Back to Journals » International Journal of Nanomedicine » Volume 14
Advances in nanomedicine for the treatment of ankylosing spondylitis
Authors Xi Y , Jiang T, Chaurasiya B , Zhou Y, Yu J, Wen J, Shen Y, Ye X, Webster TJ
Received 18 May 2019
Accepted for publication 13 August 2019
Published 29 October 2019 Volume 2019:14 Pages 8521—8542
DOI https://doi.org/10.2147/IJN.S216199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dongwoo Khang
Yanhai Xi,1,* Tingwang Jiang,2,* Birendra Chaurasiya,3 Yanyan Zhou,1 Jiangmin Yu,1 Jiankun Wen,1 Yan Shen,3 Xiaojian Ye,1 Thomas J Webster4
1Department of Spine Surgery, Changzheng Hospital, Second Military Medical University, Shanghai, People’s Republic of China; 2Department of Immunology and Microbiology, Institution of Laboratory Medicine of Changshu, Changshu, Jiangsu 215500, People’s Republic of China; 3Department of Pharmaceutics, Center for Research Development and Evaluation of Pharmaceutical Excipients and Generic Drugs, China Pharmaceutical University, Nanjing, People’s Republic of China; 4Department of Chemical Engineering, Northeastern University, Boston, MA, USA
*These authors contributed equally to this work
Correspondence: Xiaojian Ye
Department of Spine Surgery, Changzheng Hospital, Second Military Medical University, 360 Huntington Avenue, Shanghai, MA 200003, People’s Republic of China
Tel +86 1 381 734 6934
Email [email protected]
Thomas J Webster
Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA
Tel +1 617 373 6585
Email [email protected]
Abstract: Ankylosing spondylitis (AS) is a complex disease characterized by inflammation and ankylosis primarily at the cartilage–bone interface. The disease is more common in young males and risk factors include both genetic and environmental. While the pathogenesis of AS is not completely understood, it is thought to be an immune-mediated disease involving inflammatory cellular infiltrates, and human leukocyte antigen-B27. Currently, there is no specific diagnostic technique available for this disease; therefore conventional diagnostic approaches such as clinical symptoms, laboratory tests and imaging techniques are used. There are various review papers that have been published on conventional treatment approaches, and in this review work, we focus on the more promising nanomedicine-based treatment modalities to move this field forward.
Keywords: ankylosing spondylitis, pathogenesis, genetic factors, environmental factors, treatment approaches
Introduction
Ankylosing spondylitis (AS) is a type of chronic arthritis characterized by inflammation of bone, the cartilage–bone interface as well as the entheses.1 Axial skeletons are mainly affected with time via chronic inflammation developed in the spine. Likewise, sacroiliac joints are also affected. Ultimately extra bone is formed in the spine that causes fusion of vertebrae.2 The disease’s most prominent onset starts from the age of 20–30 and is most prominent in males;3 men and women are affected approximately at a ratio of 3:1.4 In about 80% of patients, the first symptom develops at the age of 30 while <5% of patients are affected at the age of >45.5
Although the etiology of AS is unknown, it is thought to arise from a combination of genetic6,7 and environmental8,9 factors. More than 90% of AS patients are believed to be affected by the specific human leukocyte antigen (HLA), mediated by an autologous peptide B27 molecule known as HLA-B27.10 In general, very few (about 2%) are afflicted, unless there is a family history of AS, in which case, the rate of incidence is higher.11 Similarly, another genetic factor ERAP1 (endoplasmic reticulum aminopeptidase 1) has been shown to play an active role in the pathogenesis of AS. ERAP1 is considered to participate in the peptide trimming required for presentation by the major histocompatibility complex (MHC) class 1, which further stabilizes and precipitates HLA-B27.7 Another causative factor is the environment and this factor may link with infection and mechanical stress in cervical bones and ligaments.
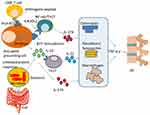
Several research groups have suggested that the bacteria Klebsiella pneumonia may be a triggering factor for the onset of AS.8 The bacteria reside mainly in the colon, and develop immune responses without overt gastrointestinal (GI) infections. There have been four studies exclusively completed in which researchers have directly correlated Klebsiella in the feces of AS patients.12–15 The results from these studies demonstrated an increase in gut permeability as well as gut inflammation in AS patients,16,17 which clearly show the role of Klebsiella AS.
Mechanical stress is another environmental factor which causes entheses damage to the ligaments. This repeated damage and repair from stress activated downstream regulates inflammation and causes bone erosion leading to spur formation.18 Clinical symptoms of this disease encompass back pain, enthesitis, asymmetrical peripheral oligoarthritis as well as chronic inflammatory bowel disease (IBD).19,20 AS is diagnosed based on clinical features, a pathological test, and imaging techniques such as X-ray and magnetic resonance imaging (MRI).
Management of this chronic disease includes combinational approaches of clinical treatment and physiotherapy, besides these, some anti-tumor necrosis factor (TNF) agents are also used.21 Despite advancements in the diagnosis and management techniques of this disease, improvements are required because these medical drugs have a high level of deleterious or even lethal side effects22 due to a lack of specificity and are expensive.23
Various literature reviews have been published based on AS pathogenesis, clinical symptoms, diagnosis, and conventional treatment, but until now, no information has been published concerning nanotechnology-based treatment. Therefore, for the first time, this review is compiled to provide brief information about the pathogenesis and diagnosis as well as provides detailed information about the possibility of nanotechnology-based treatments.
Pathogenesis
There are mainly two factors (i.e., genetic and environmental) involved in the pathogenesis of AS as described below.
Genetic factors
It has been reported that genetic factors play a major role (about 90%) in the precipitation of AS.24 There have been extensive studies completed to support gene associated AS. For example, a protein called HLA-B27 belongs to the class-1 surface antigens present on the interface of “MHC” antigenic peptides of T-cells and is strongly involved in the pathogenesis of AS.25 The exact role of HLA-B27 in the pathogenesis of AS is still under intense study but it is predicted that HLA-B27 binds with peptides present on MHC and is recognized by CD8+ T-cells which further influence the development of AS.26 There have been various hypotheses presented by scientists regarding the pathogenesis of AS with one very prominent hypothesis being the precipitation of unconventional forms of HLA-B27 as free heavy chains (FHCs).27 The endoplasmic reticulum (ER) is the main reservoir for FHCs which triggers the unfolded protein response (UPR) and further increases the production of various cellular infiltrates, especially IL-23, which plays a major role in AS pathogenesis.28
Another hypothesis postulated toward the pathogenesis of AS is the regulation of the endoplasm reticulum-associated protein degradation (ERAD) process to activate ER stress and UPR. Downregulation of ER degradation enhances α-mannosidase-like protein 1 (EDEM1) and the ERAD-related molecule, leading to increases in the number of HLA-B27 dimers which results in the pathogenesis of AS.29 Interestingly, HLA-B27 FHC dimers, located on the surface of antigen-presenting cells get stimulated by non-classical HLA-B27 molecules, positive to the IL-23 receptor which carries the killer cell immunoglobulin-like receptor (KIR) 3DL2 to produce IL-17.30 Activated KIR3DL2-expressing CD4+T cells interact with HLA-B27 dimers to promote the expression of a T helper 17 (TH17)-cell-specific transcription factor RORγt and anti-apoptotic factor B-cell lymphoma 2 (BCL-2).31 Hence, activation of KIR downregulates apoptosis of activated TH17 cells in AS.
There are various genes in the M1 family of zinc metallopeptidases which are considered to participate in the aggravation of AS,32 and these genes participate in the trimming of peptide length required by HLA molecules for protein synthesis.33,34 These genes reduce peptide cleavage time and lead to increases in the availability of antigenic peptides which are denatured by the aminopeptidase (AP) enzyme which further affect HLA antigen function in the regulation of HLA-B27 FHC and TH17 cell activation mediated by KIRs.35 The direct role of the M1 family of genes is not clear, but it was noted that these genes reduce the stability of HLA-B27.36
IL-17 has played a significant role in the pathogenesis of AS, as high levels of this cytokine have been extracted in the serum, synovial fluid, and joints in AS patients.37,38 For example, Gracey et al found increases in IL-17 levels in male patients with AS. Gracey et al conducted a sex-based genetic expression study in 94 AS patients (Table 1) to correlate the potential cytokine IL-17 and found male patients express IL-17 more than female patients (Figure 1).39
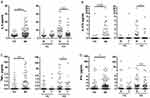 |
Figure 1 Expression of pro-inflammatory cytokines in the serum of patients with AS and HCs. The levels of IL-6 (A), IL-17A (B), TNF (C), and IFN-γ (D) in serum samples obtained from 73 patients with AS (39 male and 34 female) and 33 healthy controls (17 male and 16 female) were determined by an ELISA assay. Data are presented for the pooled cohorts and according to sex. Each symbol represents an individual subject; horizontal lines show the median (*P=0.05–0.01; **P=0.01–0.001, by a Mann–Whitney test with Benjamini-Hochberg correction). Reprinted with permission from John Wiley and Sons. Arthritis & Rheumatology, Sexual dimorphism in the Th17 signature of ankylosing spondylitis, Gracey E, Yao Y, Green B, et al, Copyright 2016.39Abbreviations: NS, not significant; AS, ankylosing spondylitis; HC, healthy control; TNF, tumor necrosis factor; IFN-γ, interferon-γ. |
 |
Table 1 Demographic of patients with AS |
Innate immunity is considered to be a primary self-protection system of the body which provides a shield for any foreign intruders in our body. This innate immunity system encompasses various kinds of cells, such as macrophages, dendritic cells (DCs), natural killer cells, and mast cells, which can recognize invaded antigens.40 Although it was mentioned above that the HLA-B27 gene is the major genetic component involved in the propagation of this disease from one generation to another, from the above trial, it can be noticed that there are also some other non-HLA-B27 factors which can aggravate the disease condition.41 The innate immune system is governed by various effector proteins called cytokines such as IL-1, TNF-α, IL-23 and others. Among these cytokines, IL-1 is composed of nine clusters which stay together in sequential arrangement. IL-α and IL-β are two potent inflammatory cytokines belonging to IL-1, which have been well studied. IL-1β can be actively secreted from all nucleated cells especially macrophages which can activate other immune cells to cause inflammation.42 During animal experiments, it was found that IL-1α and IL-1β accelerate bone-resorbing osteoclasts. It was also seen that IL-1α involved in joint bone destruction was followed by the activation of other enzymes.43 From this finding, it is clear that the AS patient synovium contains an increased amount of IL-1 and its role in pathogenesis comes from polymorphic alleles present in cytokine genes which aggravate inflammation.44
Another cytokine that belongs to the innate immunity is TNF, a pleiotropic pro-inflammatory cytokine produced by various types of cells, especially by macrophages and T cells.45 There is various evidence that backs TNF participating in AS pathogenesis. Tissue biopsies of sacroiliac have shown increased numbers of TNF-expressing macrophages and degradation of sacroiliac joint tissue considered as the hallmark of AS.46 Moreover, mice over-expressing membrane-bound TNF have developed spinal AS-like abnormalities. Opposing the finding of increased levels of TNF-α in blood, some researchers have found elevated levels of TNF linked with the pathogenesis of AS.47 The gene responsible to encode TNF localized in the class III region of the MHC is present near the HLA-B locus.48 In the promoter region, several TNF regulating polymorphism mechanisms participate in the production of TNF-α which vary in different individuals.49 In all of these polymorphism mechanisms, in the promoter region of the TNF-α gene, mostly adenine and guanine are involved and associated with AS severity.50
As explained above, IL-1 and TNF cytokines are directly responsible for the pathogenesis of AS, and it is also speculated that these cytokines are involved in the activation of APs in the ER to regulate ERAP genes. Beside HLA-B27, the ERAP1 gene is considered as a non-MHC gene responsible for the pathogenesis of AS.
