The destruction of patients’ immune systems by tumors is a multistep cooperative process. Intervention or regulation of key target molecules can trigger the rejection reaction of patients’ immune systems to tumors. A meta-analysis of 1815 ovarian cancer patients in 10 independent studies showed that the lack of lymphocyte infiltration was significantly associated with poor prognosis of cancer.12 According to the study,13 stimulating the host to initiate the immune response against tumors requires the following points: 1) a sufficient amount of effector T cells must be produced in the body to effectively recognize tumor antigens; 2) these cells can identify, present and infiltrate tumor tissue; 3) they can overcome the inhibition of the tumor microenvironment on the immune network; 4) they can directly identify tumor antigens and kill tumor cells; and 5) they can maintain the activity of antitumor T cells for a long time.14
Application Of Chimeric Antigen Receptor-Modified T Cell Therapy In Tumor
Despite the rapid development of medical research and the emergence of new technologies, the treatment of cancer remains a significant problem. Conventional therapies such as surgery, radiotherapy and chemotherapy can show some short-term efficacy, but they are usually accompanied by severe toxicity and side effects.15 In addition, several years of clinical epidemiological studies have shown that although these measures can increase the rate of remission of the tumor, in the long term, they have not significantly prolonged the survival of the cancer patient. Approximately 30–80% of cancer patients will inevitably experience recurrence in the later period of treatment and even have problems of tolerance to various chemotherapeutic drugs or radiotoxicity, which makes it difficult to treat cancer.16,17
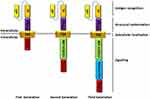
Adoptive immunocyte therapy has become the main tumor treatment method except for surgery, radiotherapy and chemotherapy, mainly including tumor infiltrating lymphocytes (TILs), T cell receptor (TCR) modified -T cells (TCR-T) and CAR-T. CAR-T cell therapy, as a representative means of tumor cell therapy, has the advantages of high specificity, not being limited by MHC,18 long-term efficacy after infusion, and good prognosis of patients. At present, CAR-T cells have been shown to have a good therapeutic effect on hematologic cancer19,20 and is expected to become a key approach for humans to conquer malignant tumors.21,22 CAR-T cells are a combination of single-chain variable fragment (scFv) that recognize tumor-associated antigens and activation motifs of T cells. They are created by gene transduction and have the ability to specifically recognize and kill tumor cells.23 The design principle of CAR-T cells is to select high affinity antibodies against tumor-specific antigens, fuse the coding sequence of the heavy chain and light chain variable region with the CD8 transmembrane region or immunoglobulin transmembrane sequence (Figure 1), a T cell activator molecule (such as CD28 or 4-1BB), and the intracellular domain of the T cell receptor complex (such as CD3) coding sequence, and construct a chimeric antigen receptor molecule, and then transfect it into T cells cultured in vitro. In recent years, the treatment of malignant hematological tumors represented by CD19-CART has achieved good results,13 especially the second generation of CART-19 targeting CD19 antigen, which has shown unprecedented clinical efficacy in recurrent and refractory acute lymphoblastic leukemia, chronic lymphoblastic leukemia and Hodgkin’s lymphoma.24–27 The results of early clinical trials of CAR-T cell therapy for cancer make it attractive to apply. In 2013, Science listed adoptive cellular immune therapy (ACT) as one of the major medical progresses of the year. On July 1, 2014, CD19-CART cell therapy, namely, CTL019, designed by the University of Pennsylvania, obtained the breakthrough designation of FDA in the United States.28 In 2017, CTL019 (Novartis) and KTE-C19 (Kite Pharma) for recurrent or refractory acute lymphoblastic leukemia in children and young adults and KTE-C19 for non-Hodgkin’s lymphoma were put into clinical use.29,30 Although the curative effect of CAR-T cell therapy on solid tumors is poor compared with that in blood tumors, previous studies have confirmed that CAR-T cell therapy also shows good curative effects in the treatment of ovarian cancer. This paper mainly summarizes the specific preparation methods of CAR-T cell therapy in ovarian cancer and discusses the clinical application and treatment prospects of CAR-T cell therapy in ovarian cancer.
