Back to Journals » OncoTargets and Therapy » Volume 13
Advances and Prospects of Vasculogenic Mimicry in Glioma: A Potential New Therapeutic Target?
Authors Cai H, Liu W, Liu X, Li Z, Feng T, Xue Y, Liu Y
Received 31 January 2020
Accepted for publication 5 May 2020
Published 21 May 2020 Volume 2020:13 Pages 4473—4483
DOI https://doi.org/10.2147/OTT.S247855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Heng Cai,1– 3,* Wenjing Liu,4,* Xiaobai Liu,1– 3 Zhiqing Li,1– 3 Tianda Feng,1– 3 Yixue Xue,5 Yunhui Liu1– 3
1Department of Neurosurgery, Shengjing Hospital of China Medical University, Shenyang 110004, People’s Republic of China; 2Liaoning Clinical Medical Research Center in Nervous System Disease, Shenyang 110004, People’s Republic of China; 3Key Laboratory of Neuro-Oncology in Liaoning Province, Shenyang 110004, People’s Republic of China; 4Department of Geriatrics, First Hospital of China Medical University, Shenyang 110001, People’s Republic of China; 5Department of Neurobiology, College of Basic Medicine, China Medical University, Shenyang 110122, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunhui Liu Email [email protected]
Abstract: Vasculogenic mimicry (VM) is the formation of a “vessel-like” structure without endothelial cells. VM exists in vascular-dependent solid tumors and is a special blood supply source involved in the highly invasive tumor progression. VM is observed in a variety of human malignant tumors and is closely related to tumor proliferation, invasion, and recurrence. Here, we review the mechanism, related signaling pathways, and molecular regulation of VM in glioma and discuss current research problems and the potential future applications of VM in glioma treatment. This review may provide a new viewpoint for glioma therapy.
Keywords: glioma, vasculogenic mimicry, glioma stem cells, hypoxia, drug delivery systems, noncoding RNAs
Introduction
Gliomas are among the most common central nervous system tumors. At present, comprehensive high-grade gliomas treatment involves surgical intervention combined with postoperative radiotherapy and chemotherapy. However, the median survival time for patients with glioblastoma is less than 14 months, as the tumors are prone to recurrence and the patient mortality rate is very high.1 High-grade gliomas are the typical vascular-dependent solid tumor,2 rich in tumor angiogenesis, and difficulty restricting tumor blood supply is one reason why clinical treatment is problematic.3
Antiangiogenic therapy has been an adjuvant therapy for high-grade gliomas for the past decade. The angiogenesis inhibitor bevacizumab has been used in the treatment of glioma. However, neuro-oncologists have found that angiogenic inhibitors have not achieved the desired therapeutic effect in clinical practice.4–6 It appears that glioma cells (GCs) exhibit “therapy resistance”, suggesting the presence of a blood supply source in gliomas that differs from traditional angiogenesis.
Discovery of Vasculogenic Mimicry (VM)
VM was first discovered by Maniotis et al in highly invasive malignant melanoma.7 VM is a matrix-rich conduit without endothelial cells (ECs), and an EC-independent tumor microcirculation model. Specifically, VM refers to a channel formed by a series of changes, including self-deformation and matrix remodeling of tumor cells to undergo “phenotypic transformation into ECs”.7 The tumor cells that comprise this channel structure show a variety of phenotypic transformations, such as dedifferentiation, where cells show the dual phenotypic characteristics of ECs and tumor cells.
Maniotis et al7 first found a grid-like structure formed by interconnections between the stroma in malignant melanoma tissue sections. Using transmission electron microscopy, they observed that the grid structure in the tumor tissue was composed of a special kind of channel. This channel contained hemoglobin and plasma components that pass through it. They further used iodate Schiff staining and found that some of the ducts were strongly positive, suggesting that they were rich in matrix components. However, there was no expression of CD34, an EC marker, in the ducts. This finding suggested that there were no vascular ECs present in these structures.
Furthermore, Maniotis et al7 found that the channels were rich in laminin, collagen IV, collagen VI, and heparan sulfate proteoglycan. Phenotypic analysis of tumor cells suggested that they had undergone a phenotypic transformation into ECs. Hemoglobin, red blood cells, platelets, and other blood components were observed in the duct, indicating that this channel was involved in the microcirculation supplied by the tumor vessels.7,8
This kind of tumor cell, with an “EC phenotype”, forms the structure of the channels through complex processes of cell deformation, proliferation, migration, and matrix remodeling, to provide the blood supply required for invasive tumor growth. This is also one of the reasons why there is a lack of necrosis in malignant melanoma tissue sections.