Recently, various studies have shown an association of ERAP in AS.51,52 Besides the trimming of antigenic peptides for HLA-B27, ERAP fragment cytokine receptors present on the surface of the cell conducted in ERAP1 knock-out mice and in AS patients failed to support this role of ERAP.53 From this finding, it looks like ERAP participate in the functioning of HLA-B27 rather than in the trimming of cytokine receptors.
Some studies have proved that DCs in HLA-B27 transgenic rats have dysfunctional properties (they do not have class II MHC expression and viability resulting in the loss of a tolerogenic CD103+ population).54,55 DCs have the ability to enhance the development of TH17 by downregulating immune synapse formation and, hence, aggravate the disease.56 CD4+T cells are released as naïve CD4+ cells into the periphery and divide into several effector T cells such as Th1, Th2, Treg, and Th17. Each of these effector T cells produces specific cytokines and master transcription factors (Figure 2). These cells develop immunity to protect from several infections caused by bacteria or fungus. The functions of TH17 are regulated by cytokines, such as IL-17A, IL-17F and IL-22, which further controls the combination of cytokines, such as IL-1β, IL-6, TGF-β and IL-23. Recently, it was found that the development of TH17 from naïve T cells is affected by several factors such as hypoxia,57 dietary and some other environmental factors.58 It was argued that due to the production of additional cytokines, the nature of TH17 has apparently become plastic and heterogenic, which make it highly pathogenic. Thus, IL-17, a cytokine, is often coupled with the production of IL-17, IL-22, IFN-γ and cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF).59,60
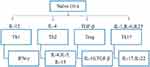 |
Figure 2 Flow diagram of the production and transcription of cytokine expression of Th17 and other CD4+ T cell subsets. Abbreviations: IFN, interferon; TGF, transforming growth factor. |
Environmental factors
Besides genetic factors, some environmental factors have also been linked with the pathogenesis of AS. There are two main environmental factors (i.e., mechanical stress and bacterial infections) hypothesized to trigger the pathogenesis of AS.61 Mechanical stress mainly at the entheses plays a role in the development in SP by repetitive damage and repair of the entheses. This repetitive damage and repair of the entheses downregulates inflammation, bone erosion and spur formation.62,63 Microbial infection with Chlamydia trachomatis, Shigella and Salmonella are well documented in AS. These microorganisms can invade mucosal surfaces and replicate intracellularly. Salmonella and Chlamydia have been found in the synovial tissues and fluids of AS patients. Nearly, 50% of patients with AS suffer from microscopic ileal inflammation and are immunologically similar to CD. Many studies, from early antibody and fecal carriage studies, advocate the role of gut Klebsiella in the pathogenesis of AS. More recent findings showing various bacterial compositions suggest a link between intestinal dysbiosis and AS, and suggest that upsetting the homeostasis between the gut microbiome and the host immune system can lead to AS.64 The pathophysiology of both factors is summarized in Figure 3.
Diagnosis and clinical symptoms
The diagnosis of AS is made by clinical, laboratory, or radiological findings. Based on clinical symptoms, patients are categorized according to the modified New York diagnosis criteria.65 However, there is no specific test available to identify AS, therefore, some precise and specific diagnostic markers are important to identify the disease.
The major clinical findings could be subtle in the early or middle stages of AS. The clinical test importantly includes measurements of forward lumber flexion, lateral lumbar flexion, and expansion of the chest together with palpating and stressing the sacroiliac joints. Moreover, the peripheral joints should also be monitored for evidence of synovitis.66
During laboratory testing, almost all patients having AS showed increased levels of C reactive protein (CRP) and erythrocyte sedimentation rates (ESR).67 However, determination of the levels of these acute phase reactants have limited value in determining the disease activity.68,69 Mild normochromic normocytic anemia may be presented in severe AS diseases. In fact, the synovial fluid of affected limbs is more similar to that of any joint inflammatory disease.
Physically, AS patients show a loss in spinal movement with a tightness in flexion, extension in the lumbar spine, and chest expansion. Due to the secondary muscle spasms, the motion is disproportionate to the degree of ankylosis.
The spinal radiographical changes show marginal vertebral erosion, squaring of vertebral bodies, and the formation of bony bridges or syndesmophytes in the interface of the adjacent vertebrae. Ossification of spinal ligaments could occur, and spinal osteopenia is a common symptom. In severe chronic diseases, almost complete fusion of the vertebral column may occur. Two-dimensional radiographic techniques like CT-scans and X-rays are used for imaging in the primary onset of the disease but further MRI can be used to image for a more confirmative result.
The initial clinical symptom generally starts with weak backbone pain. The pain is usually felt deep in the buttocks and lower lumber regions followed by morning stiffness in the same area, which improves with movement and resumes with rest. The pain becomes persistent and bilateral within a few months and is usually worse at night.70 In some patients, the primary onset starts with bone tenderness followed by back pain and stiffness. Asymmetric arthritis of other joints, predominantly of the lower limbs, can be present at any stage of the disease. Neck pain and stiffness are seen in advanced degrees of the disease.
Recent AS treatment strategies
AS is a HLA-B27 linked inflammatory disease.71 Previously, about 0.5% of the population was affected with AS with a predicted male to female ratio of about 2:1.1 AS is typically treated with the use of anti-inflammatory, immunosuppressive drugs.72,73 However, since 2000, a considerable advancement took place in order to treat AS by developing novel therapeutic approaches including biological therapies as well as efficient clinical trial design and execution. In this section, we focus on recent developments to treat AS.
NSAIDs therapy
After the observation that AS is mainly associated with the prostaglandin E receptor 4 (PTGER4) gene and prostaglandin inhibitory effects, both traditional and selective COX-2 inhibitor NSAIDs proved equally effective,74 and COX-2 inhibitor NSAIDs are considered as a first-line treatment option for AS.75 Previous reports demonstrated that COX showed two phenotypes, namely, COX-1 and COX-2, where COX-1 is responsible for prostaglandin secretion and maintenance of gastric mucosal integrity while, on the other hand, COX-2 offered inflammatory stimuli. A recent clinical study of 246 axial disease patients included an administered placebo-control, celecoxib 100 mg b.i.d. (COX-2 inhibitor) and ketoprofen 100 mg b.i.d. (non-selective anti-inflammatory drug) for a 6-week period. The results indicated significant pain reduction in the treatment as compared to the placebo-control. However, the level of poor GI tolerability was equivalent in both treatment groups.76 In another study of 3410 AS patients, Etoricoxib was found to be more effective in pain management than other NSAIDs.77 Furthermore, in another study, a preferential COX-2 inhibitor meloxicam showed a similar pain reducing result in AS patients.78 Administration of NSAIDs is debatable and, in most cases, the continuous administration of anti-inflammatory drugs showed promising results in term of disease progression (less new bone formation) as compared to patients who took drugs only when they experienced pain.79 However, controversy lies regarding NSAIDs administration. The continuous administration of NSAIDs decreased the radiographic progression as compared to the on-demand therapy.79 While in another report, Sieper et al reported that in comparison with an on-demand treatment, the continuous administration of diclofenac for about 2 years did not reduce radiographic progression,80 but treatment with NSAIDs induced various cardiovascular, GI or kidney associated complications. A meta-data of nearly 300,000 patients with NSAID treatment for different clinical conditions confirmed high vascular risk of both NSAIDs and COX, as compared to a naproxen treatment.77 Yet, a patient with inflammatory diseases (other than joints), such as inflammatory GI disorders, could be treated by administering selective COX-2 inhibitors with no potential risk of complications.81
Similarly, a Canadian study population based on a retrospective study demonstrated decreased cardiovascular risk in AS patients under NSAID treatment.82 Moreover, in order to reduce the risk of complications, it is admissible to use a combination of two different NSAIDs for 4 weeks or use the first NSAIDs for the first week and continue with the second NSAID for the remaining 3 weeks or use the first for the first 2 weeks and the second for the remaining 2 weeks to complete the therapy. Thus, selection, dosing and administration of potent NSAIDs is critical and may vary between patient-to-patient, so as its therapeutic outcome and NSAIDs treatment are justified purely on patient symptoms.83 Recently, Fattahi et al reported a randomized placebo-control trial of a new NSAID (BD-mannuronic acid). It was indicated that a 12-week ASAS response was similar to that of naproxen without renal side effects and good GI tolerability was observed.84
Analgesics therapy
Sometimes NSAIDs do not successfully control AS associated pain. In this case, analgesic drugs (such as acetaminophen and opioid drugs) could be administered as an add-on therapy with NSAIDs. A combination of Tramadol/acetaminophen was found effective and safe as an add-on therapy with selective COX-2 NSAIDs for osteoarthritis,85 chronic low back pain86 or fibromyalgia87 treatment. In this respect, in order to determine the safety and efficacy of analgesic drugs, a 12-week randomized, double-blind, placebo-controlled study of 60 AS patients were administered aceclofenac plus tablets (tramadol 37.5 mg/acetaminophen 325 mg combination). The patients were divided equally into two groups: 1) a treatment group which administered one aceclofenac plus tablet twice a day and 2) a control group which received placebo aceclofenac plus for 12 weeks. It was observed that at week 12, assessment of an AS (ASAS20, end-point) response showed a 53.3% improvement compared to the treatment group. However, a slight increase in total adverse effect was observed including dizziness, vertigo, and nausea or vomiting in the treatment group. These results confirmed that the aceclofenac plus (tramadol 37.5 mg/acetaminophen 325 mg combination tablet) could have some added effects to NSAIDs in the treatment of AS patients.88 However, in another scenario, when no other treatment option was available, then analgesic therapy (either as a single or in combination therapy) could be a potent treatment choice.
