Back to Journals » Research and Reports in Urology » Volume 11
Adult-type granulosa cell tumor of the testicle: case report
Authors Halalsheh O, Al-Mohtaseb A , Al-Karasneh AI, Al-Janabi MM, Hallak AH
Received 13 March 2019
Accepted for publication 23 May 2019
Published 24 June 2019 Volume 2019:11 Pages 189—193
DOI https://doi.org/10.2147/RRU.S208556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Omar Halalsheh,1 Alia Al-Mohtaseb,2 Anas Ibrahim Al-Karasneh,3 Mustafa Mokhalad Al-Janabi,3 Amer Hussein Hallak4
1Department of Surgery and Urology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Pathology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 3Urology, King Abdullah University Hospital, Ar Ramtha, Jordan; 4Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
Abstract: Granulosa cell tumor (GCT) is a sex-cord neoplasm of the gonads classified into either juvenile (jGrCT) or adult type (aGrCT). It is commonly arising in ovaries but is much rarer in men, with only around 50 male cases previously reported in the literature. We report on a 54-year-old male patient with a right testicular GCT measuring 10.0×8.0×6.0 cm. The tumor was treated successfully with radical orchiectomy followed by computed tomography to assess lymph node involvement, and no further treatment was done. Pathological reports showed diffuse positivity for immunohistochemical stains, inhibin, vimentin, calretinin, and CD99. The clinical and histopathological features, treatment, and prognosis of aGrCT arising in the testicle of an adult male are also reviewed in this manuscript.
Keywords: granulosa cell tumor, testicular granulosa, tumor markers, sex-cord stromal tumors
Background
The first testicular granulosa cell tumor (GCT) was reported by Laskowski et al in 1952.1 From that time on, two distinct types of testicular GCT have been identified in men: the juvenile type (jGrCT) and the adult type (aGrCT). The juvenile type, mostly presenting in the first 6 months of life, which is one of the most common congenital testicular tumors that habitually follows a benign behavior.1 The adult type, on the other hand, is so unique that only around 50 cases of aGrCT have been reported up to date.1–15 It usually follows an unpredictable clinical course, occurring at any time after puberty. Several methods of morphological, clinical and immunohistochemical assessment have been developed throughout the years to aid in the diagnosis and classification of aGrCT.
The aGrCT presents clinically as a painless, slow-growing neoplasm over an inconstant period of time and averaging an imprecise 5.4-years of enlargement.4 Moreover, it has the potential to metastasize after the initial diagnosis is made.1,2 The prevalence age ranges from 16 to 77 years and is often above 50 years old.2–4 Gynecomastia in 25% of cases decreased libido and erectile dysfunction that may be associated with symptoms generated by hormonal abnormalities, such as estrogen hypersecretion.2–5
Case report
A 54-year-old male patient with a known history of controlled type 2 diabetes mellitus, non-smoker, presented to a university hospital with a 10-year history of painless right hemi-scrotal swelling that increased in size with time. Written informed consent has been provided by the patient to have the case details and any accompanying images published. However, no institutional approval was required to publish the case details. The assessment also revealed a decreased libido and mild sexual dysfunction particularly in the last 4 months prior to presentation. There was no past history of trauma, infection, lower urinary tract symptoms, low back pain, or respiratory symptoms.
The patients’ vital signs were within normal limits, and his physical examination was remarkable for a swelling in the right hemi-scrotum with a palpable hard mass on the right testicle. The left hemi-scrotum was unremarkable with a normal epididymis and testicle. Basic laboratory tests and tumor markers were within normal limits; alpha-fetoprotein (AFP): 2.44 ng/mL, beta-human chorionic gonadotropin: 0.1 mIU/mL, lactate dehydrogenase: 310 U/L. Ultrasound examination and computed tomography revealed a tumor with multiple cysts of different sizes limited to the right testicle [Figure 1]. Therefore, standardized management of radical orchiectomy was decided. The patient was operated upon, and right total orchiectomy was performed with no immediate complications. His post-operational course was uneventful. The patient was allowed to go home on post-operative day 1 and was followed up in the outpatient clinic on monthly basis for 2 months, then once every 3 months for 1 year.