Since its initial discovery, VM has been found in other solid tumors, including hepatocellular carcinoma,9–12 Ewing’s sarcoma,12,13 acute leukemia,14 ovarian carcinoma,15,16 cervical cancer,17 prostate adenocarcinoma,18 nasopharyngeal carcinoma,19 non-small cell lung cancer,20 lung adenocarcinoma,21 osteosarcoma,22 gastric cancer,23,24 breast cancer,25,26 and renal clear cell carcinoma.27
The in vitro detection of VM involves Periodic Acid-Schiff (PAS)-CD34 double immunohistochemical staining to observe the structure of the lumen in the section. If the endothelial marker CD34 (or CD31) is present, PAS-positive staining indicates tumor vessels. If the endothelial marker CD34 (or CD31) is absent, PAS-positive staining indicates VM. Another detection method has been developed based on the Matrigel three-dimensional (3D) culture model of tumor cells in vitro. Arrangement of tumor cells in a 3D reticular structure suggests that the tumor cells have undergone phenotypic transformation into ECs. Additionally, the glycoprotein-rich VM channel was also observed in 3D in an in vitro tumor cell culture model by using X-ray tomography for 3D reconstruction.28 Through time-lapse dynamic magnetic resonance angiography combined with electron microscopy and immunohistochemistry, this VM structure has been confirmed to be involved in tumor microcirculation.29
VM Formation in Glioma
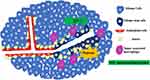
VM was discovered and reported in glioma by Yue and Chen30 in 2005. They collected 45 cases of WHO II–IV grade astrocytoma tissues and found PAS (+)/CD34 (-) channels in two high-grade astrocytoma tissues. These channels connected to the CD34 positive glioma microvessels and formed part of the microcirculation, which confirmed the existence of VM in gliomas.30 In a subsequent study of 101 glioma tissue samples, VM was found in glioma tissue sections from 13 samples. The positive rate of VM positively correlated with the degree of malignancy in these samples, indicating that patients with VM often have a poor prognosis and short survival time.31 The positive rate of VM in highly malignant adult glioblastoma specimens was higher than that in lower-grade glioma tissues.32,33 CD105 (a vascular EC marker) and CD133 double-positive GCs were also found in high-grade gliomas in children, suggesting that VM is not unique to adult high-grade glioma.34,35 VM formation in glioma is shown in Figure 1.
Differentiation of Glioma Stem Cells (GSCs)
Using gene chip technology, researchers analyzed and the expression of specific genes in GCs with VM. Their findings suggest that GCs undergoing VM may regain pluripotent characteristics, exhibit an embryonic phenotype, and undergo “transdifferentiation”.36 However, VM has been detected in some malignant tumors with bidirectional differentiation. Taken together, these studies have shown that to form VM, GCs must show “transdifferentiation” characteristics and be able to differentiate.37 Recently, Mei et al38 collected 64 glioblastoma tissue samples. Live-cell imaging confirmed that malignant GSCs could differentiate into ECs and produce VM. Among them, CD133 (+) GSCs were considered to have a stronger ability to induce VM formation than CD133 (-) GSCs.39 Wu et al40 found that bevacizumab could induce autophagy in GSCs and activate the vascular endothelial growth factor/vascular endothelial growth factor receptor-2 (VEGF/VEGFR-2) signaling pathway, which also promotes VM. This mechanism may, in part, explain the poor clinical efficacy of bevacizumab in the treatment of glioma. An increasing number of studies have shown that GSCs play a vital role in the development of VM in glioma.41 However, Ke et al42 found that non-stem-like cells of glioma were more prone to gain VM-related gene expression and phenotype than were stem-like cells of the same origin.
Based on the critical role of GSCs in glioma VM, the researchers developed a variety of drug delivery systems (Table 1), hoping to produce the dual effects of inhibiting glioma stem cell growth and glioma VM at the same time. Some receptors, such as VEGFR-2, neuropilin 1 (NRP1), ephrin A, and epidermal growth factor receptor (EGFR), are highly expressed on GSCs within the VM. Multifunctional targeted drug delivery is feasible when these receptors are efficiently targeted. Liposomes or micelles possess a high binding capacity for receptors and demonstrate superiority in tumor-homing imaging.43–57 This type of treatment is expected to become a new direction for the treatment of glioma.58
 |
Table 1 Targeting Drug Delivery Systems for Circumventing VM Formation |
Formation of Glioma VM in Hypoxic Environments
The rapid proliferation of GCs causes a relative lag in tumor angiogenesis, which then leads to the formation of a hypoxic microenvironment in localized tumor regions. In this hypoxic microenvironment, GCs are arranged autonomously into channels. These GC-arranged VM channels are the key to maintaining the malignant biological characteristics of tumors and have some EC functions and phenotypes.59,60 The hypoxic microenvironment also activates some related signaling pathways, molecules, and the prolyl hydroxylase activity decreases, allowing the hypoxia-inducible factor (HIF) subunit alpha subunit to escape von Hippel-Lindau degradation. HIF alpha subunits accumulate in the cytoplasm, where they combine with HIF beta to form heterodimers and then translocate to the nucleus to activate target gene transcription.61 Additionally, hypoxia inhibits HIF degradation, allowing HIF-1α or HIF-2α to be in the nucleus and bind to the hypoxia response element of the target gene. Activation of VEGF, cytokines, stem cell characteristic maintenance-related genes, and epithelial-mesenchymal transition (EMT) inducers could also lead to VM.62,63
Rapid cell proliferation and unorganized perfusion in glioma microcirculation can cause an increase in interstitial fluid pressure, inducing ECs to cross the blood-tumor barrier and form a hypoxic environment in the center of the glioma.61,64 This hypoxia causes GCs with “stem cell characteristics” to form VM channels, which are then connected to endothelial-dependent blood vessels to form early VM structures.65 This structure is a mixed structure in which glioma microvessels and VM coexist. The spatial and temporal correlations between the VM networks and GCs with “stem cell characteristics” suggest that these cells are the early driving forces of VM.64
Matrix Remodeling Is a Critical Step in VM
In the identification of VM, the PAS-positive matrix layer was found to cover the inner surface of the VM structure. At present, the known matrix components include laminin, collagen, mucopolysaccharide, and F tissue factor and its inhibitors. The first several components are also components of the vascular basement membrane, which promotes connection and penetration between the VM structure and glioma microvessels. The balance between F tissue factor and its inhibitors is the key regulatory mechanism controlling anticoagulant function and maintaining VM blood flow.66
Immune Cell Infiltration
GCs can recruit tumor-associated immune cells, especially M2 tumor-associated macrophages (TAMs) that express CD68 and CD206.67 GCs secrete IL-4 to activate TAMs and upregulate the expression of CD68, Arg-1, and CD204. Activated TAMs are widely recruited to, and infiltrate, VM-positive areas where they activate and upregulate cyclooxygenase-2. This further activate prostaglandin E and prostaglandin E receptor 1 through the protein kinase C pathway, and promotes VM in glioma.68,69
Regulation of VM in Glioma
Many molecules and signaling pathways are involved in the regulation and development of VM in glioma.