Preparation Of Chimeric Antigen Receptors
The preparation process of CAR-T cells is summarized as the following four steps: 1) Separation: collection of mononuclear cells from the patient’s peripheral blood by leukocyte separation;31 2) Modification: using CD3/CD28 magnetic beads to activate T cells, and then high affinity CARs of specific tumor antigens are transduced into activated T cells so that activated T cells can stably express CARs that can specifically recognize tumor antigens;31 3) Amplification: expansion of modified T cells in vitro to achieve the desired number; and 4) Retransfusion: the expanded CAR-T cells are transfused back to the pretreated patients to observe their proliferation and ability to kill cancer cells, as well as possible toxic and side effects such as tumor lysis reaction and cytokine storm and to comprehensively evaluate their therapeutic effect on cancer.
Antigen Targets
The surface antigens targeted by CARs are mainly proteins and glycolipids.32 In CAR-T cell therapy for ovarian cancer, the most common target antigens include MUC16, mesothelin, HER2 and FRα (folate receptor-alpha).
MUC16
MUC16 is usually expressed in the cornea, respiratory tract and reproductive epithelium, mainly to protect the epithelium from external pathogen invasion.33 Once the expression of MUC16 is abnormally increased, it helps cancer cells in the affected tissue escape the body’s immune surveillance.34 Overexpression of MUC16 was always found in ovarian, pancreatic, cervical and lung cancer.35,36 More than 80% of ovarian cancer is characterized by overexpression of MUC16, and the high expression of MUC16 and CA-125 is an important indicator for the early diagnosis of ovarian cancer.37
Studies have shown that MUC16-CAR-T cells have specific killing effects on MUC16+ ovarian cancer cells in vitro. Intravenous or intraperitoneal injection of MUC16-CAR-T cells can delay the progression of ovarian cancer or completely clarify the tumors in mouse tumor-bearing models.38 These studies also confirmed the research value of MUC16 as a potential target for the treatment of a class of ovarian cancer cells.
Mesothelin
Mesothelin, a group of glycoproteins anchored on the plasma membrane through the phosphatidylinositol region (GPI),39 is normally expressed in pleura, peritoneum, pericardium and mesothelial cells. Mesothelin is highly expressed in 30% of tumors, such as pancreatic cancer, ovarian cancer, mesothelioma, lung adenocarcinoma, cholangiocarcinoma, gastric cancer, colon cancer and endometrial cancer. Because mesothelin is often expressed on the surface of human normal tissues and its nonspecific toxicity is lower,40,41 researchers have designed a variety of therapeutic methods targeting mesothelin antigens, such as antitoxins, antibody-based therapy, cancer vaccines and adoptive T cell therapy. Preclinical and clinical experiments have shown good antitumor effects, thus showing that mesothelin is a potential target.42
Studies have shown that mesothelin is a promising and potential specific target in immunotherapy. Encouraging results have been achieved in the experimental phase of mesothelin-based CAR-T cell research, and clinical trials have been approved for a variety of tumors. Preclinical studies of mesothelin-based CAR-T cells in subcutaneous or in situ mouse models of mesothelioma, ovarian cancer and lung cancer transplantation were also carried out.40,41,43–45 Recently, a second-generation mesothelin-based CAR-T cell (SS1-4-BBCAR) clinical trial at the University of Pennsylvania was carried out. Four patients with pancreatic cancer and mesothelioma did not observe serious adverse reactions, such as cytokine release syndrome,46 pleurisy and pericarditis. These trials all showed that mesothelin is a safe and feasible target for treatment by CAR-T cells.41
HER2
Human epidermal growth factor receptor 2 (HER2/neu), also known as HER2 or ERBB2, is a proto-oncogene and plays an important role in the pathogenesis and clinical process of various tumors. In vitro and animal experiments have clearly shown that gene amplification and protein overexpression of HER2/neu play a key role in tumorigenic transformation and development of tumors.47 Subsequent studies have shown that HER2/neu gene amplification and overexpression are associated with other tumors, such as ovarian cancer,48 gastric cancer,49 colorectal cancer,50 salivary adenocarcinoma51 and non-small-cell lung cancer,52 while protein expression in normal tissues is negative or very low. Overexpressed HER2/neu proteins make tumors more aggressive and are independent risk factors for poor prognosis in these cancer patients.53 Research on monoclonal antibodies targeting HER2 is increasing, and the development and application of trastuzumab (Herceptin) also shows a trend of transition from traditional nonspecific chemotherapy to molecular targeting in cancer therapy. However, trastuzumab is expensive and may cause side effects, including cardiotoxicity and corneal ulcers, so its clinical application is limited. At present, HER2-specific CAR-T cell therapy has shown good therapeutic potential in the preclinical stage. However, HER2-CAR-T cell treatment in ovarian cancer is still in the clinical experimental stage.
