Back to Journals » OncoTargets and Therapy » Volume 12
Advancements of Annexin A1 in inflammation and tumorigenesis
Authors Shao G , Zhou H, Zhang Q, Jin Y, Fu C
Received 20 January 2019
Accepted for publication 1 April 2019
Published 30 April 2019 Volume 2019:12 Pages 3245—3254
DOI https://doi.org/10.2147/OTT.S202271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Gang Shao,1 Hanwei Zhou,2,3 Qiyu Zhang,2 Yuanting Jin,1 Caiyun Fu2
1College of Life Sciences, China Jiliang University, Hangzhou 310018, People’s Republic of China; 2Zhejiang Provincial Key Laboratory of Silkworm Bioreactor and Biomedicine, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, People’s Republic of China; 3Institute of Orthopedics, Xiaoshan Traditional Chinese Medical Hospital, Hangzhou 311201, People’s Republic of China
Abstract: Annexin A1 is a Ca2+-dependent phospholipid binding protein involved in a variety of pathophysiological processes. Accumulated evidence has indicated that Annexin A1 has important functions in cell proliferation, apoptosis, differentiation, metastasis, and inflammatory response. Moreover, the abnormal expression of Annexin A1 is closely related to the occurrence and development of tumors. In this review article, we focus on the structure and function of Annexin A1 protein, especially the recent evidence of Annexin A1 in the pathophysiological role of inflammatory and cancer. This summary will be very important for further investigation of the pathophysiological role of Annexin A1 and for the development of novel therapeutics of inflammatory and cancer based on targeting Annexin A1 protein.
Keywords: Annexin A1, structure, inflammation, cancer
Introduction
Annexins constitute a class of structurally homologous calcium-dependent phospholipid-binding protein superfamily. Human-derived Annexins contain Annexin A1 to A131 expressed in a variety of tissues,2,3 which are closely related to the cell membrane and cytoskeletal components.4 The structure of all Annexins is similar and includes four sets of repeating amino acid sequences with about 70 amino acid residues in the core region, except for Annexin VI whose core region consists of eight sets of repeating amino acid sequences. However, the N-terminal region of the Annexins has significant differences in amino acid sequence length and residue composition,5,6 which determines the various biological functions of Annexins, including promoting membrane fusion7 and membrane transport,8 ion channel formation,9–11 regulating cell adhesion,12 cell growth and differentiation,13 cell proliferation and apoptosis,14,15 cell migration,16 tumorigenesis,17 etc.
In the late 1970s, Annexin A1 was first identified as a member of the Annexin family.18 Initially, Annexin A1, also named as macrocortin,19 renocortin,20 lipomodulin,21 and lipocortin I,22 was identified as the inhibitor of phospholipase A2 (PLA2).18 Thus, it was mainly used as an inhibitor of pro-inflammatory factors prostaglandins (PGs) to study the inhibition of leukocyte aggregation in an inflammatory model23–26 for a long time. Annexin A1 is particularly abundant in neutrophils,27 but not abundant in lymphocytes.28–30 Annexin A1 is mainly distributed in the cytoplasm and accounts for about 2–4% of the cytoplasmic protein.31 A small amount of Annexin A1 is also found in the nucleus,32 but the Annexin A1 protein is mobilized to the cell surface when the cell is activated.27 Furthermore, Annexin A1 can also stably or reversibly bind to cytoskeletal proteins, regulating the interaction of cells with extracellular matrix.31 In this review, we mainly focus on the pathological role of Annexin A1 in inflammation and tumorigenesis.
Structure of Annexin A1
The Annexin A1 gene is located on human chromosome 9q21.13,33 which consists of a C-terminal core region and a uniquely functional N-terminal region with a molecular weight of 37 kDa.34
The C-terminal core region of Annexin A1 is composed of four homologous repeats of about 70 amino acids, and each domain consists of five α-helices that tightly compress the repeats to form a slightly curved disc-like structure.35 The four sets of repeats are arranged in a periodic manner; that is, repeats I, IV, II, and III sequentially form a structurally stable and hydrolysis-resistant compact structure by hydrophobic interaction.36–39 This core region contains multiple calcium-binding sites that bind to phospholipids in a calcium-dependent manner.36
The N-terminal domain of Annexin A1 is composed of 44 amino acids, in which the first 26 amino acids form two α-helices of Ala2-Asn16 and Glu18-Lys26 with 60° reverse tilt on Glu.17 The non-structural peptide Ser27-Asn43 plays a crucial role in linking the N-terminal region to the core region.36 Hall et al40 found that Thr,24 Ser,27 Ser,28 and Thr41 have phosphorylation sites of protein kinase C (PKC) by MS/MS. Tyr21 is the phosphorylation site of epidermal growth factor receptor kinase, Ser5 is the phosphorylation site of TRMP7, and there are glycosylation, acetylation, acrylation, and proteolytic sites on other amino residues.41,42 This post-translational modification of Annexin A1 allows Annexin A1 to be involved in the regulation of various pathophysiological processes both inside and outside the cell. The N-terminus of Annexin A1 has a similar region to the SH2 recognition domain, which may form a protein complex with a protein containing the SH2 domain and participate in intracellular signaling.43 S100A11 (also named S100C)44 is a calcium-binding protein with a molecular weight of 10 kDa, which has the ability to change the properties of Annexin A1 through binding to the N-terminal 10–14 amino acid residue of Annexin A1.1 Annexin A1 has been described as a protein with membrane aggregating properties, in which the core region of Annexin A1 binds to the cell membrane mediated by calcium ion, while the exposed N-terminus has three modes of action: 1) interaction of the exposed N-terminus with a second bilayer; 2) dimerization of two such Annexin A1 via their exposed N-terminus; 3) linking of the concave faces of two such Annexin A1 molecules via an S100A11 dimer.36,45 The pattern structure diagram of Annexin A1 was shown in Figure 1.