Hypoxia-Related Signaling Pathways
Hypoxia can induce VM. Under hypoxic conditions, leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) inhibit the EGFR mediated phosphoinositol 3-kinase (PI3K)/AKT pathway and repress the EMT.70 The inhibition of hypoxia-induced VM in gliomas has been studied.70 Under hypoxia, the rapamycin target protein is involved in VM formation in glioma through HIF-1α.63 In the in vitro hypoxia glioma model, B-cell lymphoma 2 (Bcl-2) inhibits VM formation in gliomas by inhibiting the activation of the HIF-1α-MMP-2-MMP-14 signaling pathway.71 Silencing Beclin-1 can also significantly reduce hypoxia-induced VM formation.72 Additionally, under hypoxia, some GSCs express vascular endothelial (VE)-cadherin; VE-cadherin and HIF-2α directly interact to contribute to GSC VM formation.73
The VEGF family is a group of regulatory molecules critical for angiogenesis in glioma, and it is also involved in VM regulation in gliomas.41 For example, GSCs express VEGFR-2, which is activated by VEGF and promotes tubule formation. During autophagy in GSCs, phosphorylation of VEGFR-2 is activated by the PI3K-AKT pathway, which promotes the formation of VM in GSCs.40 The role of VEGF in VM in gliomas was also detected by the dynamic 3D culture model.74
VE-cadherin, a member of the cadherin superfamily, is closely related to hypoxia-related signaling molecules. Under hypoxic conditions, VE-cadherin is upregulated in a HIF-1α- and HIF-2α-dependent manner and contributes to hypoxia-induced VM.73 Abnormal expression of VE-cadherin specifically by ECs was also found in VM glioma-like stem cells, suggesting that VE-cadherin is involved in VM.75,76
Matrix Metalloproteinases (MMPs)
MMPs play an essential role in VM formation and are essential protein targets and effectors in the VM regulatory network. It has been reported that both MMP-14 and MMP-2 degrade the gamma 2 laminin subunit into gamma 2ʹ and gamma 2x fragments and then stimulate glioma cell invasion and VM.77 In malignant glioma, MMP-14 expression and activation transform MMP-2 precursors into active MMP-2 and affect matrix remodeling, which affects VM formation in glioma.78 Histone deacetylase activates MMP proteins through the PI3K-ERK signaling pathway and promotes VM formation by regulating the expression of laminin subunit gamma 2 (LAMC2), a mimicry-related molecule in gliomas.77
Cytokine Family
Epidermal growth factor (EGF) is increased in GCs, and binding to EGFR activates its downstream pathways, including PI3K-AKT, ultimately activating LAMC2 and cyclooxygenase-2 and promoting VM.31 The inhibitory effect of LRIG1 on VM in glioma is also mediated by the EGFR signaling pathway.70 EMT plays an important role in glioma progression.79,80 Transforming growth factor beta (TGF-β) induces the development of VM,81 while the TGF-β1 inhibitor galunisertib inhibits astrocyte-induced VM in glioma.82 Additionally, the expression of insulin-like growth factor-binding protein 2 (IGFBP2) is positively correlated with VM in patients with glioma. IGFBP2 interacts with the integrin alpha5beta1 subunits and enhances CD144 expression in a FAK-ERK pathway-dependent manner, IGFBP2 can also activate CD144 and MMP2 through transcription factor SP1 activation, enhancing VM in gliomas.83 Aquaporin-1 may play a role in VM in glioblastoma, and it can be used as a new diagnostic biomarker and a potential therapeutic target.84 In oligodendroglioma, downregulation of galectin-1 gene expression, a significant decrease in brain expressed X-linked 2 expression, and inhibition of VM may present new therapeutic strategies for reducing chemotherapy resistance.85 A histone deacetylase inhibitor has also been identified as a promising candidate for VM inhibition in glioblastoma.77,86 Finally, suppression of Axin187 and curA88 have also been shown to affect VM.