Folate Receptor-α (FRα)
Folate receptor-α, as a carbonyl phosphatidylinositol anchoring protein, is highly expressed in epithelial tumors but lowly expressed in normal tissues.54 Studies have shown that in ovarian cancer, the overexpression of FRα is significantly correlated with tumor malignancy and prognosis.55 Therefore, FRα is also a potential target for CAR-T cell therapy of ovarian cancer.
At present, it has been reported that FRα showed good antitumor effects in in vitro experiments, but due to the limited homing efficiency of CAR-T cells entering the tumor, the question of how to direct them to provide a good therapeutic effect in clinical trials still needs to be further explored. Kershaw, et al56 first reported the application of CAR-T cells in FRα ovarian cancer, but no remission manifestations, such as tumor load reduction, were observed in this study. The results showed that a large number of CAR-T cells could be detected in the peripheral circulation of patients 2 days after infusion, followed by a sharp decrease in CAR-T cells, and almost no CAR-T cells could be detected 1 month after infusion. It was concluded that although a high dose of FRα-CAR-T cells is safe, its short life time in the human body and its inability to amplify are the main reasons for the failure of the clinical trial.56 Song, et al studied the coupling of the FRα-specific site scFv (MOv19) with the T cell receptor CD3ζ chain signaling module alone (MOv19-ζ) or in combination with the CD137 (4-1BB) costimulatory motif in tandem (MOv19-BBζ).7 In the coculture process of FRα(+) ovarian cancer cells, MOv19-ζ and MOv19-BBζ can increase the secretion of various inflammatory factors, such as IFN-γ, IL-2, TNF-α and IL-4.7 In addition, in animal models of FRα(+) intraperitoneal, subcutaneous, and lung metastases, MOv19-BBζ CAR-T cells also demonstrated good therapeutic effects. Modified CAR-T cells can not only proliferate steadily in vivo but also accumulate specifically in tumor tissues to enhance the antitumor effect.
Limitations Of CAR-T Cell Therapy
Although CAR-T cell therapy has shown good therapeutic potential in hematological tumors, it still needs to be further optimized in solid tumors for the following reasons: 1) off-target effects; 2) complex composition of tumor tissues and lack of ideal target antigens; 3) immunosuppressive effects of tumor microenvironment;11 4) efficiency of CAR-T cells reaching the tumor site is difficult to guarantee; and 5) cytokine release syndrome induction.
To overcome the off-target effects, researchers constructed double-targeted CAR-T cells. Two different kinds of CAR were modified on T cells to recognize different tumor surface antigen targets, one of which was responsible for the transmission of killing signals and the other for the transmission of costimulatory signals. Only when two different kinds of CAR bind to the corresponding target on the surface of the tumor at the same time can CAR-T cells be fully activated and produce effective antitumor effects.57 A double-targeted strategy can not only avoid the off-target effects of CAR-T cell therapy but also overcome immune escape and enhance anticancer activity.