Functions of Annexin A1
The role of Annexin A1 in inflammation
Inflammation is a defense response that occurs when the body’s tissues are damaged, and plays an important role in restoring tissue homeostasis,46,47 as well as inflammation has been recognized as a hallmark of cancer.48 In general, inflammation is beneficial to the body; however, when inflammation is uncontrollable or cannot be eliminated, it can also cause further damage to the body involved in many kinds of chronic diseases including asthma,49 lung injury,50 ischemia-reperfusion injury,51 atherosclerosis,52 multiple sclerosis,53 rheumatoid arthritis,54 rhinitis,55 immune dysregulation,56 and cancer.48,57
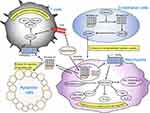
Annexin A1 exhibits anti-inflammatory and pro-inflammatory effects in a variety of inflammatory experimental models28,58 (summarized in Table 1), and its anti-inflammatory effects are mainly regulated by formyl peptide receptor family (FPRs) signaling pathways. FPRs are G-protein coupled receptors (GPCRs) with seven transmembrane structures, which are composed of FPR1 (FPR, NFPR, FMLP, FMLPR), FPR2/ALX (FPRL1, FPRH1, RFP, LXA4R, ALXR, HM63, FMLPX, FPR2A), and FPR3 (FPRL2, FPRH2, FMLPY).59 A large number of agonists (ANXA1, Ac2-26, mitochondrial formyl peptide, LL-37, lipoxin A4, MMWLL, AG-14, etc.) and antagonists (CHIPS, FLIPr, CsA, CDCA, etc.)59–61 can bind to FPRs. Annexin A1 and its N-terminal active peptide fragment Ac2-2629 bind to FPRs and initiate a downstream cascade of FPRs, promoting phosphorylation of extracellular regulated kinase (ERK) and mitogen-activated protein kinase (MAPK), thereby stimulating physiological effects.62–67 Annexin A1 and Ac2-26 exert anti-inflammatory effects on myocardial and cerebral ischemia-reperfusion injury by FPR2/ALX.68,69 Glucocorticoids are the first class of endogenous anti-inflammatory mediators that have been successfully used in therapy.70,71 It has been found that the synthesis and function of Annexin A1 are regulated by glucocorticoids,72,73 which inhibits the expression of pro-inflammatory cytokine IL-6 and TNF.74,75 Annexin A1 was released in neutrophils and macrophages by autocrine or paracrine means after glucocorticoid induction.28 Annexin A1 binds to FPR2/ALX and regulates ERK/MAPK signaling pathway which affects the activities of the downstream transcription factors AP1, NF-κB, and NFAT, thereby regulating the activity, proliferation, and differentiation of T cells and exerting corresponding anti-inflammatory effects, in contrast to the regulative effects of glucocorticoids on T cell receptors (TCR).28 Annexin A1 inhibits phospholipase A2 activity,14 prevents the formation of inflammatory precursors of arachidonic acid,76 induces the formation of anti-inflammatory factors, and inhibits the formation of COX-2 and nitric oxide synthase,77,78 inhibits neutrophil activity and migration, inhibits the synthesis and release of inflammatory factors. Moreover, the externalization of Annexin A1 provides a failure safety mechanism to promote the clearance of apoptotic cells and inhibit the secretion of pro-inflammatory factors by macrophages.79 The anti-inflammatory role of high-density lipoprotein was mediated through up-regulating Annexin A1 in vascular endothelial cells.80 Collagen IV (Col IV)–targeted nanoparticles (NPs) containing Ac2-26 prevent or attenuate inflammatory responses against advanced atherosclerosis in hypercholesterolemic mice.81 Annexin A1, which interacts with the FPR family, may have a significant role in mitigating ischemia-reperfusion injury associated complications.82 Annexin A1, Ac2-26, lipoxin A4, and ATL (15-epi-lipoxin A4) played a positive role in the return from inflammation.83–85 These anti-inflammatory mediators act at different stages of the inflammatory response and are involved in impeding leukocyte aggregation, inhibiting cytokine release, promoting apoptosis, stimulating autophagy, and vascular permeability deterioration84,85 through FPRs.60,86
 | Table 1 Anti-inflammatory and pro-inflammatory roles of Annexin A1 |
Conversely, Annexin A1 also has a pro-inflammatory effect in certain inflammations. Annexin A1 can be phosphorylated by PKC and is subsequently translocated to the nucleus of BV-2 microglial cells after oxygen-glucose deprivation/reoxygenation, resulting in the induction of pro-inflammatory cytokines.87 Annexin A1 fragment (33 kDa), formed by proteolytic hydrolysis of calpain 1 at the N-terminus of Annexin A1 (37 kDa), can activate ERK1/2 signaling activity in endothelial cells and increase the accumulation of intracellular adhesion molecule 1 (ICAM1) around neutrophils, which allows neutrophils to be immobilized on endothelial cells to enhance the transendothelial migration capacity of neutrophils.16 The N-terminal peptide WKYMVm-NH2 of Annexin A1 promotes viral replication and enhances the inflammatory response by activating FPR2/ALX in influenza A virus.88 The mechanisms of Annexin A1 involved in inflammation response are summarized in Figure 2.