Noncoding RNAs
In recent years, the regulatory roles of noncoding RNAs (ncRNAs) in glioma occurrence, metastasis, invasive growth, and angiogenesis have become the focus of glioma research. ncRNAs include long ncRNAs (lncRNAs), microRNAs (miRNAs), and PIWI-interacting RNAs.
lncRNAs are a newly discovered class of ncRNAs with lengths of more than 200 nucleotides. lncRNAHOXA-AS2 is upregulated in glioma tissues and is positively correlated with the positive rate of VM.78 HOXA-AS2 knockout attenuates the GC viability and represses VM, which may occur through VE-cadherin inhibition. Moreover, HOXA-AS2 knockout inhibits the activity of MMP-2 and MMP-9.78 In addition, LINC00339 expression in glioma positively correlates with VM formation. LINC00339 inhibits miR-539-5p expression, resulting in increased expression of twist family bHLH transcription factor 1 (TWIST1). TWIST1 upregulates MMP-2 and MMP-14 promoter activities and expression.89 The USF1 transcription factor promotes VM in glioma by regulating lincRNA-SNHG16 and linc00667. Silencing of USF1 can inhibit VM occurrence, which may be regulated by a competitive endogenous RNA mechanism.90 lncRNA SNHG20 also plays a vital role in regulating the formation of VM in glioma.91
miRNAs are also essential regulators of VM in glioma. Xue et al92 found that miR-Let-7f reduces the occurrence of VM in gliomas by inhibiting periostin-induced GC migration. Li et al93 confirmed that miR-141 expression in primary gliomas is downregulated. miR-141 regulates GC proliferation, migration, and invasion by controlling EphA2 expression, which then affects VM in gliomas. miR-584-3p plays a role in glioma inhibition by inhibiting VM formation in GCs by antagonizing hypoxia-induced ROCK1-dependent stress fiber formation.94 miR-995 and miR-26b96 can also be used as potential anti-VM molecules in GCs.
These results suggest that ncRNAs are critical VM regulatory molecules in glioma. Looking for a noncoding RNA molecule may be a potential target for glioma therapy (Figure 2 and Table 2).
 |
Table 2 The Roles of Major Noncoding RNAs in VM Formation in Glioma |
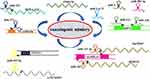 |
Figure 2 Noncoding RNAs are important regulatory molecules for VM formation in gliomas. The lncRNA-miRNA network played an essential role in regulating VM formation in glioma. |
Conclusion
The in-depth study of VM in gliomas has shown that VM can be used as a new entry point for the basic research of gliomas, and as a new direction in glioma growth inhibition. Moreover, VM has become the focus of many researchers to solve antiangiogenesis-targeted drug resistance in the treatment of gliomas.
The main issues remaining to be addressed in VM research in glioma are: (1) the glioma microenvironment and its complexity, in which the relationships among various regulatory factors, specific regulatory mechanisms, and glioma VM are not clear; (2) the relationship between GSCs and VM in glioma is not clear; and (3) at present, glioma VM research is mainly supplemental to glioma angiogenesis research, and the relationship between VM and angiogenesis and their interaction with the malignant progression of glioma have not been reported. Nevertheless, the study of the role of VM in gliomas may still provide a new direction for glioma treatment.
Abbreviations
VM, vasculogenic mimicry; ECs, endothelial cells; GCs, glioma cells; PAS, Periodic Acid-Schiff; 3D, three-dimensional; GSCs, glioma stem cells; VEGF, vascular endothelial growth factor; VEGFR-2, vascular endothelial growth factor receptor-2; NRP1, neuropilin 1; EGFR, epidermal growth factor receptor; HIF, hypoxia-inducible factor; EMT, epithelial-mesenchymal transition; TAMs, tumor-associated macrophages; LRIG1, leucine-rich repeat sequences and immunoglobulin-like domain 1; PI3K, phosphoinositol 3-kinase; Bcl-2, B-cell lymphoma; MMPs, matrix metalloproteinases; VE-cadherin, vascular endothelial-cadherin; LAMC2, laminin subunit gamma 2; TGF-β, Transforming growth factor beta; IGFBP2, insulin-like growth factor-binding protein 2; ncRNAs, noncoding RNAs; lncRNAs, long noncoding RNAs; miRNAs, microRNAs; TWIST1, transcription factor twist family bHLH transcription factor 1; IFP, interstitial fluid pressure.
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (81702488), China Postdoctoral Science Foundation (2018M641745), Doctoral Start-up Foundation of Liaoning Province (No. 20170520020), Shenyang Science and Technology Bureau (No. 19-112-4-044), and 345 Talent Project fund of Shengjing Hospital of China Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362(1):1–7. doi:10.1016/j.canlet.2015.03.015
2. Das S, Marsden PA. Angiogenesis in glioblastoma. N Engl J Med. 2013;369(16):1561–1563. doi:10.1056/NEJMcibr1309402
3. Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2014;9:CD008218.