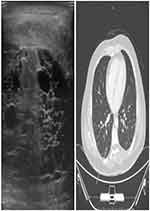 | Figure 1 Ultrasonography of the tumor on the right testicle reveals multiple cysts of variable size. Computed tomography of lung nodule (arrow) sized 5–6 mm in the lower lobe of the right lung. |
Upon follow up, computed tomography demonstrated a 5–6 mm nodule in the lower lobe of the right lung. Radiological reports stated that the nodule was stable, solitary and exhibited no significant signs of metastatic testicular origin. However, follow up of every 3 months was recommended for additional investigations and monitoring [Figure 1].
Pathological findings
Macroscopically, the orchiectomy specimen weighed 377 g with a testicle that measured 8.0×6.5×5.0 cm. The epididymis measured 4.0×3.0 cm and the spermatic cord length was 14.0 cm with a diameter of 2.0 cm. The tumor, sized 10.0×8.0×6.0 cm, was hard and pale on cross-sections with multiple cysts of variable sizes and no necrosis or hemorrhage.
Specimen was received as frozen section and microscopic report exhibited a heterogenous, well-circumscribed nodule that measured 10.0×8.0×6.0 cm with solid, microcystic areas confined to the testicle and not adherent to the tunica vaginalis [Figure 2]. Further assessment deduced a mixed macro-follicular and trabecular pattern of cells distributed in collagenous material. Upon closer inspection, individual cells had an oval-shaped nucleus with a central groove, giving them a coffee bean-like appearance. Cells surrounded areas of amorphous eosinophilic material which seemed like Call-Exner bodies [Figure 3].
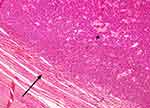 | Figure 2 Tumor lesion 4x. Heterogenous, well-circumscribed tumor confined to the testicle (*) and not adherent to the tunica vaginalis (black arrow). |
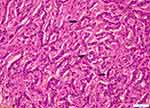 | Figure 3 Tumor lesion 40x. Ovoid tumor cells sometimes with nuclear grooves. Call-Ener bodies between tumor cells. |
For definitive characterization of the tumor, immunohistochemical stains were performed. Immunoprofile showed strong positivity for Inhibin, Calretinin, Vimentin, and CD99 [Figure 4]. Nevertheless, cells were negative for pancytokeratin, CD30, CD117, LCA and PLAP immunohistochemical stains.
Discussion
Granulosa cell tumors are classified under the rare entity of sex-cord stromal tumors. In the ovaries, it commonly arises in the adult form (95%), but the opposite seems to occur in men, where 25% constitute the juvenile form (jGrCT).6 Only around 50 cases have been reported in the literature corresponding to the adult type.1–15
The diagnosis of testicular aGrCT is mostly based on histological and immunohistochemical findings, since the outer appearance and preoperative lab workup are inappreciable. Typically, multiple microscopical growth patterns featuring aGrCT in the testicle are present; macrofollicular, microfollicular, diffuse, insular, trabecular, gyriform, solid, pseudomembranous, or predominantly cystic patterns may exist solely or in combination.1,5,6 Other hallmarks include the presence of Call-Exner bodies, which are cavities filled with hyalinized basement membrane material haphazardly scattered between well-differentiated granulosa cells. Granulosa cells are distinguished by their “coffee-bean nuclei”; oval-shaped nuclei with a central groove, resembling a coffee-bean. One must keep in mind when analyzing immunohistochemistry results that all types of sex-cord stromal tumors, with only a few peculiarities, display reactivity to the same antibodies.8