Different tumors have various tumor microenvironment (TME), which can be divided into two categories: hot tumors and cold tumors. “Hot tumors” (inflamed) are associated with better prognosis and response to antitumor immunotherapy than “cold tumors” (noninflamed). Because of the immunosuppressive cells in the tumor microenvironment (TME), including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), the anti-tumor immune function of ovarian cancer patients is greatly attenuated.58 Thus, patients have the poorest outcomes after receiving immunotherapy. This may be limitations of CAR-T cell therapy in ovarian cancer, but it can be hope to reprogramming tumor from an immunologically cold, or T cell excluded, to an inflamed tumor.59
The occurrence of cytokine release syndrome is mainly due to the overactivation of CAR-T cells and lack of effective control, which means that the regulation of CAR-T cells is particularly important. Currently, there are two main methods: 1) introduce suicide genes to modify T cells to remove overactivated T cells to achieve a balance between CAR-T cell therapy toxicity and antitumor activity. Recently, researchers have developed suicide gene systems for CAR-T cells, such as HSV-TK, iCasp9 and CD20, to control adverse reactions caused by CAR-T cells in treatment.60 2) Construction of CAR-T cells carrying negative regulatory receptors, which can reduce the activity of overactivated T cells when recognizing the corresponding ligands on the surface of tumors. Wu et al divided CAR-T cells into two parts: the extracellular antigen binding domain (scFv) and the intracellular signal region (ITAM), the receptors of which can reversibly bind small molecular particles such as kanamycin.61 The activation of T cells requires the binding of tumor surface antigens and small molecule particles to the corresponding receptors so that the activation time and intensity of CAR-T cells can be regulated by increasing or decreasing the concentration of molecule particles without affecting the anticancer activity.
Conclusion
CAR-T cell therapy has made a breakthrough in the treatment of hematologic tumors, which has great potential value in the field of tumor research. However, the treatment of solid tumors is not mature enough to achieve the desired results. To enhance the anticancer activity of CAR-T cells and avoid the related toxicity of treatment, it has become a research hotspot in this field to find suitable tumor surface antigens, overcome the microenvironment of tumor immunosuppression, optimize the combination of costimulatory molecules, construct the fourth generation of CAR, design suicide genes, and reduce adverse reactions after reinfusion.
Abbreviations
CAR-T, Chimeric antigen receptor T; TILs, tumor infiltrating lymphocytes; scFv, single-chain variable fragment; ACT, adoptive cellular immune therapy; FRα, folate receptor-alpha; HER2, human epidermal growth factor receptor 2; TAAs, tumor-associated antigens.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Webb PM, Jordan J. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3. doi:10.1016/j.bpobgyn.2016.08.006
3. Bookman MA. Standard treatment in advanced ovarian cancer in 2005: the state of the art. Int J Gynecol Cancer. 2005;15(Suppl 3):212–220. doi:10.1111/j.1525-1438.2005.00444.x
4. Chekmasova AA, Brentjens RJ. Adoptive T cell immunotherapy strategies for the treatment of patients with ovarian cancer. Discov Med. 2010;9(44):62–70.
5. Michael K, Bruce LL, David LP, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73.
6. Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16 ecto directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13(1):102. doi:10.1186/s12967-015-0541-x
7. Song D-G, Ye Q, Carpenito C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res. 2011;71(13):4617–4627. doi:10.1158/0008-5472.CAN-11-0422
8. Narod S. Can advanced-stage ovarian cancer be cured?. Nat Rev Clin Oncol. 2016;13(4):255. doi:10.1038/nrclinonc.2015.224
9. Ledermann JA, Embleton AC, Raja F, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387(10023):1066–1074. doi:10.1016/S0140-6736(15)01167-8
10. Chan JK, Brady MF, Penson RT, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016;71(8):738–748. doi:10.1056/NEJMoa1505067
11. O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167. doi:10.1038/s41571-018-0142-8
12. Wei-Ting H, Adams SF, Emin T, Hagemann IS, George C. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi:10.1016/j.ygyno.2011.09.039
13. Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10(5):267–276. doi:10.1038/nrclinonc.2013.46
14. Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8(4):7175–7180. doi:10.18632/oncotarget.12739
15. Neuman BJ, Ailon T, Scheer JK, et al. Development and validation of a novel adult spinal deformity surgical invasiveness score: analysis of 464 patients. Neurosurgery. 2017;82(6):847–853.