4. Soda Y, Myskiw C, Rommel A, Verma IM. Mechanisms of neovascularization and resistance to anti-angiogenic therapies in glioblastoma multiforme. J Mol Med (Berl). 2013;91(4):439–448. doi:10.1007/s00109-013-1019-z
5. Angara K, Borin TF, Arbab AS. Vascular mimicry: a novel neovascularization mechanism driving Anti-Angiogenic Therapy (AAT) resistance in glioblastoma. Transl Oncol. 2017;10(4):650–660. doi:10.1016/j.tranon.2017.04.007
6. Xue W, Du X, Wu H, et al. Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment. Sci Rep. 2017;7(1):13894. doi:10.1038/s41598-017-14341-9
7. Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. doi:10.1016/S0002-9440(10)65173-5
8. Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156(2):361–381. doi:10.1016/S0002-9440(10)64739-6
9. Sun T, Sun BC, Zhao XL, et al. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology. 2011;54(5):1690–1706. doi:10.1002/hep.24543
10. Sun T, Zhao N, Zhao XL, et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51(2):545–556. doi:10.1002/hep.23311
11. Meng J, Chen S, Lei YY, et al. Hsp90beta promotes aggressive vasculogenic mimicry via epithelial-mesenchymal transition in hepatocellular carcinoma. Oncogene. 2019;38(2):228–243. doi:10.1038/s41388-018-0428-4
12. Puerto-Camacho P, Amaral AT, Lamhamedi-Cherradi SE, et al. Preclinical efficacy of endoglin-targeting antibody-drug conjugates for the treatment of ewing sarcoma. Clin Cancer Res. 2019;25(7):2228–2240. doi:10.1158/1078-0432.CCR-18-0936
13. van der Schaft DW, Hillen F, Pauwels P, et al. Tumor cell plasticity in ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65(24):11520–11528. doi:10.1158/0008-5472.CAN-05-2468
14. Mirshahi P, Rafii A, Vincent L, et al. Vasculogenic mimicry of acute leukemic bone marrow stromal cells. Leukemia. 2009;23(6):1039–1048. doi:10.1038/leu.2009.10
15. Sood AK, Fletcher MS, Coffin JE, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190(4):899–909. doi:10.1016/j.ajog.2004.02.011
16. Sood AK, Fletcher MS, Zahn CM, et al. The clinical significance of tumor cell-lined vasculature in ovarian carcinoma: implications for anti-vasculogenic therapy. Cancer Biol Ther. 2002;1(6):661–664. doi:10.4161/cbt.316
17. Wan HY, Li QQ, Zhang Y, et al. MiR-124 represses vasculogenic mimicry and cell motility by targeting amotL1 in cervical cancer cells. Cancer Lett. 2014;355(1):148–158. doi:10.1016/j.canlet.2014.09.005
18. Sharma N, Seftor RE, Seftor EA, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50(3):189–201. doi:10.1002/pros.10048
19. Xiang T, Lin YX, Ma W, et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun. 2018;9(1):5009. doi:10.1038/s41467-018-07308-5
20. Williamson SC, Metcalf RL, Trapani F, et al. Vasculogenic mimicry in small cell lung cancer. Nat Commun. 2016;7:13322. doi:10.1038/ncomms13322
21. Peng Z, Wang J, Shan B, et al. The long noncoding RNA LINC00312 induces lung adenocarcinoma migration and vasculogenic mimicry through directly binding YBX1. Mol Cancer. 2018;17(1):167. doi:10.1186/s12943-018-0920-z
22. Cai XS, Jia YW, Mei J, Tang RY. Tumor blood vessels formation in osteosarcoma: vasculogenesis mimicry. Chin Med J (Engl). 2004;117(1):94–98.
23. Sun B, Qie S, Zhang S, et al. Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol. 2008;39(3):444–451. doi:10.1016/j.humpath.2007.07.018
24. Li Y, Wu Z, Yuan J, et al. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. doi:10.1016/j.canlet.2017.02.035
25. Gong W, Sun B, Zhao X, et al. Nodal signaling promotes vasculogenic mimicry formation in breast cancer via the Smad2/3 pathway. Oncotarget. 2016;7(43):70152–70167. doi:10.18632/oncotarget.12161
26. Hendrix MJ, Seftor EA, Kirschmann DA, Seftor RE. Molecular biology of breast cancer metastasis. Molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res. 2000;2(6):417–422. doi:10.1186/bcr88
27. Bai J, Yeh S, Qiu X, et al. TR4 nuclear receptor promotes clear cell renal cell carcinoma (ccRCC) vasculogenic mimicry (VM) formation and metastasis via altering the miR490-3p/vimentin signals. Oncogene. 2018;37(44):5901–5912. doi:10.1038/s41388-018-0269-1
28. Racordon D, Valdivia A, Mingo G, et al. Structural and functional identification of vasculogenic mimicry in vitro. Sci Rep. 2017;7(1):6985. doi:10.1038/s41598-017-07622-w