Hormonal and disease-modifying anti-rheumatic (DMARDs), antitumor and antibiotic therapy
Hormonal therapy (such as oral or injectable corticosteroids (cortisone)) is an effective anti-inflammatory agent and could be employed for active AS together with other inflammatory disorders. Sometimes a local intra-articular steroid injection into the sacroiliac and peripheral joint can offer relief.89 However, the long-term use of corticosteroids might induce severe adverse effects including cataracts, osteoporosis, easy bruising, diabetes, thinning of the skin and destruction of the hips.90,91 On the other hand, a local glucocorticoid injection could be administered for skeletal muscle inflammation, such as enthesitis, but the systematic injection of steroids is not recommended and81 also has not proved effective for AS.89
Disease-modifying anti-rheumatic drugs (DMARDs), including leflunomide and methotrexate (MTX), due to their low efficacy are not generally administered to treat AS. Interestingly, MTX, also known as amethopterin, is also a chemotherapeutic and immunosuppressant drug widely used to treat various types of cancers, such as breast cancer, lung cancer, leukemia, lymphoma, and osteosarcoma. It has also been employed to treat autoimmune diseases including rheumatoid arthritis (RA), psoriasis and Crohn’s disease.92–94 In this respect, one study showed that MTX at a high dose (20 mg s.c.) did not show any improvement in AS patients with axial symptoms.95 However, some evidence suggests that sulfasalazine could be beneficial in peripheral arthritis and early morning stiffness.96 Therefore, it is speculated that DMARDs might benefit AS patients with peripheral arthritis but not of axial disease. Furthermore, in a study of 207 AS patients, it was also demonstrated that a high dose of thalidomide could induce peripheral neuropathy within a year after AS treatment.97 However, a 6-month open-label trial including 13 subjects with different subtypes of active AS with psoriasis showed that all of the subjects were resistant to conventional non-biological therapies, such as NSAIDs, MTX and sulfasalazine. The thalidomide proved to be a promising treatment for active AS patients, who were resistant to conventional therapies.98 Recently, a meta-analysis reported that a low dose of oral MTX (15 mg) could be more effective than a placebo, when taken for 6 months. However, the effects of a higher dose of MTX have not been measured or reported in a randomized placebo‐controlled trial.99 Furthermore, another 1-year open-trial study also showed similar results where thalidomide was administered in AS resistant patients at a dosage of 200 mg/day.100 Thus, it could be concluded that DMARDs (especially thalidomide) are potent drugs to treat resistant AS.
In many countries, it has been reported that enterobacteria (K. pneumoniae) can induce AS. Therefore, numerous antibacterial drugs have been investigated for AS treatment. Fluoroquinolones are synthetic, broad-spectrum antimicrobial agents used for treating various infections. Furthermore, they also showed some immunomodulatory effects.101 In this respect, fluoroquinolone moxifloxacin (MXF) is active against both Gram-negative and Gram-positive bacteria. Long-term open label-trials were performed, administering MXF in patients with AS to observe its therapeutic efficacy. The patients treated with MXF showed significant and sustained improvement after 12 weeks. Recently, another study demonstrated the efficacy of co‐amoxiclav in two AS patients. Co‐amoxiclav was administered orally for 7 days to both AS patients. Similarly, the results indicated significant improvements in all AS and physical examination parameters. Thus, antibacterial therapy could have the potential to improve AS along with other inflammatory diseases.102,103
Tumor necrosis factor (TNF-α) inhibitor therapy
TNF-α is also known as a pro-inflammatory cytokine, produced by monocytes, macrophages and T cells. It is mainly responsible for the activation of lymphocytes, release of IL-1 and IL-6 cytokines, metalloproteases and prostaglandins. Recent data also suggested a role for TNF-α in the pathophysiology of AS and TNF-α mRNA and was also found to be upregulated in the sacroiliac joints of AS patients.104 Furthermore, TNF-α levels along with other inflammatory cytokines were found to be high in AS patient as compared to mechanical back pain patients.105 Therefore, if a patient continues to suffer high AS disease symptoms and the above treatments are not effective; then, biological treatments which inhibit TNF inhibitors can be an option (such as adalimumab, etanercept, certolizumab pegol, infliximab and golimumab). The anti-TNF therapy not only effectively treats AS but it also decreases inflammation and improves spinal mobility. Furthermore, all anti-TNF biologics including Remicade, Simponi, Enbrel and Humira markedly treat AS and have shown sustained effects that last for years after treatment.
However, a major drawback of anti-TNF therapy is that if the disease returns after discontinuation of treatment within a year, one needs to resume the therapy which gathers attention and calls for a rational anti-TNF therapy. In this respect, infliximab has demonstrated safety and efficacy for about 5 years in an open-label study in severe to moderate AS patients and has demonstrated a sustained BASDAI treatment score of 2.5 after 5 years of treatment and about 34% of AS patients were in clinical remission.106 In other placebo-controlled studies, results showed a treatment efficiency of Infliximab for 2 years and 1 year of adalimumab.107,108 In another report, MRI scans showed a reduction in spinal inflammation after anti-TNF therapy.66 It was estimated that up to 60% of AS patients showed a better response to these biological agents and patients reported a decreased pain score, improved physical activity and well tolerated-ability to the disease.89 In addition to spinal inflammation, pain and stiffness, there was also a significant improvement in peripheral arthritis and enthesitis. MRI scans showed notable suppression of juxta-articular bony inflammation.109–111 Although in a small continuous study, less radiographic progression was observed after 2 and 4 years as compared to conventional AS therapy.1,110 Other clinical indicators (such as ESR and CRP levels) were higher in more active AS conditions. Importantly, both the ESR and CRP levels declined with anti-TNF therapy.46,112 Generally, Infliximab is administered via intravenous infusion every 6–8 weeks. In addition to the positive effects on peripheral and axial joint symptoms, there are also additional beneficial effects on IBD, psoriasis and uveitis,113,114 while Etanercept is administered weekly or biweekly by subcutaneous injection, which showed promising results in AS. Etanercept was also effective in treating psoriasis, but less effective for the symptoms of IBD and uveitis. On the other hand, adalimumab was also injected subcutaneously every other week. Like Infliximab, it was also effective in psoriasis and IBD;115 however, at this moment, insufficient reports are available regarding uveitis.
Currently, two anti-TNF-α drugs for the treatment of RA exist: 1) infliximab (mAb cA2), a neutralizing monoclonal antibody116 and 2) etanercept, consisting of 2 molecules of the p75 TNF-α receptor.117 Both have been tested for AS in 6 open and 3 placebo-controlled trials. In these studies, patients received 3 infusions of infliximab (5 mg/kg at weeks 0, 2 and 6 but in a Canadian study it was only administered at 3 mg/kg), followed by a maintenance dose after every 14 weeks in a 1-year follow-up study, and etanercept was subcutaneously administered twice-weekly (25 mg) for 4 or 6 months.118 All of these studies demonstrated a dramatic improvement in all clinical and laboratory variables of AS patients after anti-TNF application and these beneficial effects were observed a few days after the first infliximab infusion or etanercept injection. Interestingly, the PLANETAS study observed a comparable result in AS patients in terms of ASAS20 and ASAS4057.
As an extension, a study also demonstrated that switching from Infliximab to its biosimilar did not negatively affect safety and efficacy.119 These results were further confirmed in a PLANETRA study, carried out in patients with administered RA59.120 Various studies also suggested that the short-term AS disease and younger patients are more likely to respond to anti-TNF therapy.121 Talking about the dark side of the TNF blocker therapy, early and reliable diagnosis of AS is an important issue. In various large-pooled studies, the main adverse effects were very low and minor (including moderate infection such as reactivation of tuberculosis was more common),1 along with manageable injection site reactions; although patients with these malignancies may not be suitable candidates for anti-TNF therapy due to a lack of outcome information with these agents. The other notable factor which restricts this therapy is its high cost. Table 2 summarizes these novel biological approaches with their current status to treat AS.
 |
Table 2 New treatment approaches for AS and their current status |
IL inhibitors and monoclonal antibody therapy
IL-17 is involved in the pathogenesis of AS and patients with AS experience a high-level of IL-17.122 As a humanized monoclonal antibody, secukinumab, suppresses IL-17 and thus is a promising approach to treat AS.123 In addition, antigen-presenting cells secrete IL-23, which stimulate the Th-17 cells and are responsible for the production of IL-17.124 Recently, two placebo-controlled double-blind Phase III clinical trials MEASURE-1 and MEASURE-2 were performed. In both trials, a total of 590 AS patients received either s.c. secukinumab (150, 75 mg) or a placebo every 4 weeks, after an initiation phase of 4-weeks with an i.v. of secukinumab (at weeks 0, 2, 4 in MEASURE-1) or s.c. administration (at weeks 0, 1, 2, 3, 4 in MEASURE-2). The results at week 16 reached 61%, 60%, and 29% in the MEASURE-1 trial for 150, 75 mg secukinumab and placebo, respectively; whereas, in MEASURE-2, the outcomes were 61%, 41%, and 28%. Thus, secukinumab led to a comparable improvement in the signs and symptoms of AS and represents a major improvement for 2 years in AS patients, combined with a significant safety profile.125,126 Similarly, in another randomized double-blind Phase III clinical study, MEASURE-3 showed notable improvement after a 16-week follow-up period, which lasted for 52 weeks in AS patients as compared with the control and no major side effects were observed.127 Currently, a MEASURE-5 randomized double-blind Phase III clinical trial is ongoing, comparing the tolerability and safety profile of secukinumab in AS patients against a placebo.128 Another antibody, Brodalumab, active against IL-17R was effective for psoriatic arthritis, however, the induction of suicidal tendencies in patients stopped its development.81
IL-23 is directly associated with the development of enthesitis,129 and the IL-23/IL-17 axis is mainly involved in AS pathogenesis. In this respect, the humanized monoclonal antibody Ustekinumab targets the p40 subunit of IL-12 and IL-23. Ustekinumab is considered as the most effective treatment for psoriasis,130 however, its efficacy in AS did not show expected results. Although an open-label, single-arm proof-of-concept clinical trial (TOPAS) showed a reduction in the sign and symptoms in AS patients.131 An immunoglobulin molecule ABT-122 which targets both IL-17A and TNF-α demonstrated efficacy in Phase I and II clinical trials for RA and psoriatic arthritis132,133 and due to the involvement of IL-17 in the pathogenesis of AS, it is expected that AS patients will be performed soon. Furthermore, another dual targeting fusion protein antibody COVA-32274, thought to be a promising therapy for AS, will soon be studied.77 In AS patients, a selective inhibitor of CBP/p300 bromodomains CBP-3052 suppresses the secretion of cytokines by Th-17 cells. Sarilumab is another humanized monoclonal antibody which blocks α-receptors of IL-6 and showed a lack of efficiency for AS treatment in an ALIGN study.134
Finally, in order to investigate predictors of the selective response of AS patients against anti-TNF and IL therapies, further studies are requested to identify the first and second lines of treatment for AS.