The role of Annexin A1 in tumorigenesis
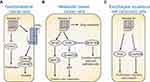
Recent studies have shown that the expression of Annexin A1 in tumors is tissue specific. Annexin A1 is highly expressed in colorectal cancer,89 lung adenocarcinoma,90 pancreatic cancer,91 liver cancer,92 and glioma,93 down-regulation or deletion in cervical cancer,94 thyroid cancer,95 laryngeal cancer,96 prostate cancer,97 head and neck cancer.98 In addition, the expression of Annexin A1 in gastric cancer,77,99–101 breast cancer,102,103 esophageal cancer,104–106 cholangiocarcinoma77,107–109 is still controversial. The mechanism of Annexin A1 involved in tumorigenesis is summarized in Figure 3.
Annexin A1 and gastrointestinal cancer
The expression of Annexin A1 was analyzed from 1,072 Chinese gastric cancer patients using immunohistochemistry, which showed that complete loss of Annexin A1 expression was observed in 691 (64%) of the 1,072 primary tumors and 146 (86%) of 169 nodal metastases correlated significantly with poor survival rates.99 Annexin A1 was widely expressed in adult gastrointestinal tissue.77 Exogenous overexpression of Annexin A1 significantly inhibited growth rate, colony formation, and migration ability, while interference with Annexin A1 by shRNA increased the viability of N87 cells, indicating the growth inhibition effect of Annexin A1 in gastrointestinal cancer. The negative correlation between Annexin A1 and COX-2 (cyclooxygenase-2) indicated that Annexin A1 can regulate COX-2 production to inhibit cell proliferation of gastrointestinal cancer.77 However, Cheng et al showed that the high expression of Annexin A1 is significantly associated with stage IV disease, peritoneal metastasis, and serosal invasion of gastric cancer, and the high expression of Annexin A1 is an independent risk factor for poor overall survival of gastric cancer patients, which can promote the migration and invasion of gastric cancer cells. They also found that FPR1, FPR2/ALX, and FPR3 were up-regulated and increased phosphorylation of ERK1/2 and ITGB1BP1, indicating that Annexin A1 activates ERK/ITGB1BP1 signaling pathway through FPRs to induce gastric cancer cell invasion.100 In gastric and colon cancer, up-regulated Annexin A1 expression is involved in cancer invasion and lymph node metastasis implicated in poor prognosis of patients.110
Annexin A1 and breast cancer
The expression of Annexin A1 is reduced in primary breast cancer but it is significantly elevated in metastatic breast cancer.103 Annexin A1 is heterogeneously expressed in benign epithelium and is lost in both in situ carcinoma and invasive carcinoma, indicating a possible role for Annexin A1 in the early events of malignant transformation.111 Annexin A1 can induce the epithelial-to-mesenchymal transition (EMT) of tumor cells by activating the TGFβ pathway, thereby enhancing the mobility and invasiveness of tumor cells in breast cancer.112 Annexin A1 has the ability to increase the Smad2 phosphorylation induced by TGFβ and to increase Smad3/Smad4 transcription in the MCF7 cell line, whereas the TGFβ inhibitor SB-431542 can transform tumor cells back into epithelial cells. Interference of Annexin A1 in breast cancer cells reduces the metastasis of MTLn3 and 4T1 cells and impaires the TGFβ/Smad signaling pathway.113 Annexin A1 is a constitutive activator of NF-κB, which can increase the expression of MMP-9 by activating NF-κB,114 thereby promoting the invasion and metastasis of breast cancer cells.115,116 Metastasis and invasion of breast cancer cells cause membrane damage and activate the plasma membrane repair system, which induces the formation of Annexin A2-S100A11 complex to promote actin aggregation to repair the plasma membrane and remove the lesion membrane marked by Annexin A1.117–119 Epidermal growth factor receptor (EGFR) activity is closely related to breast cancer progression, and Annexin A1 and Annexin A2 are mediators of EGFR endocytosis.120 Anti-Annexin A2 antibody can suppress EGFR tyrosine phosphorylation and endocytosis, as well as inhibit EGFR-dependent PI3K-AKT and Raf-MEK-ERK downstream pathways to reduce cell proliferation and migration.121 Anti-Annexin A2 antibody also prevents growth of human breast cancer xenograft by inhibiting neoangiogenesis.122 Annexin A1 also stimulates drug resistance in breast cancer cells.123 Therefore, Annexin A1 may play a multifaceted role in breast cancer development, progression, and metastases.102
Annexin A1 and esophageal cancer