29. Shirakawa K, Kobayashi H, Heike Y, et al. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62(2):560–566.
30. Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005;53(8):997–1002. doi:10.1369/jhc.4A6521.2005
31. Liu XM, Zhang QP, Mu YG, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105(2):173–179. doi:10.1007/s11060-011-0578-5
32. El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133(Pt 4):973–982. doi:10.1093/brain/awq044
33. Liu Z, Li Y, Zhao W, Ma Y, Yang X. Demonstration of vasculogenic mimicry in astrocytomas and effects of endostar on U251 cells. Pathol Res Pract. 2011;207(10):645–651. doi:10.1016/j.prp.2011.07.012
34. Smith SJ, Tilly H, Ward JH, et al. CD105 (endoglin) exerts prognostic effects via its role in the microvascular niche of paediatric high grade glioma. Acta Neuropathol. 2012;124(1):99–110. doi:10.1007/s00401-012-0952-1
35. Chen Y, Jing Z, Luo C, et al. Vasculogenic mimicry-potential target for glioblastoma therapy: an in vitro and in vivo study. Med Oncol. 2012;29(1):324–331. doi:10.1007/s12032-010-9765-z
36. Seftor EA, Meltzer PS, Schatteman GC, et al. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44(1):17–27. doi:10.1016/S1040-8428(01)00199-8
37. Scully S, Francescone R, Faibish M, et al. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci. 2012;32(37):12950–12960. doi:10.1523/JNEUROSCI.2017-12.2012
38. Mei X, Chen YS, Chen FR, Xi SY, Chen ZP. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol. 2017;19(8):1109–1118. doi:10.1093/neuonc/nox016
39. Chiao MT, Yang YC, Cheng WY, Shen CC, Ko JL. CD133+ glioblastoma stem-like cells induce vascular mimicry in vivo. Curr Neurovasc Res. 2011;8(3):210–219. doi:10.2174/156720211796558023
40. Wu HB, Yang S, Weng HY, et al. Autophagy-induced KDR/VEGFR-2 activation promotes the formation of vasculogenic mimicry by glioma stem cells. Autophagy. 2017;13(9):1528–1542. doi:10.1080/15548627.2017.1336277
41. Yao X, Ping Y, Liu Y, et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS One. 2013;8(3):e57188. doi:10.1371/journal.pone.0057188
42. Ke C, Luo JR, Cen ZW, et al. Dual antivascular function of human fibulin-3 variant, a potential new drug discovery strategy for glioblastoma. Cancer Sci. 2020;111(3):940–950. doi:10.1111/cas.14300
43. Huang D, Zhang S, Zhong T, et al. Multi-targeting NGR-modified liposomes recognizing glioma tumor cells and vasculogenic mimicry for improving anti-glioma therapy. Oncotarget. 2016;7(28):43616–43628. doi:10.18632/oncotarget.9889
44. Liu Y, Mei L, Yu Q, et al. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7(30):16792–16801. doi:10.1021/acsami.5b04596
45. Yang L, Song X, Gong T, et al. Development a hyaluronic acid ion-pairing liposomal nanoparticle for enhancing anti-glioma efficacy by modulating glioma microenvironment. Drug Deliv. 2018;25(1):388–397. doi:10.1080/10717544.2018.1431979
46. Liu Y, Mei L, Xu C, et al. Dual receptor recognizing cell penetrating peptide for selective targeting, efficient intratumoral diffusion and synthesized anti-glioma therapy. Theranostics. 2016;6(2):177–191. doi:10.7150/thno.13532
47. Li XY, Zhao Y, Sun MG, et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials. 2014;35(21):5591–5604. doi:10.1016/j.biomaterials.2014.03.049
48. Shi K, Long Y, Xu C, et al. Liposomes combined an integrin alphavbeta3-specific vector with pH-responsible cell-penetrating property for highly effective antiglioma therapy through the blood-brain barrier. ACS Appl Mater Interfaces. 2015;7(38):21442–21454. doi:10.1021/acsami.5b06429
49. Feng X, Yao J, Gao X, et al. Multi-targeting peptide-functionalized nanoparticles recognized vasculogenic mimicry, tumor neovasculature, and glioma cells for enhanced anti-glioma therapy. ACS Appl Mater Interfaces. 2015;7(50):27885–27899. doi:10.1021/acsami.5b09934
50. Ying M, Zhan C, Wang S, et al. Liposome-based systemic glioma-targeted drug delivery enabled by all-d peptides. ACS Appl Mater Interfaces. 2016;8(44):29977–29985. doi:10.1021/acsami.6b10146
51. Ran D, Mao J, Shen Q, et al. GRP78 enabled micelle-based glioma targeted drug delivery. J Control Release. 2017;255:120–131. doi:10.1016/j.jconrel.2017.03.037
52. Mao J, Ran D, Xie C, Shen Q, Wang S, Lu W. EGFR/EGFRvIII dual-targeting peptide-mediated drug delivery for enhanced glioma therapy. ACS Appl Mater Interfaces. 2017;9(29):24462–24475. doi:10.1021/acsami.7b05617