Physical therapy, exercise and behavioral changes
Physical therapy has also proven beneficial in AS, including different exercises to maintain proper posture, stretching exercises, and deep breathing for lung expansion in order to improve joints and spinal mobility. As demonstrated, AS induces a forward curvature, therefore AS patients are guided to perform a couple of back-extension exercises as well as instructed to maintain an erect position as much as possible. In addition, AS patients are also instructed to avoid using a pillow during sleep and to try to sleep on a flat and firm mattress. As reported previously, AS could involve areas where ribs attach to the upper spine and vertebral joints, which limits breathing capacity. Therefore, AS patients are suggested to frequently expand their back as much as possible every day. Various swimming programs have also been designed for individual patients. In this respect, swimming is suggested as an exercise for AS patients because it avoids a jarring impact of the spine. AS patients have been instructed to take part in aerobic sports, while taking necessary precautions in athletics. Aerobic exercises promote the expansion of the chest, breathing muscles and opens airways of the lungs.135 AS patients are strongly discouraged to smoke cigarettes since it effects lung expansion capacity as well as lung scarring and induces breathing difficulties. Thus, AS patients with lung disease are advised to take oxygen supplements to improve breathing.67 AS patients are also suggested to modify their daily life and workplace activities such as adjusting their work chair and table at their office to maintain proper posture, for driving use wide rear-view mirrors and prism glasses to limit spinal movement. Importantly patients with AS are encouraged to eat foods rich in calcium and Omega-3, eat vegetables and fruits, eat more herbs and spices, avoid excessive amounts of sugar, fat and sodium, limit alcohol consumption and consider probiotics.136,137
Surgical intervention
Research has shown that the biomechanical changes due to AS that take place in the spine predisposes one to fractures, possibly leading to spinal deformity and spondylodiscitis. AS involves caudo-rostral progression, altering the biochemical properties of the spine and diminishing its resistance through remodeling, which is associated with ligamentary ossification, osteoporosis, vertebral joint fusion, and finally spinal deformity. Thus, the ankylosed spine tends to be fractured even after minor trauma. Furthermore, these unstable fractures require treatment in order to avoid neurological injury, deformity and disability.138–140 In a retrospective study, AS patients who underwent spinal surgeries at a tertiary university hospital in Brazil included a total of 8 patients (3 men and 5 women) with a mean age of 57 years. Six patients were found positive for acute-phase protein (75%), while 2 patients showed HLA-B27 (25%). Four patients had radiological diagnosis of spondylodiscitis (50%) and underwent spinal disc biopsy. Three patients were faced with no pain with analgesic treatment in their last follow-up. While an additional three patients had spinal fractures surgically treated (37.5%) and one patient was operated due to cervical kyphotic deformity (12.5%).141 Furthermore, a Secondary Cohort Analysis of a Nationwide, Population‐Based Health Claim Database, included 3462 AS patients and 17,310 patients without AS (Table 3). The important three variables (i.e., spine surgery, cervical surgery, and lumbar surgery) were observed at a significantly higher incidence in the AS cohort than in the comparison cohort (incidence rate ratio [IRR] 2.34 [95% CI 1.92–2.87] for any spinal surgery, IRR 2.36 [95% CI 1.55–3.59] for cervical spine surgery, and IRR 2.33 [95% CI 1.85–2.93] for lumbar spine surgery). In addition, the incidence of IRRs was the largest in the youngest age group (individuals in their 20s and 30s). The data indicated that the AS patients, especially in their 20s and 30s, had a significantly higher risk of spinal surgery, cervical spine surgery, and lumbar spine surgery, as compared the patients without AS.142 However, the surgical approach for AS patients shifted for cervical fractures with anterior-posterior surgery transitioning from the most popular to the least popular approach from 2003 to 2014. For thoracolumbar fractures, posterior surgery remained the preferred approach. In addition, patients who undergo anterior-posterior surgery are at a high risk of pulmonary complication rates in both thoracolumbar and cervical fractures.143 These data suggest that surgical intervention could be performed in the most severe cases of AS and the outcome could be promising through accurate and specific surgeries.
 |
Table 3 Incidence rates and incidence rate ratios (IRR) of spinal surgery for the AS cohort and comparison cohort, with and without stratification by sex (n=20,772) |
Nanotechnology-based treatment approaches for AS
Conventional medication can provide fast relief to patients clinically, but, when thinking from a long-term safety prospective, conventional treatment approaches are not suitable because this requires frequent medication which can increase financial burden and due to frequent medication, decrease a patient’s quality of life. Moreover, these approaches have high degradation and clearance from the body due to the high and frequent dosing required further increasing many complications and toxicity.144 Recently, various nanotechnology-based drug delivery approaches have been applied to combat these problems and deliver drugs to treat chronic inflammatory diseases. These nanotechnology-based drug delivery systems have shown better therapeutic compliance in comparison to conventional formulations by prolonging drug retention time at the site of action.145 Unfortunately, currently, no nanotechnology-based formulations have been reported for AS but there are many formulations which have been studied for other inflammatory diseases such as osteoarthritis, RA, and backache that can be used for AS.146 The conventional treatment approaches for these diseases are the same as AS. As AS is also classified as a chronic inflammatory disease, we can hypothesize that nanotechnology-based formulations prepared for the treatment of other inflammatory diseases can also work for AS.
Recently, various nanotechnology-based drug delivery systems have been established to deliver drugs in a safe mode to treat various diseases including cancer, fibrosis, lung diseases, diabetes and chronic inflammation.147–153 Well-established nano preparations include liposomes, polymeric nanoparticles, and hydrogels.154
Liposomes
Liposomes are round vesicles made of phospholipids and have the ability to hold both hydrophilic and hydrophobic drugs. Generally, hydrophilic drugs are encapsulated in an internal aqueous chamber and hydrophobic drugs are entrapped into a surface bilayer structure.155 Liposomes are generally prepared from biodegradable nontoxic lipids and have good retention ability at the site of action. Some liposome-based NSAIDs preparations reported for the successful treatment of RA and osteoarthritis are indomethacin, diclofenac and ibuprofen. These liposomal nano-preparation increase the half-life of the drug in comparison to conventional preparations. For example, liposomal iohexol declined biexponentially with an elimination half-life of 134 hrs as compared with free iohexol.156 Other work reported by Inbar Elron-Gross improved the retention time of diclofenac and they used collagomers to prepare collagen-lipid conjugates to encapsulate diclofenac for slow release in the synovial area after injection.157 In their other work, they used the corticosteroid dexamethasone for encapsulation along with diclofenac to reduce the activity of COX for the treatment of osteoarthritis. Similarly, to counteract the GI side effects caused by indomethacin during treatment of RA, lipid-based microspheres were prepared.158
Gottschalk et al prepared MTX encapsulated cationic liposomes and observed its anti-inflammatory and anti-angiogenic efficacy. In an antigen-induced arthritis model, the cationic liposome demonstrated a significant and superior reduction in leukocyte and platelet interaction, functional capillary density and knee joint diameters.159 Recently, MTX-DG liposomes (methotrexate-diglyceride ester lipophilic prodrug) were synthesized for antitumor efficacy in T-cell leukemic lymphoma. The results indicated that the mice plasma concentration-time and AUC of MTX after i.v. administration of MTX-DG liposomes increased up to 2.5-fold as compared to the intact MTX. Furthermore, the MTX-DG liposomes retarded T-cell lymphoma and did not induce significant toxicity as compared with free MTX.160 Previously, a fluoroquinolone-liposomal formulation was extensively used for antibacterial effects and showed promising antibacterial control and successfully treated bacterial infections.161–164
TNF-α-mediated liposomal formulations showed exceptional therapeutic potential to treat numerous tumors including long-circulating stealth liposomes, cationic liposomes, surface modified liposomes or a combination of liposomes (such as TNF-α + anticancer agent). The stealth liposome encapsulating a low dose of TNF-α augmented the antitumor effect of doxorubicin in soft tissue sarcoma-bearing rats.165 On the other hand, the TNF-α liposome enhanced the radiation effects against a human colon cancer xenograft, 166 while cytokine GM-CSF and TNF-alpha encapsulated liposomes showed improved pharmacokinetics and biological activity at a minimal toxicity in mice.167 Recently, Rakeshchandra et al, synthesized a peptide (ART-1) coated IL-27 encapsulated liposome (ART-1-IL-27). The ART-1-IL-27 liposomes displayed significant binding to the endothelial cells and better homing to arthritic joints as compared to the control liposomes, in vivo. Moreover, following i.v. administration in arthritic rats at an equivalent dose of IL-27, the ART-1-IL-27 liposomes effectively suppressed disease progression and significantly improved the therapeutic index of IL-27, as compared to the control-IL-27 liposomes without the ART-1 peptide coating and free IL-27, as shown in Figure 4.169
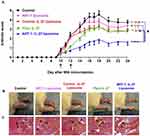 |
Figure 4 Arthritis treatment with ART-1-IL-27 liposomes. A Lewis rats arthritis model was used to i.v. administer free IL-27, ART-1-IL-27 liposomes, control-IL-27 liposomes, or ART-1 liposomes and an untreated PBS control. (A) The differences in arthritic scores of the 4 pairs of groups were significant (*P<0.01): ART-1-IL-27 liposomes v/s control rats, ART-1-IL-27 liposomes v/s ART-1 liposomes without IL-27, ART-1-IL-27 liposomes v/s control-IL-27 liposomes, and ART-1-IL-27 liposomes v/s free IL-27. (B) Photographs of the rat’s hind paw at the end of the treatment and (C) H&E-staining of hind paw sections, the histopathological features represented as: B: bone, C: cartilage, JS: joint space, P: pannus. Reprinted with permission from Elsevier, JControlled Release, Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis, Meka RR, Venkatesha SH, Moudgil KD. Copyright 2018.168 |
Finally, these observations provide a proof-of-concept for the use of nanoliposomes to successfully deliver numerous NSAIDs, MTX, TNF-α, and IL-27 to target and treat various inflammation or infection conditions. Therefore, the presently described targeted liposomal platform could be extremely promising for use in AS patients and should be developed as soon as possible.
Polymeric nanoparticles
Polymeric nanoparticles are generally prepared from different polymers such as chitosan, poly-lactic acid (PLA), poly-lactic glycolic acid (PLGA), poly-δ-valerolactona (PV), etc.169 Tuncay et al prepared diclofenac sodium injection incorporated PLGA microspheres for the treatment of chronic inflammatory diseases.170 Diclofenac is a very common NSAID used in the treatment of various inflammatory conditions in different forms such as oral, rectal or intramuscular preparation. The clinical efficacy of diclofenac has been compared with various other NSAIDs (such as naproxen, ibuprofen, sulindac, and diflunisal) for the treatment of osteoarthritis and the obtained data suggest that it is well tolerable by a patient but the dosing frequency is higher in comparison to other NSAIDs. So, to reduce the dosing frequency, a slow-release PLGA microparticle was prepared for oral administration for the treatment of osteoarthritis.171 Similarly, Saravanan et al have prepared diclofenac sodium gelatin magnetic microspheres to target joint inflammation through intra-articular administration. The prepared microparticles show better therapeutic efficacy in comparison to conventional diclofenac sodium injection therapy, which could be even better through the use of nanoparticles.172 Zhao et al fabricated MTX loaded folate receptor-targeted pH-responsive polymeric nanoparticles (FA-PPLNPs/Mtx) to treat RA. The FA-PPLNPs/Mtx exhibited improved cellular uptake and enhanced cytotoxicity toward activated macrophages. Furthermore, in vivo experiments using an adjuvant-induced arthritis rat model (AIA) further confirmed FA-PPLNPs/Mtx effectiveness as shown in Figure 5.