The expression level of Annexin A1 is high in normal esophageal epithelium and down-regulation in esophageal squamous cell carcinoma.124 The down-regulation of Annexin A1 was further confirmed by other groups in esophageal squamous cell carcinoma by mRNA detection and immunohistochemistry, and the increased expression of Annexin A1 is consistent with the higher degree of tumor differentiation.104,106 However, Wang et al found that the expression of Annexin A1 was higher in adenocarcinoma at the esophagus and esophagogastric junction.105 The increased expression of Annexin A1 promotes the proliferation, migration, and invasion of esophageal squamous cell carcinoma by up-regulating the expression of Snail and down-regulating the expression of E-cadherin, indicating that Annexin A1 can regulate the metastasis and invasion of esophageal squamous cell carcinoma through the Snail/E-cadherin pathway.125
Prospects
Annexin A1 was first identified as an anti-inflammation factor. Now, evidence showed that Annexin A1 has two-sided effects of anti-inflammatory and pro-inflammatory through different molecular mechanisms. It is intriguing that more than 20 years of study on the roles of Annexin A1 in cancers have not provided a detailed understanding of its roles and mechanisms in various cancers, even in same cancer. Annexin A1 has been described as a “double-face” protein, because of its numerous, diverse, and sometimes opposing functions. The roles of Annexin A1 in inflammation and cancers are vacant depending on its different distribution among cytoplasm, nucleus, and cell surface. In this review, we have summarized the functional progress of Annexin A1 in inflammation and cancers, although there are not many remarkable achievements in recent years. Thus, it is urgent to further investigate the roles and mechanisms of Annexin A1 involved in inflammation and cancers, as well as other diseases to complete understanding of Annexin A1 pathophysiological involvements, which could lead to new models and therapeutic approaches in treating various diseases related to Annexin A1.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81770176), the New Century 151 Talent Project of Zhejiang Province, the 521 Talent Foundation of Zhejiang Sci-Tech University.
Disclosure
The authors declare no competing interests exist in this work.
References
1. Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–371. doi:10.1152/physrev.00030.2001
2. Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197(1):63–93.
3. Seaton BA, Dedman JR. Annexins. BioMetals. 1998;11(4):399–404.
4. Clark GB, Morgan RO, Fernandez MP, Roux SJ. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012;196(3):695–712. doi:10.1111/j.1469-8137.2012.04308.x
5. Smith PD, Moss SE. Structural evolution of the annexin supergene family. Trends Genet. 1994;10(7):241–246.
6. Crompton MR, Moss SE, Crumpton MJ. Diversity in the lipocortin/calpactin family. Cell. 1988;55(1):1–3.
7. Creutz CE. The annexins and exocytosis. Science. 1992;258(5084):924–931.
8. Gruenberg J, Emans N. Annexins in membrane traffic. Trends Cell Biol. 1993;3(7):224–227.
9. Demange P, Voges D, Benz J, et al. Annexin V: the key to understanding ion selectivity and voltage regulation? Trends Biochem Sci. 1994;19(7):272–276.
10. Pollard HB, Rojas E. Ca2+-activated synexin forms highly selective, voltage-gated Ca2+ channels in phosphatidylserine bilayer membranes. Proc Natl Acad Sci U S A. 1988;85(9):2974–2978.
11. Rojas E, Pollard HB, Haigler HT, Parra C, Burns AL. Calcium-activated endonexin II forms calcium channels across acidic phospholipid bilayer membranes. J Biol Chem. 1990;265(34):21207–21215.
12. Moss SE. Annexins taken to task. Nature. 1995;378(6556):446–447. doi:10.1038/378446a0
13. Flower RJ. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988;94(4):987–1015.
14. Perretti M, Solito E. Annexin 1 and neutrophil apoptosis. Biochem Soc Trans. 2004;32(Pt3):507–510. doi:10.1042/BST0320507
15. Alldridge LC, Bryant CE. Annexin 1 regulates cell proliferation by disruption of cell morphology and inhibition of cyclin D1 expression through sustained activation of the ERK1/2 MAPK signal. Exp Cell Res. 2003;290(1):93–107.
16. Williams SL, Milne IR, Bagley CJ, et al. A proinflammatory role for proteolytically cleaved Annexin A1 in neutrophil transendothelial migration. J Immunol. 2010;185(5):3057–3063. doi:10.4049/jimmunol.1000119
17. Guo C, Liu S, Sun MZ. Potential role of Anxa1 in cancer. Future Oncol. 2013;9(11):1773–1793. doi:10.2217/fon.13.114
18. Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278(5703):456–459.
19. Blackwell GJ, Carnuccio R, Di Rosa M, Flower RJ, Parente L, Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980;287(5778):147–149.
20. Rothhut B, Russo-Marie F, Wood J, Di Rosa M, Flower RJ. Further characterization of the glucocorticoid-induced antiphospholipase protein “renocortin”. Biochem Biophys Res Commun. 1983;117(3):878–884.
21. Hirata F, del Carmine R, Nelson CA, et al. Presence of autoantibody for phospholipase inhibitory protein, lipomodulin, in patients with rheumatic diseases. Proc Natl Acad Sci U S A. 1981;78(5):3190–3194.