53. Chen T, Song X, Gong T, et al. nRGD modified lycobetaine and octreotide combination delivery system to overcome multiple barriers and enhance anti-glioma efficacy. Colloids Surf B Biointerfaces. 2017;156:330–339. doi:10.1016/j.colsurfb.2017.05.038
54. Ruan H, Chai Z, Shen Q, et al. A novel peptide ligand RAP12 of LRP1 for glioma targeted drug delivery. J Control Release. 2018;279:306–315. doi:10.1016/j.jconrel.2018.04.035
55. Ying M, Wang S, Zhang M, et al. Myristic Acid-Modified (D)A7R peptide for whole-process glioma-targeted drug delivery. ACS Appl Mater Interfaces. 2018;10(23):19473–19482. doi:10.1021/acsami.8b05235
56. Zhang Y, Sun X, Huang M, Ke Y, Wang J, Liu X. A novel bispecific immunotoxin delivered by human bone marrow-derived mesenchymal stem cells to target blood vessels and vasculogenic mimicry of malignant gliomas. Drug Des Devel Ther. 2015;9:2947–2959. doi:10.2147/DDDT.S79475
57. Ying M, Shen Q, Liu Y, et al. Stabilized heptapeptide A7R for enhanced multifunctional liposome-based tumor-targeted drug delivery. ACS Appl Mater Interfaces. 2016;8(21):13232–13241. doi:10.1021/acsami.6b01300
58. Ju RJ, Mu LM, Lu WL. Targeting drug delivery systems for circumventing multidrug resistance of cancers. Ther Deliv. 2013;4(6):667–671. doi:10.4155/tde.13.39
59. Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18(10):2726–2732. doi:10.1158/1078-0432.CCR-11-3237
60. Mahase S, Rattenni RN, Wesseling P, et al. Hypoxia-mediated mechanisms associated with antiangiogenic treatment resistance in glioblastomas. Am J Pathol. 2017;187(5):940–953. doi:10.1016/j.ajpath.2017.01.010
61. Wang Z, Dabrosin C, Yin X, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl):S224–S243. doi:10.1016/j.semcancer.2015.01.001
62. Olenyuk BZ, Zhang GJ, Klco JM, Nickols NG, Kaelin WG
63. Huang M, Ke Y, Sun X, et al. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1alpha. Oncol Rep. 2014;32(5):1973–1980. doi:10.3892/or.2014.3454
64. Sun B, Zhang D, Zhao N, Zhao X. Epithelial-to-endothelial transition and cancer stem cells: two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget. 2017;8(18):30502–30510. doi:10.18632/oncotarget.8461
65. Shao R, Taylor SL, Oh DS, Schwartz LM. Vascular heterogeneity and targeting: the role of YKL-40 in glioblastoma vascularization. Oncotarget. 2015;6(38):40507–40518. doi:10.18632/oncotarget.5943
66. Ruf W, Seftor EA, Petrovan RJ, et al. Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Res. 2003;63(17):5381–5389.
67. Zhang L, Xu Y, Sun J, et al. M2-like tumor-associated macrophages drive vasculogenic mimicry through amplification of IL-6 expression in glioma cells. Oncotarget. 2017;8(1):819–832. doi:10.18632/oncotarget.13661
68. Epron G, Ame-Thomas P, Le Priol J, et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia. 2012;26(1):139–148. doi:10.1038/leu.2011.179
69. Rong X, Huang B, Qiu S, Li X, He L, Peng Y. Tumor-associated macrophages induce vasculogenic mimicry of glioblastoma multiforme through cyclooxygenase-2 activation. Oncotarget. 2016;7(51):83976–83986. doi:10.18632/oncotarget.6930
70. Zhang X, Song Q, Wei C, Qu J. LRIG1 inhibits hypoxia-induced vasculogenic mimicry formation via suppression of the EGFR/PI3K/AKT pathway and epithelial-to-mesenchymal transition in human glioma SHG-44 cells. Cell Stress Chaperones. 2015;20(4):631–641. doi:10.1007/s12192-015-0587-y
71. Li J, Ke Y, Huang M, Huang S, Liang Y. Inhibitory effects of B-cell lymphoma 2 on the vasculogenic mimicry of hypoxic human glioma cells. Exp Ther Med. 2015;9(3):977–981. doi:10.3892/etm.2014.2162
72. Duan S. Silencing the autophagy-specific gene Beclin-1 contributes to attenuated hypoxia-induced vasculogenic mimicry formation in glioma. Cancer Biomark. 2018;21(3):565–574. doi:10.3233/CBM-170444
73. Mao XG, Xue XY, Wang L, et al. CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia. Neuro Oncol. 2013;15(7):865–879. doi:10.1093/neuonc/not029
74. Smith SJ, Ward JH, Tan C, Grundy RG, Rahman R. Endothelial-like malignant glioma cells in dynamic three dimensional culture identifies a role for VEGF and FGFR in a tumor-derived angiogenic response. Oncotarget. 2015;6(26):22191–22205. doi:10.18632/oncotarget.4339
75. Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. doi:10.1038/nature09624
76. Delgado-Bellido D, Serrano-Saenz S, Fernandez-Cortes M, Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer. 2017;16(1):65. doi:10.1186/s12943-017-0631-x