16. Yin Q, Shen J, Zhang Z, Yu H, Li Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Deliv Rev. 2013;65(13–14):1699–1715. doi:10.1016/j.addr.2013.04.011
17. Rong L, Xia P, Chang JY, et al. MiRNA-related genetic variations associated with radiotherapy-induced toxicities in patients with locally advanced non–small cell lung cancer. PLoS One. 2016;11(3):e0150467. doi:10.1371/journal.pone.0150467
18. Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. 2016;37(3):220–230. doi:10.1016/j.tips.2015.11.004
19. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi:10.1016/S0140-6736(14)61403-3
20. Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127(26):3312. doi:10.1182/blood-2016-02-629063
21. Kunert A, Obenaus M, Lamers CH, Blankenstein T, Debets R. T cell receptors for clinical therapy: in vitro assessment of toxicity risk. Clin Cancer Res. 2017;23(20):6012–6020.
22. Hay KA, Turtle CJ. Chimeric antigen receptor (CAR) T cells: lessons learned from targeting of CD19 in B-cell malignancies. Drugs. 2017;77(3):237–245. doi:10.1007/s40265-017-0690-8
23. Morgan RA, Yang JC, Mio K, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi:10.1038/mt.2010.24
24. Nabil A, Brawley VS, Meenakshi H, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi:10.1200/JCO.2014.58.0225
25. Feng K, Yang L, Guo Y, et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2017;5:1–10.
26. Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2013;257(1):107–126. doi:10.1111/imr.12131
27. Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155(1):29. doi:10.1053/j.gastro.2018.03.029
28. Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263(1):68–89. doi:10.1111/imr.12243
29. Ruella M, Kenderian SS. Next-generation chimeric antigen receptor T-cell therapy: going off the shelf. Biodrugs. 2017;31(6):473–481. doi:10.1007/s40259-017-0247-0
30. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447
31. Mcguirk J, Waller EK, Qayed M, et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 2017;19(9):1015–1024. doi:10.1016/j.jcyt.2017.06.001
32. Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi:10.1016/j.coi.2009.02.009
33. Dhanya H, Ponnusamy MP, Seema C, Imayavaramban L, Parthasarathy S, Batra SK. MUC16: molecular analysis and its functional implications in benign and malignant conditions. Faseb J. 2014;28(10):4183–4199. doi:10.1096/fj.14-257352
34. Liu Q, Cheng Z, Luo L, et al. C-terminus of MUC16 activates Wnt signaling pathway through its interaction with β-catenin to promote tumorigenesis and metastasis. Oncotarget. 2016;7(24):36800–36813. doi:10.18632/oncotarget.9191
35. Togami S, Nomoto M, Higashi M, et al. Expression of mucin antigens (MUC1 and MUC16) as a prognostic factor for mucinous adenocarcinoma of the uterine cervix. J Obstet Gynaecol Res. 2010;36(3):588–597. doi:10.1111/j.1447-0756.2010.01221.x
36. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43(10):1755–1763. doi:10.1016/j.humpath.2012.01.005
37. Rao TD, Tian H, Ma X, et al. Expression of the carboxy-terminal portion of MUC16/CA125 induces transformation and tumor invasion. PLoS One. 2015;10(5):e0126633. doi:10.1371/journal.pone.0126633
38. Alena AC, Thapi DR, Yan N, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16(14):3594–3606. doi:10.1158/1078-0432.CCR-10-0192
39. Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time?. J Clin Oncol. 2016;34(34):4171–4179. doi:10.1200/JCO.2016.68.3672