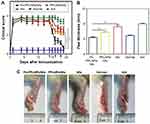 |
Figure 5 Anti-arthritic efficacy of FA-PPLNPs/Mtx, PPLNPs/Mtx and free Mtx in AIA rats. (A) Scores of RA in days after administration of FA-PPLNPs/Mtx, PPLNPs/Mtx, free Mtx and saline. **P<0.01. (B) Paw thickness after all treatments. (C) AIA rat paws from the different AIA groups. Copyright © 2017. Dove Medical Press. Reprinted from Zhao J, Zhao M, Yu C, et al. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int J Nanomedicine. 2017;12:6735-6746.173 Abbreviations: RA, rheumatoid arthritis; MTX, methotrexate. |
These results suggested that the multifunctional folate receptor-targeting and pH-responsive nanocarriers are promising for the treatment of RA.173 On the other hand, Zhang et al encapsulated ursolic acid (UA) into polymeric nanoparticles (UA-NPs) by using amphiphilic-methoxy poly (ethylene glycol) polycaprolactone (mPEG–PCL) block-copolymers. The UA-NPs successfully delivered UA in SGC7901 cells and induced cell death by the activation of caspase-3 activity. The mechanistic study showed that UA-NPs strongly inhibited the COX-2 receptors and activated the caspase cascade, therefore, inducing tumor cell death, as shown in Figure 6.174
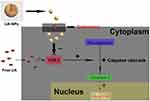 |
Figure 6 A schematic representation of UA-NPs induced cell death by inhibiting the COX-2 and activating the caspase-3 cascade. Reprinted with permission from Elsevier: Int J Pharm, Delivery of UA in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2), Zhang H, Li X, Ding J, et al. Copyright 2013.174Abbreviations: UA, ursolic acid; COX-2, cyclooxygenase 2. |
On the other hand, in term of biological delivery via polymeric nanoparticles, Shoaib Iqbal et al orally delivered TNF-α by poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) (PEG5K-b-PLGA10K) NPs by a double emulsion method aminated nanoparticles (ANPs), as shown in Figure 7. The in vivo experiments in a murine acute ulcerative colitis model showed that ANPs were mainly centered in inflamed colons and significantly decreased TNF-α secretion as well as mRNA expression, while maintaining colon histology.175
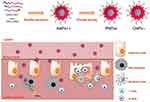 |
Figure 7 Schematic illustration of the tenable surface charge nanoparticles for anti-TNF-α siRNA oral delivery for treating ulcerative colitis. Reprinted with permission from Springer Nature: Nano Res, Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis, Iqbal S, Du X, Wang J, Li H, Yuan Y, Wang J. Copyright 2017.175Abbreviation: TNF, tumor necrosis factor. |
In another report, Stephanie et al successfully delivered Leukemia Inhibitory Factor (LIF) by fabricating poly(ethylene glycol)–poly(lactic acid) (PEG-PLA) polymer backbone polymeric nanoparticles (NanoLIF) and modified their surfaces with a CD-11b antibody (CD-11b-NanoLIF) to target peripheral-macrophages. The results indicated that the CD11-b-NanoLIF successfully targeted the activated peripheral-macrophages and significantly decreased inflammation by inhibiting M1-cell growth over 72 hrs as compared to the free LIF.176 In conclusion, polymeric nanoparticles either surface modified or naked could be an important strategy to treat AS and localized inflammatory disorders.
Hydrogels
Hydrogels are three-dimensional, hydrophilic, polymeric networks composed of homopolymers or copolymers having the capacity to hold a high amount of water or biological fluids. After crosslinking, they form stable vehicles which cannot be soluble in water but can swell in a large volume in the presence of water.177 This hydrogel technique was used to prepare 50% crosslinked Hyal hydrogels to overcome the problem of rapid clearance of polysaccharide hyaluronic acid for the treatment of osteoarthritis.178
Hydrogels are novel drug delivery systems and play a pivotal role by tackling the problems associated with conventional dosage forms including stability, drug loading, in vitro release kinetics and in vivo pharmacokinetics. In recent years, numerous hydrogel formulations have been employed locally, orally and systemically to treat various types of inflammation. In this respect, Xiong-Bin et al prepared a microemulsion-based hydrogel (MBH) of 3,5,4′-trimethoxy-trans-stilbene (BTM) for a topical drug delivery system to treat osteoarthritis. In papain induced OA rabbit models, the results indicated that the topical administration of BTM-MBH showed remarkable anti-OA effects, with decreased levels of pro-inflammatory cytokines. Such results indicated that the BTM-MBH hydrogel formulation could be a promising strategy for the treatment of OA, as shown in Figure 8.179 In another report, Ahmad et al fabricated chitosan-based thermosensitive hydrogels for the sustained delivery of loxoprofen. The hydrogel formulation was stable in the rat after i.v. administration and in vivo experiments showed loxoprofen release for up to 144 hrs.180
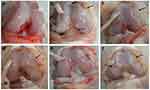 |
Figure 8 Macroscopic images of the right knee surface of rabbits on day 28. Where a: normal, b: OA, c: OA + 1% Diclofenac-gel, d: OA + 0.5% BTM-MBH, e: OA + 1% BTM-MBH, and f: OA + 1% BTM-EG. The black arrows indicate the damaged areas. Reprintedwith permission from Springer Nature: Drug Deliv Transl res, Topical delivery of 3,5,4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model, Hu XB, Kang RR, Tang TT, et al, Copyright 2019.179 |
Furthermore, the hydrogel-based drug delivery system was successfully employed to inhibit TNF-α secretion. In this respect, Lei et al produced a transplantable cationic-hydrogel to deliver antisense oligodeoxynucleotides (ASOs) to target mRNA of TNF-α. The in vivo study demonstrated that the ASOs specifically targeted the spleen at up to a 50-fold higher concentration as compared to the naked ASO. Moreover, this was demonstrated for three different types of animal models including adjuvant-induced arthritis (AA), carrageen/lipopolysaccharide-induced arthritis and collagen-induced arthritis models. Surprisingly, the ASOs significantly not only decreased inflammatory cytokines but also decreased joint swelling and tissue damage,181 as shown in Figure 9.
 |
Figure 9 Therapeutic effects of ASO delivered by c-agarose gel on the development of AA. Row A are photographs of the inflamed feet of AA. Row B represents the X-ray examination of the inflamed feet. Row C shows the sections of inflamed feet tissue. All the results were taken on the 15th day after ASO administration and n=10, while, the clinical indexes are summarized in the 7th column. *P<0.05 v/s AA animals without the treatment of ASO. Reprinted with permission from Elsevier: Biomaterials, Spleen-specific suppression of TNF-α by cationic hydrogel-delivered antisense nucleotides for the prevention of arthritis in animal models, Dong L, Xia S, Chen H, Chen J, Zhang J, Copyright 2009.181 |
In another report, Marie-Claude et al developed a hydrophobic three-dimensional porous scaffold-based drug delivery system to deliver an anti-TNF-α antibody. Their major finding was no deleterious effect of the drug delivery system on antibody affinity and anti-TNF-α release which was much higher after the first minutes in the aqueous medium followed by a sustained release of anti-TNF-α antibody up to the next 30 days. However, the histopathological examination showed a fibrous pseudo-capsule and chronic inflammation but without granuloma formation around the implants.182
In conclusion, all these findings open new possibilities for the delivery of biological agents to treat AS using nanomedicine.
To date, however, there have been no specific nanotechnology-based formulation reported but there are several nanotechnology-based formulations that have been reported for the treatment of osteoarthritis and RA which are a similar type of chronic joint inflammatory disease, like AS. Some of the developed nanotechnology-based formulations for osteoarthritis and RA are listed in Table 4 which should be immediately tested for AS.
 |
Table 4 Nanotechnology-based formulations for osteoarthritis and rheumatoid arthritis |
Conclusion
In order to understand the pathogenesis of AS, various studies have been carried out over the most recent 3 years. These data lay the foundation to design new therapeutic strategies to treat AS and consequently improve the quality of life of patients with AS. The more we know about AS, the more we understand its complexity. AS is a kind of autoimmune disease, which could lead to spinal pain, spinal deformity and could induce extra-articular and systemic abnormalities. Investigations into AS include testing of inflammatory biomarkers and HLA-B27. Notably, in the early stages of AS disease, radiographic investigations could be normal, therefore, MRI scans should be performed to reach a definitive conclusion. AS treatment should include an anti-inflammatory agent as the first line of defense and in advanced cases, biological therapy could be more promising. Finally, therapeutic options that target specific IL must be developed and more comprehensive clinical studies should be performed to specifically identify key factors and develop therapeutic regimens accordingly. As described here, nanotechnology plays a central role with much promise for improved AS treatment.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant no. 81472071 and Grant no. 81301537), Shanghai Changzheng Hospital Foundation (Grant no. 2017CZQN05), Youth Medical Talent Project of Jiangsu (QNRC2016214), and Key Technologies of Prevention and the Control for Major and Infectious Diseases (No. GWZX201604).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shaikh SA. Ankylosing spondylitis: recent breakthroughs in diagnosis and treatment. J Can Chiropr Assoc. 2007;51(4):249–260.
2. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–1390. doi:10.1016/S0140-6736(07)60635-7
3. Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2.
4. Gran JT, Husby G, Hordvik M. Prevalence of ankylosing spondylitis in males and females in a young middle-aged population of Tromsø, northern Norway. Ann Rheum Dis. 1985;44(6):359–367. doi:10.1136/ard.44.6.359
5. Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23(2):61–66. doi:10.1007/s00296-002-0237-4
6. Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57(1):44–51. doi:10.1016/j.molimm.2013.07.013
7. Keidel S, Chen L, Pointon J, Wordsworth P. ERAP1 and ankylosing spondylitis. Curr Opin Immunol. 2013;25(1):97–102. doi:10.1016/j.coi.2012.11.002
8. Rashid T, Ebringer A. Ankylosing spondylitis is linked to Klebsiella – the evidence. Clin Rheumatol. 2007;26(6):858–864. doi:10.1007/s10067-006-0488-7
9. Li Y, Wang P, Xie Z, et al. Whole genome expression profiling and signal pathway screening of MSCs in ankylosing spondylitis. Stem Cells Int. 2014;2014.
10. Sheehan NJ. The ramifications of HLA-B27. J R Soc Med. 2004;97(1):10–14. doi:10.1258/jrsm.97.1.10
11. Benjamin R, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142.
12. Ebringer R, Cooke D, Cawdell DR, Cowling P, Ebringer A. Ankylosing Spondylitis: Klebsiella and HL-A B27. Rheumatol Rehabil. 1977;16(3):190–196.
13. Eastmond C, Willshaw HE, Burgess SE, Shinebaum R, Cooke EM, Wright V. Frequency of faecal Klebsiella aerogenes in patients with ankylosing spondylitis and controls with respect to individual features of the disease. Ann Rheum Dis. 1980;39(2):118–123. doi:10.1136/ard.39.2.118
14. Hunter T, Harding GKM, Kaprove RE, Schroeder M-L. Fecal carriage of various Klebsiella and Enterobacter species in patients with active ankylosing spondylitis. Arthritis Rheum. 1981;24(1):106–108. doi:10.1002/(ISSN)1529-0131
15. Morse HG, Morse HG, Rate RG, Bonnell MD. Increased recovery of Klebsiella from the gastrointestinal tract of Reiter’s syndrome and ankylosing spondylitis patients. Rheumatology. 1983;XXII(suppl_2):85–90. doi:10.1093/rheumatology/XXII.suppl_2.85
16. Cuvelier C, Veys EM, Cuvelier C, de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Rheumatology. 1988;XXVII(suppl_2):95–105. doi:10.1093/rheumatology/XXVII.suppl_2.95
17. Martínez-González O, Cantero-Hinojosa J, Paule-Sastre P, Gómez-Magán JC, Salvatierra-Ríos D.> Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Rheumatology. 1994;33(7):644–647. doi:10.1093/rheumatology/33.7.644
18. Tam L-S, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nature Rev Rheumatol. 2010;6:399. doi:10.1038/nrrheum.2010.79
19. McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352(9134):1137–1140. doi:10.1016/S0140-6736(97)12004-9
20. Martin TM, Smith JR, Rosenbaum JT. Anterior uveitis: current concepts of pathogenesis and interactions with the spondyloarthropathies. Curr Opin Rheumatol. 2002;14(4):337–341.