77. Liu X, Wang JH, Li S, et al. Histone deacetylase 3 expression correlates with vasculogenic mimicry through the phosphoinositide3-kinase/ERK-MMP-laminin5gamma2 signaling pathway. Cancer Sci. 2015;106(7):857–866. doi:10.1111/cas.12684
78. Gao Y, Yu H, Liu Y, et al. Long non-coding RNA HOXA-AS2 regulates malignant glioma behaviors and vasculogenic mimicry formation via the MiR-373/EGFR axis. Cell Physiol Biochem. 2018;45(1):131–147. doi:10.1159/000486253
79. Chow KH, Park HJ, George J, et al. S100A4 is a biomarker and regulator of glioma stem cells that is critical for mesenchymal transition in glioblastoma. Cancer Res. 2017;77(19):5360–5373. doi:10.1158/0008-5472.CAN-17-1294
80. Iser IC, Pereira MB, Lenz G, Wink MR. The epithelial-to-mesenchymal transition-like process in glioblastoma: an updated systematic review and in silico investigation. Med Res Rev. 2017;37(2):271–313. doi:10.1002/med.21408
81. Ling G, Ji Q, Ye W, Ma D, Wang Y. Epithelial-mesenchymal transition regulated by p38/MAPK signaling pathways participates in vasculogenic mimicry formation in SHG44 cells transfected with TGF-beta cDNA loaded lentivirus in vitro and in vivo. Int J Oncol. 2016;49(6):2387–2398. doi:10.3892/ijo.2016.3724
82. Zhang C, Chen W, Zhang X, et al. Galunisertib inhibits glioma vasculogenic mimicry formation induced by astrocytes. Sci Rep. 2016;6:23056. doi:10.1038/srep23056
83. Liu Y, Li F, Yang YT, et al. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene. 2019;38(11):1815–1831. doi:10.1038/s41388-018-0525-4
84. Yang WY, Tan ZF, Dong DW, et al. Association of aquaporin1 with tumor migration, invasion and vasculogenic mimicry in glioblastoma multiforme. Mol Med Rep. 2018;17(2):3206–3211. doi:10.3892/mmr.2017.8265
85. Le Mercier M, Fortin S, Mathieu V, et al. Galectin 1 proangiogenic and promigratory effects in the Hs683 oligodendroglioma model are partly mediated through the control of BEX2 expression. Neoplasia. 2009;11(5):485–496. doi:10.1593/neo.81526
86. Pastorino O, Gentile MT, Mancini A, et al. Histone deacetylase inhibitors impair vasculogenic mimicry from glioblastoma cells. Cancers (Basel). 2019;11(6). doi:10.3390/cancers11060747
87. Liu X, Wang X, Du W, et al. Suppressor of fused (Sufu) represses Gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget. 2014;5(22):11681–11694. doi:10.18632/oncotarget.2585
88. Liang Y, Huang M, Li J, et al. Curcumin inhibits vasculogenic mimicry through the downregulation of erythropoietin-producing hepatocellular carcinoma-A2, phosphoinositide 3-kinase and matrix metalloproteinase-2. Oncol Lett. 2014;8(4):1849–1855. doi:10.3892/ol.2014.2401
89. Guo J, Cai H, Liu X, et al. Long non-coding RNA LINC00339 stimulates glioma vasculogenic mimicry formation by regulating the miR-539-5p/TWIST1/MMPs axis. Mol Ther Nucleic Acids. 2018;10:170–186. doi:10.1016/j.omtn.2017.11.011
90. Wang D, Zheng J, Liu X, et al. Knockdown of USF1 inhibits the vasculogenic mimicry of glioma cells via stimulating SNHG16/miR-212-3p and linc00667/miR-429 axis. Mol Ther Nucleic Acids. 2019;14:465–482. doi:10.1016/j.omtn.2018.12.017
91. Li X, Xue Y, Liu X, et al. ZRANB2/SNHG20/FOXK1 axis regulates vasculogenic mimicry formation in glioma. J Exp Clin Cancer Res. 2019;38(1):68. doi:10.1186/s13046-019-1073-7
92. Xue H, Gao X, Xu S, et al. MicroRNA-Let-7f reduces the vasculogenic mimicry of human glioma cells by regulating periostin-dependent migration. Oncol Rep. 2016;35(3):1771–1777. doi:10.3892/or.2016.4548
93. Li G, Huang M, Cai Y, Ke Y, Yang Y, Sun X. miR141 inhibits glioma vasculogenic mimicry by controlling EphA2 expression. Mol Med Rep. 2018;18(2):1395–1404. doi:10.3892/mmr.2018.9108
94. Xu S, Zhang J, Xue H, et al. MicroRNA-584-3p reduces the vasculogenic mimicry of human glioma cells by regulating hypoxia-induced ROCK1 dependent stress fiber formation. Neoplasma. 2017;64(1):13–21. doi:10.4149/neo_2017_102
95. Song Y, Mu L, Han X, et al. MicroRNA-9 inhibits vasculogenic mimicry of glioma cell lines by suppressing Stathmin expression. J Neurooncol. 2013;115(3):381–390. doi:10.1007/s11060-013-1245-9
96. Wu N, Zhao X, Liu M, et al. Role of microRNA-26b in glioma development and its mediated regulation on EphA2. PLoS One. 2011;6(1):e16264. doi:10.1371/journal.pone.0016264
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.