40. Gregory LB, Andrew RH, Marcela VM, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–120. doi:10.1158/2326-6066.CIR-13-0170
41. Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016;6(2):133–146. doi:10.1158/2159-8290.CD-15-0583
42. Okla K, Surowka J, Fraszczak K, et al. Assessment of the clinicopathological relevance of mesothelin level in plasma, peritoneal fluid, and tumor tissue of epithelial ovarian cancer patients. Tumour Biol. 2018;40(10):1010428318804937. doi:10.1177/1010428318804937
43. Sonia G, Xi C, Aviv M, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124(7):1070–1080. doi:10.1182/blood-2013-10-535245
44. Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi:10.1126/scitranslmed.3010162
45. Servais EL, Colovos C, Kachala SS, Adusumilli PS. Pre-clinical mouse models of primary and metastatic pleural cancers of the lung and breast and the use of bioluminescent imaging to monitor pleural tumor burden. Curr Protoc Pharmacol. 2011;chapter 14:
46. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi:10.1038/nrclinonc.2017.148
47. Martin V, Cappuzzo F, Mazzucchelli L, Frattini M. HER2 in solid tumors: more than 10 years under the microscope; where are we now?. Future Oncol. 2014;10(8):1469–1486. doi:10.2217/fon.14.19
48. Chang KL, Lee MY, Chao WR, Han CP. The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma. Hum Genomics. 2016;10(1):40. doi:10.1186/s40246-016-0096-9
49. Kanayama K, Imai H, Usugi E, Shiraishi T, Hirokawa YS, Watanabe M. Association of HER2 gene amplification and tumor progression in early gastric cancer. Virchows Arch. 2018;473(5):559–565. doi:10.1007/s00428-018-2433-y
50. Jeong JH, Kim J, Hong YS, et al. HER2 amplification and cetuximab efficacy in patients with metastatic colorectal cancer harboring wild-type RAS and BRAF. Clin Colorectal Cancer. 2017;16(3):e147–e152. doi:10.1016/j.clcc.2017.01.005
51. Kondo Y, Kikuchi T, Esteban JC, et al. Intratumoral heterogeneity of HER2 protein and amplification of HER2 gene in salivary duct carcinoma. Pathol Int. 2014;64(9):453–459. doi:10.1111/pin.12195
52. Grob TJ, Kannengiesser I, Tsourlakis MC, et al. Heterogeneity of ERBB2 amplification in adenocarcinoma, squamous cell carcinoma and large cell undifferentiated carcinoma of the lung. Mod Pathol. 2012;25(12):1566. doi:10.1038/modpathol.2012.125
53. Cai Y, Wang J, Zhang L, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med Oncol. 2015;32(1):391. doi:10.1007/s12032-014-0391-z
54. Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015;34(1):41–52. doi:10.1007/s10555-014-9539-8
55. Kurosaki A, Hasegawa K, Kato T, et al. Serum folate receptor alpha as a biomarker for ovarian cancer: implications for diagnosis, prognosis and predicting its local tumor expression. Int J Cancer. 2016;138(8):1994–2002. doi:10.1002/ijc.29937
56. Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi:10.1158/1078-0432.CCR-06-1183
57. Oble DA, Loewe R, Yu P, Mihm
58. McCloskey CW, Rodriguez GM, Galpin KJC, Vanderhyden BC. Ovarian cancer immunotherapy: preclinical models and emerging therapeutics. Cancers. 2018;10(8). doi:10.3390/cancers10110400
59. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves Anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119 e1110. doi:10.1016/j.cell.2017.08.027
60. Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol. 2014;5:254. doi:10.3389/fphar.2014.00254
61. Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350(6258):aab4077. doi:10.1126/science.aab4077
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.