Exclusion of differential diagnoses for testicular GCT initially starts by classifying the neoplasm in hand into either adult or juvenile type through histopathological investigations. Conversely, the gross appearance of juvenile type is multi-cystic and lacks the histologic criterion the adult type has.1 Moreover, other possibilities like germ cell tumors particularly yolk sac tumor (YST) or fibrothecoma and even metastatic carcinoma need to be taken into consideration. YST of the germ cell tumor category may show a wide variety of growth implementations. However, YST is positive for PLAP on immunostudies and AFP in serum most of the time, albeit it has the potential to be positive for inhibin which may raise suspicion when trying to reach a definitive diagnosis.1–15 Just as any other testicular neoplasm, fibrothecoma in males may present as a painless testicular mass. In addition, signs and symptoms of estrogen hypersecretion such as gynecomastia, decreased libido, and sexual dysfunction may also ensue. Upon histological studies, acellular fibrohyalinized zones with spindle-shaped cells without any discriminant Sertoli, Leydig, or granulosa cell component are used to classify fibrothecoma. Immunohistochemistry of fibrothecoma expresses positivity for smooth muscle actin, CD99, PS100, inhibin, and keratins.7 Metastatic carcinoma, however, favors infiltration into the interstitium between testicular tubules and majority does not form solid tumor masses.8
In 1993, Jiminez-Quintero et al conducted the most comprehensive case series published on sex-cord stromal tumors.10 It was the first to contemplate the histopathological characteristics and features that may predict malignancy. One of which was a size >7.0 cm, others included lymphovascular invasion, necrosis and hemorrhage. On the other hand, Hanson et al reviewed 28 reported cases of GCTs in an attempt to analyze variables and their association with malignancy to conclude that sizes of 5.0 cm or greater were statistically significant to be fearsome.9 Despite the aforementioned studies, the behavior and prognosis of testicular aGrCT are unfortunately still fragmentary owing to the scarcity of reported cases. This is reflected in the fact that the presented case has a large-sized tumor (10.0×8.0×6.0) with no proven malignant complications.
According to Miliaras et al, aGrCT tend to have a comparatively more aggressive deportment than its juvenile counterpart.6 Furthermore, it can cause distal metastasis even after many years. Retroperitoneal lymph nodes are the most common site of metastasis in male cases, but liver, bones, and lungs have also been reported.11,12
As for any solid testicular mass, the standardized initial management is radical orchiectomy. Retroperitoneal lymph nodes dissection (RPLND) has been additionally performed in a minority of cases with lymph node metastasis in testicular GCT.2–4,6,14 Conversely, Mosharafa et al argue that “prophylactic” RPLND is only a triumphant option in early stages of the disease, but not in the higher stages when lymph nodes are already involved; on account of most patients studied died of metastatic disease despite the addition of chemotherapy and radiotherapy.13
In the majority of cases, surgical resection of sex-cord stromal tumors is sufficient as a primary treatment and is regularly curative, with no additional radio/chemotherapeutic intervention necessary.7 However, there seems to be no clear-cut consensus about the regimen of approaching metastatic disease with adjuvant therapy as treatment whether by chemotherapy or radiotherapy. Nonetheless, several cases reported the use of the agents like bleomycin, etoposide, and cisplatin post-operatively.3,11 Jimenez-Quintero et al suggested that etoposide-based chemotherapy with adjuvant radiotherapy may be a curative possibility for widespread disease.10 Interestingly, Harrison et al reported an advanced case of testicular aGrCT fractionally responding to an angiogenesis inhibitor (pazopanib) after initial resistance to cytotoxic chemotherapy.14
Conclusion
This report highlights another case of the aGrCT. Diagnosis of such a tumor has proven to challenge the diagnostic skills of surgical pathologists due to its rarity and difficulty in predicting behavior, prognosis, and management. Therefore, further reporting every case of aGrCT is necessary to allow further analysis and devising a generally agreed regimen for treatment of benign and the possibly complicated metastatic disease.