21. van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo‐controlled trial (ASSERT). Arthritis Rheum. 2005;52(2):582–591. doi:10.1002/(ISSN)1529-0131
22. Fleischmann RM, Iqbal I, Stern RL. Considerations with the use of biological therapy in the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2004;3(5):391–403. doi:10.1517/14740338.3.5.391
23. Merkesdal S, Ruof J, Mittendorf T, Zeidler H. Cost-effectiveness of TNF-α-blocking agents in the treatment of rheumatoid arthritis. Expert Opin Pharmacother. 2004;5(9):1881–1886. doi:10.1517/14656566.5.9.1881
24. Reveille JD. 57 – Spondyloarthritis, in Clinical Immunology.
25. Skalska U, Kozakiewicz A, Maśliński W, Jurkowska M. HLA-B27 detection – comparison of genetic sequence-based method and flow cytometry assay. Reumatologia. 2015;53(2):74–78. doi:10.5114/reum.2015.51506
26. Khan MA. Polymorphism of HLA-B27: 105 subtypes currently known. Curr Rheumatol Rep. 2013;15(10):362. doi:10.1007/s11926-013-0362-y
27. Rysnik O, McHugh K, van Duivenvoorde L, et al. Non-conventional forms of HLA-B27 are expressed in spondyloarthritis joints and gut tissue. J Autoimmun. 2016;70:12–21. doi:10.1016/j.jaut.2016.03.009
28. Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010;233(1):181–202. doi:10.1111/j.0105-2896.2009.00865.x
29. Guiliano DB, Fussell H, Lenart I, et al. Endoplasmic reticulum degradation-enhancing α-mannosidase-like protein 1 targets misfolded HLA–B27 dimers for endoplasmic reticulum-associated degradation. Arthritis Rheumatol. 2014;66(11):2976–2988. doi:10.1002/art.38809
30. Bowness P, Ridley A, Shaw J, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186(4):2672–2680. doi:10.4049/jimmunol.1002653
31. Ridley A, Hatano H, Wong-Baeza I, et al. Activation-induced killer cell immunoglobulin-like receptor 3DL2 binding to HLA–B27 licenses pathogenic T cell differentiation in spondyloarthritis. Arthritis Rheumatol. 2016;68(4):901–914. doi:10.1002/art.39515
32. The Australo-Anglo-American Spondyloarthritis, C., et al.Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci.Nat Genet.2010;42(p):123. doi:10.1038/ng.513
33. Saveanu L, Carroll O, Lindo V, et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6:689. doi:10.1038/ni1208
34. Chang S-C, Momburg F, Bhutani N, Goldberg AL. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci U S A. 2005;102(47):17107–17112. doi:10.1073/pnas.0500721102
35. Chen L, Ridley A, Hammitzsch A, et al. Silencing or inhibition of endoplasmic reticulum aminopeptidase 1 (ERAP1) suppresses free heavy chain expression and Th17 responses in ankylosing spondylitis. Ann Rheum Dis. 2016;75(5):916–923. doi:10.1136/annrheumdis-2014-206996
36. García-Medel N, Sanz-Bravo A, Van Nguyen D, et al. Functional interaction of the ankylosing spondylitis-associated endoplasmic reticulum aminopeptidase 1 polymorphism and HLA-B27 in vivo. Mol Cell Proteomics. 2012;11(11):1416–1429. doi:10.1074/mcp.M112.019588
37. Wendling D, Cedoz J-P, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–305. doi:10.1016/j.jbspin.2006.11.005
38. Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30(2):269–273. doi:10.1007/s10067-010-1647-4
39. Gracey E, Yao Y, Green B, et al. Sexual dimorphism in the Th17 signature of ankylosing spondylitis. Arthritis Rheumatol. 2016;68(3):679–689. doi:10.1002/art.39464
40. Turvey SE, Broide DH. Innate immunity. J Allergy Clin Immunol. 2010;125(2, Supplement 2)):S24–S32. doi:10.1016/j.jaci.2009.07.016
41. Robinson PC, Brown MA. Genetics of ankylosing spondylitis. Mol Immunol. 2014;57(1):2–11. doi:10.1016/j.molimm.2013.06.013
42. Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family – balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37. doi:10.1016/j.cyto.2015.06.017
43. Loo FAJVD, Joosten LAB, Van Lent PLEM, Arntz OJ, Van Den Berg WB. Role of interleukin-1, tumor necrosis factor α, and interleukin-6 in cartilage proteoglycan metabolism and destruction effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995;38(2):164–172. doi:10.1002/(ISSN)1529-0131
44. DANIS VA, MILLINGTON M, HYLAND VJ, GRENNAN D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clinical & Experimental Immunology. 1995;99(2):303–310. doi:10.1111/(ISSN)1365-2249
45. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10(1):411–452. doi:10.1146/annurev.iy.10.040192.002211
46. Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38(4):499–505. doi:10.1002/(ISSN)1529-0131
47. Duivenvoorde LMV, Ambarus CA, Masdar H, et al. A2.15 relative overexpression of transmembrane versus soluble TNF in human and experimental spondyloarthritis. Ann Rheum Dis. 2013;72(Suppl 1):A9–A10. doi:10.1136/annrheumdis-2013-203215.15
48. Campbell RD, Trowsdale J. Map of the human MHC. Immunol Today. 1993;14(7):349–352. doi:10.1016/0167-5699(93)90234-C
49. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997;94(7):3195–3199. doi:10.1073/pnas.94.7.3195
50. El-Tahan RR, Ghoneim AM, El-Mashad N. TNF-α gene polymorphisms and expression. SpringerPlus. 2016;5(1):1508. doi:10.1186/s40064-016-3197-y
51. Mahmoudi M, Jamshidi AR, Amirzargar AA, et al. Association between endoplasmic reticulum aminopeptidase-1 (ERAP-1) and susceptibility to ankylosing spondylitis in Iran. Iran J Allergy Asthma Immunol. 2012;11(4);294–300.
52. Bettencourt BF, Rocha FL, Alves H, et al. Protective effect of an ERAP1 haplotype in ankylosing spondylitis: investigating non-MHC genes in HLA-B27-positive individuals. Rheumatology. 2013;52(12):2168–2176. doi:10.1093/rheumatology/ket269
53. Haroon N, Tsui FWL, Chiu B, Tsui HW, Inman RD. Serum cytokine receptors in ankylosing spondylitis: relationship to inflammatory markers and endoplasmic reticulum aminopeptidase polymorphisms. J Rheumatol. 2010;37(9):1907–1910. doi:10.3899/jrheum.100019
54. Hacquard‐Bouder C, Falgarone G, Bosquet A, et al. Defective costimulatory function is a striking feature of antigen‐presenting cells in an HLA-B27-transgenic rat model of spondylarthropathy. Arthritis Rheum. 2004;50(5):1624–1635. doi:10.1002/(ISSN)1529-0131
55. Hacquard‐Bouder C, Chimenti M-S, Giquel B, et al. Alteration of antigen‐independent immunologic synapse formation between dendritic cells from HLA-B27-transgenic rats and CD4+ T cells: selective impairment of costimulatory molecule engagement by mature HLA–B27. Arthritis Rheum. 2007;56(5):1478–1489. doi:10.1002/(ISSN)1529-0131
56. Glatigny S, Fert I, Blaton MA, et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis‐prone HLA-B27-transgenic rats. Arthritis Rheum. 2012;64(1):110–120. doi:10.1002/art.33321
57. Pan F, Barbi J, Pardoll DM. Hypoxia-inducible factor 1: a link between metabolism and T cell differentiation and a potential therapeutic target. Oncoimmunology. 2012;1(4):510–515. doi:10.4161/onci.19457
58. Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106. doi:10.1038/nature06881
59. Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nature Rev Immunol. 2009;9:811. doi:10.1038/nri2654
60. El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi:10.1038/ni.2031
61. Rudwaleit M, Höhler T. Cytokine gene polymorphisms relevant for the spondyloarthropathies. Curr Opin Rheumatol. 2001;13(4):250–254.
62. Benjamin M, McGONAGLE D. Basic concepts of enthesis biology and immunology. J Rheumatol Suppl. 2009;83:12–13. doi:10.3899/jrheum.090211
63. Benjamin M, Toumi H, Suzuki D, Redman S, Emery P, McGonagle D. Microdamage and altered vascularity at the enthesis–bone interface provides an anatomic explanation for bone involvement in the HLA-B27-associated spondylarthritides and allied disorders. Arthritis Rheum. 2007;56(1):224–233. doi:10.1002/(ISSN)1529-0131
64. Costello M-E, Robinson PC, Benham H, Brown MA. The intestinal microbiome in human disease and how it relates to arthritis and spondyloarthritis. Best Pract Res Clin Rheumatol. 2015;29(2):202–212. doi:10.1016/j.berh.2015.08.001
65. Khan MA, van Der Linden SM, Kushner I, Valkenburg HA, Cats A. Spondylitic disease without radiologic evidence of sacroiliitis in relatives of HLA‐B27 positive ankylosing spondylitis patients. Arthritis Rheum. 1985;28(1):40–43. doi:10.1002/(ISSN)1529-0131
66. McVeigh CM, Cairns AP. Diagnosis and management of ankylosing spondylitis. BMJ. 2006;333(7568):581–585. doi:10.1136/bmj.38954.689583.DE
67. Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis. 2002;61(suppl 3):iii8–iii18. doi:10.1136/ard.61.suppl_3.iii8
68. Spoorenberg A, Dougados M, Mielants H. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol. 1999;26(4):980–984.
69. Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol. 1999;26(4):966–970.
70. Underwood M, Dawes P. Inflammatory back pain in primary care. Rheumatology. 1995;34(11):1074–1077. doi:10.1093/rheumatology/34.11.1074
71. Dougados M, Linden SVD, Juhlin R, et al. The European spondylarthropathy study group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10):1218–1227. doi:10.1002/(ISSN)1529-0131
72. Wong RS. Disease-modifying effects of long-term and continuous use of nonsteroidal anti-inflammatory drugs (NSAIDs) in Spondyloarthritis. Adv Pharmacol Sci. 2019;2019.
73. Nunes GPS, Cunha PDS, Bosco DPD, Ribeiro SLE. Challenging management of hepatitis B infection in ankylosing spondylitis patients in an endemic area during immunosuppressive therapy. Rev Soc Bras Med Trop. 2019;52.
74. Kroon FP, van der Burg LRA, Ramiro S, et al. Nonsteroidal antiinflammatory drugs for axial spondyloarthritis: a cochrane review. J Rheumatol. 2016;43(3):607–617. doi:10.3899/jrheum.150721
75. Evans DM, Spencer CCA, Pointon JJ, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43(8):761. doi:10.1038/ng.873
76. Dougados M, Béhier J-M, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2–specific inhibitor, in the treatment of ankylosing spondylitis: a six‐week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44(1):180–185. doi:10.1002/(ISSN)1529-0131
77. Coxib and traditional NSAID Trialists (CNT) Collaboration, Bhala N, et al. Vascular and Upper Gastrointestinal Effects of Non-steroidal Anti-inflammatory Drugs: Meta-analyses of Individual Participant Data from Randomised Trials. Lancet. 2013 Aug 31;382(9894):769–779. doi:10.1016/S0140-6736(13)60900-9.