22. Wallner BP, Mattaliano RJ, Hession C, et al. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986;320(6057):77–81. doi:10.1038/320077a0
23. Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120(6):1075–1082. doi:10.1038/sj.bjp.0701029
24. Bandeira-Melo C, Bonavita AG, Diaz BL, et al. A novel effect for annexin 1-derived peptide ac2-26: reduction of allergic inflammation in the rat. J Pharmacol Exp Ther. 2005;313(3):1416–1422. doi:10.1124/jpet.104.080473
25. Babbin BA, Laukoetter MG, Nava P, et al. Annexin A1 regulates intestinal mucosal injury, inflammation, and repair. J Immunol. 2008;181(7):5035–5044.
26. Souza DG, Fagundes CT, Amaral FA, et al. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179(12):8533–8543.
27. Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2(11):1259–1262.
28. Perretti M, D‘Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70. doi:10.1038/nri2470
29. Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158(4):936–946. doi:10.1111/j.1476-5381.2009.00483.x
30. Mulla A, Leroux C, Solito E, Buckingham JC. Correlation between the antiinflammatory protein annexin 1 (lipocortin 1) and serum cortisol in subjects with normal and dysregulated adrenal function. J Clin Endocrinol Metab. 2005;90(1):557–562. doi:10.1210/jc.2004-1230
31. Perretti M, Flower RJ. Annexin 1 and the biology of the neutrophil. J Leukoc Biol. 2004;76(1):25–29. doi:10.1189/jlb.1103552
32. Perretti M, Christian H, Wheller SK, et al. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 2000;24(3):163–174. doi:10.1006/cbir.1999.0468
33. Rodrigo JP, Garcia-Pedrero JM, Fernandez MP, Morgan RO, Suarez C, Herrero A. Annexin A1 expression in nasopharyngeal carcinoma correlates with squamous differentiation. Am J Rhinol. 2005;19(5):483–487.
34. Perez DA, Vago JP, Athayde RM, et al. Switching off key signaling survival molecules to switch on the resolution of inflammation. Mediators Inflamm. 2014;2014:829851. doi:10.1155/2014/829851
35. Liemann S, Huber R. Three-dimensional structure of annexins. Cell Mol Life Sci. 1997;53(6):516–521.
36. Rosengarth A, Gerke V, Luecke H. X-ray structure of full-length annexin 1 and implications for membrane aggregation. J Mol Biol. 2001;306(3):489–498. doi:10.1006/jmbi.2000.4423
37. Glenney JR
38. Johnsson N, Nguyen Van P, Soling HD, Weber K. Functionally distinct serine phosphorylation sites of p36, the cellular substrate of retroviral protein kinase; differential inhibition of reassociation with p11. Embo J. 1986;5(13):3455–3460.
39. Boudhraa Z, Bouchon B, Viallard C, D‘Incan M, Degoul F. Annexin A1 localization and its relevance to cancer. Clin Sci (Lond). 2016;130(4):205–220. doi:10.1042/CS20150415
40. Hall SC, Smith DM, Masiarz FR, et al. Mass spectrometric and Edman sequencing of lipocortin I isolated by two-dimensional SDS/PAGE of human melanoma lysates. Proc Natl Acad Sci U S A. 1993;90(5):1927–1931.
41. D‘Acunto CW, Gbelcova H, Festa M, Ruml T. The complex understanding of Annexin A1 phosphorylation. Cell Signal. 2014;26(1):173–178. doi:10.1016/j.cellsig.2013.09.020
42. Schlaepfer DD, Haigler HT. In vitro protein kinase C phosphorylation sites of placental lipocortin. Biochemistry. 1988;27(12):4253–4258.
43. Monastyrskaya K, Babiychuk EB, Draeger A. The annexins: spatial and temporal coordination of signaling events during cellular stress. Cell Mol Life Sci. 2009;66(16):2623–2642. doi:10.1007/s00018-009-0027-1
44. Rety S, Osterloh D, Arie JP, et al. Structural basis of the Ca(2+)-dependent association between S100C (S100A11) and its target, the N-terminal part of annexin I. Structure. 2000;8(2):175–184.
45. Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21(4):968–975. doi:10.1096/fj.06-7464rev
46. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771–776.
47. Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi:10.1038/sigtrans.2017.23
48. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309. doi:10.1038/nri.2017.142
49. Chung KF. Inflammatory biomarkers in severe asthma. Curr Opin Pulm Med. 2012;18(1):35–41. doi:10.1097/MCP.0b013e32834d09a5
50. Li Y, Huang X, Huang S, et al. Central role of myeloid MCPIP1 in protecting against LPS-induced inflammation and lung injury. Signal Transduct Target Ther. 2017;2:17066. doi:10.1038/sigtrans.2017.66
51. Eltzschig HK, Eckle T. Ischemia and reperfusion – from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi:10.1038/nm.2507
52. Van-Assche T, Huygelen V, Crabtree MJ, Antoniades C. Gene therapy targeting inflammation in atherosclerosis. Curr Pharm Des. 2011;17(37):4210–4223.
53. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–919. doi:10.1038/ni1507
54. Waldburger JM, Firestein GS. Garden of therapeutic delights: new targets in rheumatic diseases. Arthritis Res Ther. 2009;11(1):206. doi:10.1186/ar2684
55. Mandhane SN, Shah JH, Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int Immunopharmacol. 2011;11(11):1646–1662. doi:10.1016/j.intimp.2011.07.005
56. Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. 2018;11(2):56. doi:10.3390/ph11020056
57. Pearson JRD, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2:17040. doi:10.1038/sigtrans.2017.40
58. Vago JP, Nogueira CR, Tavares LP, et al. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012;92(2):249–258. doi:10.1189/jlb.0112008
59. Ye RD, Boulay F, Wang JM, et al. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61(2):119–161. doi:10.1124/pr.109.001578
60. Chiang N, Serhan CN, Dahlen SE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463–487. doi:10.1124/pr.58.3.4
61. Li Y, Ye D. Molecular biology for formyl peptide receptors in human diseases. J Mol Med (Berl). 2013;91(7):781–789. doi:10.1007/s00109-013-1005-5
62. Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107(5):2123–2130. doi:10.1182/blood-2005-08-3099
63. Odobasic D, Jia Y, Kao W, et al. Formyl peptide receptor activation inhibits the expansion of effector T cells and synovial fibroblasts and attenuates joint injury in models of rheumatoid arthritis. Int Immunopharmacol. 2018;61:140–149. doi:10.1016/j.intimp.2018.05.028
64. Jia Y, Morand EF, Song W, Cheng Q, Stewart A, Yang YH. Regulation of lung fibroblast activation by Annexin A1. J Cell Physiol. 2013;228(2):476–484. doi:10.1002/jcp.24156
65. Marmorato MP, Gimenes AD, Andrade FEC, Oliani SM, Gil CD. Involvement of the Annexin A1-Fpr anti-inflammatory system in the ocular allergy. Eur J Pharmacol. 2018;842:298–305. doi:10.1016/j.ejphar.2018.11.008
66. Dalli J, Consalvo AP, Ray V, et al. Proresolving and tissue-protective actions of Annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J Immunol. 2013;190(12):6478–6487. doi:10.4049/jimmunol.1203000
67. McArthur S, Gobbetti T, Kusters DH, Reutelingsperger CP, Flower RJ, Perretti M. Definition of a novel pathway centered on lysophosphatidic acid to recruit monocytes during the resolution phase of tissue inflammation. J Immunol. 2015;195(3):1139–1151. doi:10.4049/jimmunol.1500733
68. Vital SA, Becker F, Holloway PM, et al. Formyl-peptide receptor 2/3/lipoxin A4 receptor regulates neutrophil-platelet aggregation and attenuates cerebral inflammation: impact for therapy in cardiovascular disease. Circulation. 2016;133(22):2169–2179. doi:10.1161/CIRCULATIONAHA.115.020633
69. Qin C, Yang YH, May L, et al. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharmacol Ther. 2015;148:47–65. doi:10.1016/j.pharmthera.2014.11.012
70. Ronchetti S, Migliorati G, Bruscoli S, Riccardi C. Defining the role of glucocorticoids in inflammation. Clin Sci. 2018;132(14):1529–1543. doi:10.1042/CS20171505
71. Cruz-Topete D, Cidlowski JA. Glucocorticoids: molecular mechanisms of action. In: Riccardi C, Levi-Schaffer F, Tiligada E, editors. Immunopharmacology and Inflammation. Springer; 2018:249–266.
72. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi:10.1056/NEJMra050541
73. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi:10.1210/edrv.21.1.0389
74. Damazo AS, Yona S, D‘Acquisto F, Flower RJ, Oliani SM, Perretti M. Critical protective role for annexin 1 gene expression in the endotoxemic murine microcirculation. Am J Pathol. 2005;166(6):1607–1617. doi:10.1016/S0002-9440(10)62471-6
75. Yang YH, Aeberli D, Dacumos A, Xue JR, Morand EF. Annexin-1 regulates macrophage IL-6 and TNF via glucocorticoid-induced leucine zipper. J Immunol. 2009;183(2):1435–1445. doi:10.4049/jimmunol.0804000
76. Kamal AM, Flower RJ, Perretti M. An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):39–47. doi:10.1590/S0074-02762005000900008
77. Gao Y, Chen Y, Xu D, Wang J, Yu G. Differential expression of ANXA1 in benign human gastrointestinal tissues and cancers. BMC Cancer. 2014;14:520. doi:10.1186/1471-2407-14-520
78. Parente L, Solito E. Annexin 1: more than an anti-phospholipase protein. Inflammation Res. 2004;53(4):125–132. doi:10.1007/s00011-003-1235-z
79. Blume KE, Soeroes S, Keppeler H, et al. Cleavage of Annexin A1 by ADAM10 during secondary necrosis generates a monocytic “find-me” signal. J Immunol. 2012;188(1):135–145. doi:10.4049/jimmunol.1004073
80. Pan B, Kong J, Jin J, et al. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating Annexin A1 in vascular endothelial cells. Biochim Biophys Acta. 2016;1861(6):501–512.