Disclosure
The authors report no conflicts of interest in regard to this work.
References
1. Song Z, Vaughn DJ, Bing Z. Adult type granulosa cell tumor in adult testis: report of a case and review of the literature. Rare Tumors. 2011;3(4):e37. doi:10.4081/rt.2011.e37
2. Al-Alao O, Gul T, Al-Ani A, Bozom IA, Al-Jalham K. Adult-type granulosa cell tumour of the testis: report of a case and review of the literature. Arab J Urol. 2016;14(1):44–49. doi:10.1016/j.aju.2015.12.002
3. Elbachiri M, Taleb A, Derrabi N, et al. Adult-type granulosa cell tumor of the testis: report of a case and review of literature. Pan Afr Med J. 2017;26:198. doi:10.11604/pamj.2017.26.38.10312
4. Chen W-C, Lin Y-H, Yeh S-D, Wu -C-C. Testicular adult type granulosa cell tumor: a very rare case report and review of literature. Urol Androl Open J. 2016;1(1):12–14. doi:10.17140/UAOJ-1-103
5. Kucukodaci Z, Keles M, Yapanoglu T, Alp BF, Aksoy Y, Ozbey I. Adult type granulosa cell tumor of the testis: case report and review of the literature. Eurasian J Med. 2008;40(1):39–41.
6. Miliaras D, Anagnostou E, Moysides I. Adult type granulosa cell tumor: a very rare case of se-cord tumor of the testis with review of the literature. Case Rep Pathol. 2013;2013:932086.
7. Sourial M, Sabbagh R, Doueik A, Ponsot Y. A 17 year old male with a testicular fibrothecoma: a case report. Diagn Pathol. 2013;8(152). doi:10.1186/1746-1596-8-152
8. Schubert TE, Stoehr R, Hartmann A, Schöne S, Löbelenz M, Mikuz G. Adult type granulosa cell tumor of the testis with a heterologous sarcomatous component: case report and review of the literature. Diagn Pathol. 2014;9:107. doi:10.1186/1746-1596-9-107
9. Hanson JA, Ambaye AB. Adult testicular granulosa cell tumor: a review of the literature for clinicopathologic predictors of malignancy. Arch Pathol Lab Med. 2011;135:143–146. doi:10.1043/2009-0512-RSR.1
10. Jimenez-Quintero LP, Ro JY, Zavala-Pompa A, et al. Granulosa cell tumor of the adult testis: a clinicopathologic study of seven cases and a review of the literature. Hum Pathol. 1993;24(10):1120–1125. doi:10.1016/0046-8177(93)90193-K
11. Hammerich KH, Hille S, Ayala GE, et al. Malignant advanced granulosa cell tumor of the adult testis: case report and review of the literature. Hum Pathol. 2008;39:701–709. doi:10.1016/j.humpath.2007.09.015
12. Suppiah A, Musa MM, Morgan DR, North AD. Adult granulosa cell tumour of the testis and bony metastasis. A report of the first case of granulosa cell tumour of the testicle metastasizing to bone. Urol Int. 2005;75:91–93. doi:10.1159/000085936
13. Mosharafa AA, Foster RS, Bihrle R, et al. Does retroperitoneal lymph node dissection have a curative role for patients with sex cord-stromal testicular tumors? Cancer. 2003;98:753–757. doi:10.1002/(ISSN)1097-0142
14. Harrison MR, Huang W, Liu G, Gee J. Response to antiangiogenesis therapy in a patient with advanced adult-type testicular granulosa cell tumor. Oncology (Williston Park). 2009;23:792–795.
15. Urazan Murcia J, Urazan Murcia J, Sanchez Ochoa M, Vargas Manrique I, Silva Herrera J. Pure yolk sac testicular tumor in an adult patient: case report. Oncology Cancer Case Rep. 2017;03(03). doi:10.4172/2471-8556.1000139
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