78. Dougados M, Gueguen A, Nakache JP, et al. Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford). 1999;38(3):235–244. doi:10.1093/rheumatology/38.3.235
79. Wanders A, Heijde DVD, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756–1765. doi:10.1002/(ISSN)1529-0131
80. Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016;75(8):1438–1443. doi:10.1136/annrheumdis-2015-207897
81. Moon K-H, Kim Y-T. Medical treatment of ankylosing spondylitis. Hip Pelvis. 2014;26(3):129–135. doi:10.5371/hp.2014.26.3.129
82. Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med. 2015;163(6):409–416. doi:10.7326/M14-2470
83. Rademacher J, Poddubnyy D. Emerging drugs for the treatment of axial spondyloarthritis. Expert Opin Emerg Drugs. 2018;23(1):83–96. doi:10.1080/14728214.2018.1445719
84. Fattahi MJ, Jamshidi AR, Mahmoudi M, et al. Evaluation of the efficacy and safety of β-d-mannuronic acid in patients with ankylosing spondylitis: a 12-week randomized, placebo-controlled, phase I/II clinical trial. Int Immunopharmacol. 2018;54:112–117. doi:10.1016/j.intimp.2017.11.003
85. Emkey R, Rosenthal N, Wu S-C, Jordan D, Kamin M. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double-blind, placebo-controlled trial. J Rheumatol. 2004;31(1):150–156.
86. Peloso PM, Fortin L, Beaulieu A, Kamin M, Rosenthal N. Analgesic efficacy and safety of tramadol/acetaminophen combination tablets (Ultracet) in treatment of chronic low back pain: a multicenter, outpatient, randomized, double blind, placebo controlled trial. J Rheumatol. 2004;31(12):2454–2463.
87. Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114(7):537–545. doi:10.1016/s0002-9343(03)00116-5
88. Chang J-K, Yu C-T, Lee M-Y, et al. Tramadol/acetaminophen combination as add-on therapy in the treatment of patients with ankylosing spondylitis. Clin Rheumatol. 2013;32(3):341–347. doi:10.1007/s10067-012-2125-y
89. Farrouq Mahmood PH. Ankylosing spondylitis: a review. Eur Med J. 2017;2(4):134–139.
90. Ryall NH, Helliwell P. A critical review of ankylosing spondylitis. Crit Rev™ Phys Rehabil Med. 1998;10:3.
91. sailaja AK. An overall review on ankylosing spondylitis. Int J Current Pharm Sci. 2014;1(1):1–5.
92. Xie J, Fan Z, Li Y, et al. Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy. Int J Nanomedicine. 2018;13:1381–1398. doi:10.2147/IJN.S152312
93. Nosrati H, Salehiabar M, Davaran S, Danafar H, Manjili HK. Methotrexate-conjugated L-lysine coated iron oxide magnetic nanoparticles for inhibition of MCF-7 breast cancer cells. Drug Dev Ind Pharm. 2018;44(6):886–894. doi:10.1080/03639045.2017.1417422
94. Abdelrady H, Hathout RM, Osman R, Saleem I, Mortada ND. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. EurJ Pharm Sci. 2019;133:115–126. doi:10.1016/j.ejps.2019.03.016
95. Haibel H, Brandt HC, Song IH, et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis. 2007;66(3):419–421. doi:10.1136/ard.2006.054098
96. Chen J, Lin S, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2014;11.
97. Xue H-X, Fu W-Y, Cui H-D, Yang -L-L, Zhang N, Zhao L-J. High-dose thalidomide increases the risk of peripheral neuropathy in the treatment of ankylosing spondylitis. Neural Regener Res. 2015;10(5):814–818. doi:10.4103/1673-5374.156988
98. Wei J-C-C, Chan TW, Lin H-S, Huang F, Chou C-T. Thalidomide for severe refractory ankylosing spondylitis: a 6-month open-label trial. J Rheumatol. 2003;30(12):2627–2631.
99. Wilsdon TD, Whittle SL, Thynne TRJ, Mangoni AA. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1.
100. Huang F, Gu J, Zhao W, Zhu J, Zhang J, Yu DTY. One-year open-label trial of thalidomide in ankylosing spondylitis. Arthritis Care Res (Hoboken). 2002;47(3):249–254. doi:10.1002/(ISSN)1529-0131
101. Nabeel AI, Moawed FSM, Hassan H. Immunomodulatory effect of new quinolone derivative against cisplatin/gamma radiation-induced renal and brain toxicity in mice. J Photochem Photobiol B. 2018;184:54–60. doi:10.1016/j.jphotobiol.2018.05.013
102. Öğrendik M. Treatment of Ankylosing Spondylitis with Moxifloxacin. South Med Assoc. 2007;100(4):366–370. doi:10.1097/SMJ.0b013e31802fa2a8
103. Öğrendik M. Treatment of ankylosing spondylitis with co-amoxiclav. Clin Exp Pharmacol Physiol. 2018;45(7):742–744. doi:10.1111/1440-1681.12952
104. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26):2563–2574. doi:10.1056/NEJMra1406182
105. Gratacós J, Collado A, Filella X, et al.. Serum cytokines (IL-6, TNF-α, IL-1β and IFN-γ) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Rheumatology. 1994;33(10):927–931. doi:10.1093/rheumatology/33.10.927
106. Baraliakos X, et al. Persistent clinical efficacy and excellent safety over 5 years of treatment with the anti-TNF alpha agent infliximab in patients with ankylosing spondylitis. In: Arthritis and Rheumatism. Hoboken, NJ: Wiley-Liss Div John Wiley & Sons Inc; 2006. doi:10.1136/ard.2007.075879
107. Braun J, Deodhar A, Dijkmans B, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two‐year period. Arthritis Care Res. 2008;59(9):1270–1278. doi:10.1002/art.v59:9
108. Van der Heijde D, et al. Long-term adalimumab treatment reduces signs and symptoms in ankylosing spondylitis (AS) patients – results from the ATLAS trial. In: Leiden University Medical Center, editor. Annals of the Rheumatic Diseases. London, UK: BMJ Publishing Group British Med Assoc House; 2006.
109. Braun J, Landewé R, Hermann K-GA, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum. 2006;54(5):1646–1652. doi:10.1002/(ISSN)1529-0131
110. Baraliakos X, Listing J, Rudwaleit M, Brandt J, Sieper J, Braun J. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor α antibody infliximab. Ann Rheum Dis. 2005;64(10):1462–1466. doi:10.1136/ard.2004.033472
111. Haibel H, Baraliakos X, Listing J, et al. Persistent reduction of spinal inflammation as assessed by magnetic resonance imaging in patients with ankylosing spondylitis after 2 yrs of treatment with the anti-tumour necrosis factor agent infliximab. Rheumatology. 2005;44(12):1525–1530. doi:10.1093/rheumatology/kei046
112. Davis JC
113. Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006;20(3):451–471. doi:10.1016/j.berh.2006.03.010
114. Voulgari PV, Venetsanopoulou AI, Epagelis EK, Alamanos Y, Takalou I, Drosos AA. Infliximab in refractory psoriatic arthritis with severe psoriasis: a 2-year experience. Ann Rheum Dis. 2007;66(2):270–271. doi:10.1136/ard.2006.058735
115. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–333. doi:10.1053/j.gastro.2005.11.030
116. Markham A, Lamb HM. Infliximab. Drugs. 2000;59(6):1341–1359. doi:10.2165/00003495-200059060-00010
117. Cook DA. J.B.D., D.C. Gallagher. Etanercept Rheumatoid Arthritis Drugs R & D. 1999;1(1):75–77.
118. Toussirot É, Wendling D. Recent progress in ankylosing spondylitis treatment. Expert Opin Pharmacother. 2003;4(1):1–12. doi:10.1517/14656566.4.1.1
119. Park W, Yoo DH, Miranda P, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017;76(2):346–354. doi:10.1136/annrheumdis-2015-208783
120. Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76(2):355–363. doi:10.1136/annrheumdis-2015-208786
121. Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor α blockers in ankylosing spondylitis. Ann Rheum Dis. 2004;63(6):665–670. doi:10.1136/ard.2003.016386
122. Liu W, Wu Y-H, Zhang L, et al. Elevated serum levels of IL-6 and IL-17 may associate with the development of ankylosing spondylitis. Int J Clin Exp Med. 2015;8(10):17362–17376.
123. Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76(6):1070–1077. doi:10.1136/annrheumdis-2016-209730
124. Yago T, Nanke Y, Kawamoto M, Kobashigawa T, Yamanaka H, Kotake S. IL-23 and Th17 disease in inflammatory arthritis. J Clin Med. 2017;6:9.
125. Baraliakos X, Kivitz AJ, Deodhar AA, et al. Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the Phase 3 MEASURE 1 trial. Clin Exp Rheumatol. 2018;36(1):50–55.
126. Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active Ankylosing Spondylitis through two years: results from a phase III study. Arthritis Care Res (Hoboken). 2017;69(7):1020–1029. doi:10.1002/acr.23233
127. Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19(1):285. doi:10.1186/s13075-017-1490-y
128. El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101(2):311. doi:10.1111/ajg.2006.101.issue-2
129. Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–1076. doi:10.1038/nm.2817
130. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–789. doi:10.1016/S0140-6736(13)60594-2
131. Poddubnyy D, Hermann K-GA, Callhoff J, Listing J, Sieper J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis. 2014;73(5):817–823. doi:10.1136/annrheumdis-2013-204248
132. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69(12):2283–2291. doi:10.1002/art.40319
133. Genovese MC, Weinblatt ME, Mease PJ, et al. Dual inhibition of tumour necrosis factor and interleukin-17A with ABT-122: open-label long-term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatology (Oxford). 2018;57(11):1972–1981. doi:10.1093/rheumatology/key173
134. Sieper J, Braun J, Kay J, et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann Rheum Dis. 2015;74(6):1051–1057. doi:10.1136/annrheumdis-2013-204963
135. 6 daily stretches for ankylosing spondylitis that can help ease pain. Available from: https://creakyjoints.org/diet-exercise/ankylosing-spondylitis-stretches-exercises/. Accessed September 4, 2019.
136. American College of Rheumatology Releases New Ankylosing Spondylitis & Non-Radiographic Axial Spondyloarthritis Treatment Recommendations; 2015. Available from: https://www.rheumatology.org/About-Us/Newsroom/Press-Releases/ID/679. Accessed September 4, 2019.
137. Versus Arthritis. What is ankylosing spondylitis (AS)?. 2015. Available from: https://www.versusarthritis.org/about-arthritis/conditions/ankylosing-spondylitis/. Accessed September 4, 2019.