81. Fredman G, Kamaly N, Spolitu S, et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7(275):275ra220. doi:10.1126/scitranslmed.aad3106
82. Ansari J, Kaur G, Gavins FNE. Therapeutic potential of Annexin A1 in ischemia reperfusion injury. Int J Mol Sci. 2018;19(4):1211. doi:10.3390/ijms19041211
83. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi:10.1038/ni1276
84. Trentin PG, Ferreira TP, Arantes AC, et al. Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice. Br J Pharmacol. 2015;172(12):3058–3071. doi:10.1111/bph.13109
85. Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi:10.1146/annurev.immunol.25.022106.141647
86. Gavins FN. Are formyl peptide receptors novel targets for therapeutic intervention in ischaemia-reperfusion injury? Trends Pharmacol Sci. 2010;31(6):266–276. doi:10.1016/j.tips.2010.04.001
87. Zhao B, Wang J, Liu L, et al. Annexin A1 translocates to nucleus and promotes the expression of pro-inflammatory cytokines in a PKC-dependent manner after OGD/R. Sci Rep. 2016;6:27028. doi:10.1038/srep27028
88. Tcherniuk S, Cenac N, Comte M, et al. Formyl peptide receptor 2 plays a deleterious role during influenza a virus infections. J Infect Dis. 2016;214(2):237–247. doi:10.1093/infdis/jiw127
89. Su N, Xu XY, Chen H, et al. Increased expression of Annexin A1 is correlated with K-ras mutation in colorectal cancer. Tohoku J Exp Med. 2010;222(4):243–250.
90. Liu YF, Zhang PF, Li MY, Li QQ, Chen ZC. Identification of Annexin A1 as a proinvasive and prognostic factor for lung adenocarcinoma. Clin Exp Metastasis. 2011;28(5):413–425. doi:10.1007/s10585-011-9380-1
91. Bai XF, Ni XG, Zhao P, et al. Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol. 2004;10(10):1466–1470. doi:10.3748/wjg.v10.i10.1466
92. Masaki T, Tokuda M, Ohnishi M, et al. Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology. 1996;24(1):72–81. doi:10.1053/jhep.1996.v24.pm0008707286
93. Cheng SX, Tu Y, Zhang S. FoxM1 promotes glioma cells progression by up-regulating Anxa1 expression. PLoS One. 2013;8(8):e72376. doi:10.1371/journal.pone.0072376
94. Wang LD, Yang YH, Liu Y, Song HT, Zhang LY, Li PL. Decreased expression of Annexin A1 during the progression of cervical neoplasia. J Int Med Res. 2008;36(4):665–672. doi:10.1177/147323000803600407
95. Petrella A, Festa M, Ercolino SF, et al. Annexin-1 downregulation in thyroid cancer correlates to the degree of tumor differentiation. Cancer Biol Ther. 2006;5(6):643–647.
96. Silistino-Souza R, Rodrigues-Lisoni FC, Cury PM, et al. Annexin 1: differential expression in tumor and mast cells in human larynx cancer. Int J Cancer. 2007;120(12):2582–2589. doi:10.1002/ijc.22639
97. Patton KT, Chen HM, Joseph L, Yang XJ. Decreased annexin I expression in prostatic adenocarcinoma and in high-grade prostatic intraepithelial neoplasia. Histopathology. 2005;47(6):597–601. doi:10.1111/j.1365-2559.2005.02300.x
98. Garcia Pedrero JM, Fernandez MP, Morgan RO, et al. Annexin A1 down-regulation in head and neck cancer is associated with epithelial differentiation status. Am J Pathol. 2004;164(1):73–79. doi:10.1016/S0002-9440(10)63098-2
99. Yu G, Wang J, Chen Y, et al. Tissue microarray analysis reveals strong clinical evidence for a close association between loss of Annexin A1 expression and nodal metastasis in gastric cancer. Clin Exp Metastasis. 2008;25(7):695–702. doi:10.1007/s10585-008-9178-y
100. Cheng TY, Wu MS, Lin JT, et al. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer. 2012;118(23):5757–5767. doi:10.1002/cncr.27565
101. Jorge YC, Mataruco MM, Araujo LP, et al. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediators Inflamm. 2013;2013:152860. doi:10.1155/2013/152860
102. Yom CK, Han W, Kim SW, et al. Clinical significance of Annexin A1 expression in breast cancer. J Breast Cancer. 2011;14(4):262–268. doi:10.4048/jbc.2011.14.4.262
103. Shen D, Nooraie F, Elshimali Y, et al. Decreased expression of Annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37(12):1583–1591. doi:10.1016/j.humpath.2006.06.001
104. Hu N, Flaig MJ, Su H, et al. Comprehensive characterization of annexin I alterations in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10(18 Pt 1):6013–6022. doi:10.1158/1078-0432.CCR-04-0317
105. Wang KL, Wu TT, Resetkova E, et al. Expression of Annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin Cancer Res. 2006;12(15):4598–4604. doi:10.1158/1078-0432.CCR-06-0483
106. Han G, Tian Y, Duan B, Sheng H, Gao H, Huang J. Association of nuclear Annexin A1 with prognosis of patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(2):751–759.
107. Wang AG, Yoon SY, Oh JH, et al. Identification of intrahepatic cholangiocarcinoma related genes by comparison with normal liver tissues using expressed sequence tags. Biochem Biophys Res Commun. 2006;345(3):1022–1032. doi:10.1016/j.bbrc.2006.04.175
108. Wang D, Zhang H, Fang Z, Yu G. Annexin-1 downregulation is associated with clinical outcome in Chinese patients with hilar cholangiocarcinoma. Eur Surg Res. 2010;45(3–4):151–157. doi:10.1159/000320237
109. Hongsrichan N, Rucksaken R, Chamgramol Y, et al. Annexin A1: a new immunohistological marker of cholangiocarcinoma. World J Gastroenterol. 2013;19(16):2456–2465. doi:10.3748/wjg.v19.i16.2456
110. Sato Y, Kumamoto K, Saito K, et al. Up-regulated Annexin A1 expression in gastrointestinal cancer is associated with cancer invasion and lymph node metastasis. Exp Ther Med. 2011;2(2):239–243. doi:10.3892/etm.2011.210
111. Cao Y, Li Y, Edelweiss M, et al. Loss of Annexin A1 expression in breast cancer progression. Appl Immunohistochem Mol Morphol. 2008;16(6):530–534. doi:10.1097/PAI.0b013e31817432c3
112. Maschler S, Gebeshuber CA, Wiedemann EM, et al. Annexin A1 attenuates EMT and metastatic potential in breast cancer. EMBO Mol Med. 2010;2(10):401–414. doi:10.1002/emmm.201000095
113. de Graauw M, van Miltenburg MH, Schmidt MK, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci U S A. 2010;107(14):6340–6345. doi:10.1073/pnas.0913360107
114. Kang H, Ko J, Jang SW. The role of Annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun. 2012;423(1):188–194. doi:10.1016/j.bbrc.2012.05.114
115. Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8(2):395–405.
116. Duffy MJ, Maguire TM, Hill A, McDermott E, O‘Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2(4):252–257.
117. Jaiswal JK, Lauritzen SP, Scheffer L, et al. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat Commun. 2014;5:3795. doi:10.1038/ncomms5972
118. Jaiswal JK, Nylandsted J. S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle. 2015;14(4):502–509. doi:10.1080/15384101.2014.995495
119. Lauritzen SP, Boye TL, Nylandsted J. Annexins are instrumental for efficient plasma membrane repair in cancer cells. Semin Cell Dev Biol. 2015;45:32–38. doi:10.1016/j.semcdb.2015.10.028
120. de Graauw M, Cao L, Winkel L, et al. Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. 2014;33(20):2610–2619. doi:10.1038/onc.2013.219
121. Chaudhary P, Thamake SI, Shetty P, Vishwanatha JK. Inhibition of triple-negative and herceptin-resistant breast cancer cell proliferation and migration by annexin A2 antibodies. Br J Cancer. 2014;111(12):2328–2341. doi:10.1038/bjc.2014.542
122. Sharma M, Blackman MR, Sharma MC. Antibody-directed neutralization of annexin II (ANX II) inhibits neoangiogenesis and human breast tumor growth in a xenograft model. Exp Mol Pathol. 2012;92(1):175–184. doi:10.1016/j.yexmp.2011.10.003
123. Wang Y, Serfass L, Roy MO, Wong J, Bonneau AM, Georges E. Annexin-I expression modulates drug resistance in tumor cells. Biochem Biophys Res Commun. 2004;314(2):565–570.
124. Xia SH, Hu LP, Hu H, et al. Three isoforms of annexin I are preferentially expressed in normal esophageal epithelia but down-regulated in esophageal squamous cell carcinomas. Oncogene. 2002;21(43):6641–6648. doi:10.1038/sj.onc.1205818
125. Han G, Lu K, Huang J, et al. Effect of Annexin A1 gene on the proliferation and invasion of esophageal squamous cell carcinoma cells and its regulatory mechanisms. Int J Mol Med. 2017;39(2):357–363. doi:10.3892/ijmm.2016.2840
126. Patel HB, Kornerup KN, Sampaio AL, et al. The impact of endogenous Annexin A1 on glucocorticoid control of inflammatory arthritis. Ann Rheum Dis. 2012;71(11):1872–1880. doi:10.1136/annrheumdis-2011-201180
127. Paschalidis N, Iqbal AJ, Maione F, et al. Modulation of experimental autoimmune encephalomyelitis by endogenous Annexin A1. J Neuroinflammation. 2009;6:33. doi:10.1186/1742-2094-6-1
128. Locatelli I, Sutti S, Jindal A, et al. Endogenous Annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology. 2014;60(2):531–544. doi:10.1002/hep.27141
129. Perretti M. Editorial: to resolve or not to resolve: Annexin A1 pushes resolution on track. J Leukoc Biol. 2012;92(2):245–247. doi:10.1189/jlb.0312128
130. Leoni G, Neumann PA, Kamaly N, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125(3):1215–1227. doi:10.1172/JCI76693
131. Saito N, Qiao H, Yanagi T, et al. An Annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014;6(245):245ra295. doi:10.1126/scitranslmed.3008227
132. D‘Acquisto F, Paschalidis N, Raza K, Buckley CD, Flower RJ, Perretti M. Glucocorticoid treatment inhibits annexin-1 expression in rheumatoid arthritis CD4+ T cells. Rheumatology (Oxford). 2008;47(5):636–639. doi:10.1093/rheumatology/ken062
133. Butcher MJ, Galkina EV. wRAPping up early monocyte and neutrophil recruitment in atherogenesis via Annexin A1/FPR2 signaling. Circ Res. 2015;116(5):774–777. doi:10.1161/CIRCRESAHA.115.305920
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.