138. Goie The HS, Steven MM, van der Linden SM, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. Br J Rheumatol. 1985;24(3):242–249. doi:10.1093/rheumatology/24.3.242
139. Jacobs WB, Fehlings MG. Ankylosing spondylitis and spinal cord injury: origin, incidence, management, and avoidance. Neurosurg Focus. 2008;24(1):E12. doi:10.3171/FOC/2008/24/1/E12
140. Bron JL, de Vries MK, Snieders MN, van der Horst-Bruinsma IE, van Royen BJ. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol. 2009;28(8):883–892. doi:10.1007/s10067-009-1151-x
141. Britto NMF, Renor BS, Ghizoni E, Tedeschi H, Joaquim AF. Spine surgery in patients with ankylosing spondylitis. Rev Assoc Med Bras. 2018;64(4):379–383. doi:10.1590/1806-9282.64.04.379
142. Lu MC, Koo M, Lai NS. Incident spine surgery in patients with ankylosing spondylitis: a secondary cohort analysis of a nationwide, population-based health claims database. Arthritis Care Res (Hoboken). 2018;70(9):1416–1420. doi:10.1002/acr.23478
143. Kurucan E, Bernstein DN, Mesfin A. Surgical management of spinal fractures in ankylosing spondylitis. J Spine Surg. 2018;4(3):501–508. doi:10.21037/jss.2018.06.15
144. Mountziaris PM, Kramer PR, Mikos AG. Emerging intra-articular drug delivery systems for the temporomandibular joint. Methods. 2009;47(2):134–140. doi:10.1016/j.ymeth.2008.09.001
145. Leone G, Fini M, Torricelli P, Giardino R, Barbucci R. An amidated carboxymethylcellulose hydrogel for cartilage regeneration. J Mater Sci. 2008;19(8):2873–2880. doi:10.1007/s10856-008-3412-7
146. Huang Y-C, Tsai S-K, Huang C-H, et al. Intravenous tenoxicam reduces uterine cramps after Cesarean delivery. Can J Anesthesia. 2002;49(4):384–387. doi:10.1007/BF03017327
147. Mohammad IS, He W, Yin L. A smart paclitaxel-disulfiram nanococrystals for efficient MDR reversal and enhanced apoptosis. Pharm Res. 2018;35(4):77. doi:10.1007/s11095-018-2370-0
148. Mohammad IS, He W, Yin L. Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed Pharmacother. 2018;100:335–348. doi:10.1016/j.biopha.2018.02.038
149. Mohammad IS, Hu H, Yin L, He W. Drug nanocrystals: fabrication methods and promising therapeutic applications. Int J Pharm. 2019;562:187–202. doi:10.1016/j.ijpharm.2019.02.045
150. Mohammad IS, Teng C, Chaurasiya B, Yin L, Wu C, He W. Drug-delivering-drug approach-based codelivery of paclitaxel and disulfiram for treating multidrug-resistant cancer. Int J Pharm. 2019;557:304–313. doi:10.1016/j.ijpharm.2018.12.067
151. DiSanto RM, Subramanian V, Gu Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(4):548–564. doi:10.1002/wnan.1329
152. Johnson BM, Junkins RD, Ting JPY. A spike in inflammation. Nat Nanotechnol. 2018;13(11):980–981. doi:10.1038/s41565-018-0292-y
153. Guan S, Munder A, Hedtfeld S, et al. Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat Nanotechnol. 2019;14(3):287–297. doi:10.1038/s41565-018-0358-x
154. Chevalier X. Intraarticular treatments for osteoarthritis: new perspectives. Curr Drug Targets. 2010;11(5):546–560.
155. Bhardwaj U, Burgess DJ. Physicochemical properties of extruded and non-extruded liposomes containing the hydrophobic drug dexamethasone. Int J Pharm. 2010;388(1):181–189. doi:10.1016/j.ijpharm.2010.01.003
156. Zhang Z, Huang G. Micro- and nano-carrier mediated intra-articular drug delivery systems for the treatment of osteoarthritis. J Nanotechnol. 2012;2012.
157. Elron-Gross I, Glucksam Y, Margalit R. Liposomal dexamethasone–diclofenac combinations for local osteoarthritis treatment. Int J Pharm. 2009;376(1):84–91. doi:10.1016/j.ijpharm.2009.04.025
158. Srinath P, Vyas SP, Diwan PV. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev Ind Pharm. 2000;26(3):313–321. doi:10.1081/DDC-100100359
159. Gottschalk O, Metz P, Dao Trong ML, et al. Therapeutic effect of methotrexate encapsulated in cationic liposomes (EndoMTX) in comparison to free methotrexate in an antigen-induced arthritis study in vivo. Scand J Rheumatol. 2015;44(6):456–463. doi:10.3109/03009742.2015.1030448
160. Alekseeva AA, Moiseeva E, Onishchenko N, et al. Liposomal formulation of a methotrexate lipophilic prodrug: assessment in tumor cells and mouse T-cell leukemic lymphoma. Int J Nanomedicine. 2017;12:3735. doi:10.2147/IJN
161. Gaidukevich SK, Mikulovich YL, Smirnova TG, et al. Antibacterial effects of liposomes containing phospholipid cardiolipin and fluoroquinolone levofloxacin on mycobacterium tuberculosis with extensive drug resistance. Bull Exp Biol Med. 2016;160(5):675–678. doi:10.1007/s10517-016-3247-z
162. Vázquez JL, Montero MT, Trias J, Hernàndez-Borrell J. 6-Fluoroquinolone–liposome interactions: fluorescence quenching study using iodide. Int J Pharm. 1998;171(1):75–86. doi:10.1016/S0378-5173(98)00154-9
163. Grancelli A, Morros A, Cabañas ME, et al. Interaction of 6-fluoroquinolones with dipalmitoylphosphatidylcholine monolayers and liposomes. Langmuir. 2002;18(24):9177–9182. doi:10.1021/la025837h
164. Furneri PM, Fresta M, Puglisi G, Tempera G. Ofloxacin-loaded liposomes: in vitro activity and drug accumulation in bacteria. Antimicrob Agents Chemother. 2000;44(9):2458–2464. doi:10.1128/aac.44.9.2458-2464.2000
165. Ten Hagen TLM, Van Der Veen AH, Nooijen PT, Van Tiel ST, Seynhaeve AL, Eggermont AM. Low-dose tumor necrosis factor-α augments antitumor activity of stealth liposomal doxorubicin (DOXIL®) in soft tissue sarcoma-bearing rats. Int J Cancer. 2000;87(6):829–837. doi:10.1002/1097-0215(20000915)87:6<829::aid-ijc12>3.0.co;2-c
166. Kim DW, Andres ML, Li J, et al. Liposome-encapsulated tumor necrosis factor-α enhances the effects of radiation against human colon tumor xenografts. J Interferon Cytokine Res. 2001;21(11):885–897. doi:10.1089/107999001753289497
167. Kedar E, Palgi O, Golod G, Babai I, Barenholz Y. Delivery of cytokines by liposomes. III. Liposome-encapsulated GM-CSF and TNF-alpha show improved pharmacokinetics and biological activity and reduced toxicity in mice. J Immunother. 1997;20(3):180–193.
168. Meka RR, Venkatesha SH, Moudgil KD. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J Controlled Release. 2018;286:279–288. doi:10.1016/j.jconrel.2018.08.007
169. Lin S-Y, Chen KS, Teng HH, Li MJ. In vitro degradation and dissolution behaviours of microspheres prepared by three low molecular weight polyesters. J Microencapsul. 2000;17(5):577–586. doi:10.1080/026520400417630
170. Tunçay M, Caliş S, Kaş HS, Ercan MT, Peksoy I, Hincal AA. Diclofenac sodium incorporated PLGA (50:50) microspheres: formulation considerations and in vitro/in vivo evaluation. Int J Pharm. 2000;195(1):179–188.
171. Skoutakis VA, Carter CA, Mickle TR, et al. Review of diclofenac and evaluation of its place in therapy as a nonsteroidal antiinflammatory agent. Drug Intell Clin Pharm. 1988;22(11):850–859. doi:10.1177/106002808802201102
172. Saravanan M, Bhaskar K, Maharajan G, Pillai KS. Development of gelatin microspheres loaded with diclofenac sodium for intra-articular administration. J Drug Target. 2011;19(2):96–103. doi:10.3109/10611861003733979
173. Zhao J, Zhao M, Yu C, et al. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int J Nanomedicine. 2017;12:6735. doi:10.2147/IJN
174. Zhang H, Li X, Ding J, et al. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int J Pharm. 2013;441(1):261–268. doi:10.1016/j.ijpharm.2012.11.034
175. Iqbal S, Du X, Wang J, Li H, Yuan Y, Wang J. Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis. Nano Res. 2018;11(5):2872–2884. doi:10.1007/s12274-017-1918-3
176. Davis SM, Reichel D, Bae Y, Pennypacker KR. Leukemia inhibitory factor-loaded nanoparticles with enhanced cytokine metabolic stability and anti-inflammatory activity. Pharm Res. 2018;35(1):6. doi:10.1007/s11095-017-2282-4
177. Peppas N, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46.
178. Barbucci R, Lamponi S, Borzacchiello A, et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials. 2002;23(23):4503–4513. doi:10.1016/s0142-9612(02)00194-1
179. Hu X-B, Kang -R-R, Tang -T-T, et al. Topical delivery of 3,5,4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model. Drug Deliv Transl Res. 2019;9(1):357–365. doi:10.1007/s13346-018-00604-z
180. Ahmad U, Sohail M, Ahmad M, et al. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: development, characterization and in-vivo evaluation. Int J Biol Macromol. 2019;129:233–245. doi:10.1016/j.ijbiomac.2019.02.031
181. Dong L, Xia S, Chen H, Chen J, Zhang J. Spleen-specific suppression of TNF-α by cationic hydrogel-delivered antisense nucleotides for the prevention of arthritis in animal models. Biomaterials. 2009;30(26):4416–4426. doi:10.1016/j.biomaterials.2009.04.045
182. Robert M-C, Frenette M, Zhou C, et al. A drug delivery system for administration of anti-TNF-α antibody. Transl Vis Sci Technol. 2016;5(2):11. doi:10.1167/tvst.5.2.11
183. Zhang Z, Huang G. Intra-articular lornoxicam loaded PLGA microspheres: enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Deliv. 2012;19(5):255–263. doi:10.3109/10717544.2012.700962
184. Ko J-Y, Choi Y-J, Jeong G-J, Im G-I. Sulforaphane–PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials. 2013;34(21):5359–5368. doi:10.1016/j.biomaterials.2013.03.066
185. Bozdag S, Caliş S, Kaş HS, Ercan MT, Peksoy I, Hincal AA. In vitro evaluation and intra-articular administration of biodegradable microspheres containing naproxen sodium. J Microencapsul. 2001;18(4):443–456. doi:10.1080/02652040010018641
186. Elron-Gross I, Glucksam Y, Biton IE, Margalit R. A novel Diclofenac-carrier for local treatment of osteoarthritis applying live-animal MRI. J Controlled Release. 2009;135(1):65–70. doi:10.1016/j.jconrel.2008.12.005
187. Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, García AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33(30):7665–7675. doi:10.1016/j.biomaterials.2012.06.101
188. Dong J, Jiang D, Wang Z, Wu G, Miao L, Huang L. Intra-articular delivery of liposomal celecoxib–hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int J Pharm. 2013;441(1):285–290. doi:10.1016/j.ijpharm.2012.11.031
189. Hui JH, Chan S-W, Li J, et al. Intra-articular delivery of chondroitin sulfate for the treatment of joint defects in rabbit model. J Mol Histol. 2007;38(5):483–489. doi:10.1007/s10735-007-9120-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.