Back to Journals » OncoTargets and Therapy » Volume 9
Administration of polysaccharide from Panax notoginseng prolonged the survival of H22 tumor-bearing mice
Authors Li H, Gu L, Zhong Y, Chen Y, Zhang L, Zhang AR, Sobol RW, Chen T, Li J
Received 17 December 2014
Accepted for publication 7 March 2016
Published 8 June 2016 Volume 2016:9 Pages 3433—3441
DOI https://doi.org/10.2147/OTT.S79427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Huaiyu Li,1,* Longlong Gu,1,2,* Yuanyuan Zhong,1 Yajuan Chen,1 Lei Zhang,1 Annie R Zhang,3 Robert W Sobol,4,5 Tong Chen,1,6 Jianfeng Li4,5
1Yunnan Key Laboratory of Pharmacology for Natural Products, School of Pharmaceutical Sciences, 2Haiyuan College, Kunming Medical University, Kunming, Yunnan, People’s Republic of China; 3Graduate School of Applied and Professional Psychology, Rutgers, The State University of New Jersey, Piscataway, NJ, 4Department of Oncologic Sciences, 5Mitchell Cancer Institute, University of South Alabama, Mobile, AL, USA; 6Yunnan Panax notoginseng (Burk) F.H. Chen Biotechnology and Pharmaceutical Engineering Research Center, Kunming, Yunnan, People’s Republic of China
*These authors contributed equally to this work
Background: Polysaccharides from various sources are being considered potential sources for the treatment of liver cancer. The aim of this study was to investigate the impact of polysaccharide isolated from Panax notoginseng (PPN) on the proliferation of H22 liver cancer cells and the survival of the tumor-bearing mice transplanted with H22 cells.
Materials and methods: Polysaccharide from PPN was added to the culture medium of mouse hepatoma H22 cells at different doses. Cell proliferation was assayed with a standard MTT assay. Survival rates of tumor-bearing mice were recorded. Peripheral blood lymphocytes were assayed by flow cytometry. Serum interleukin-2 levels in peripheral blood were measured by enzyme-linked immunosorbent assay.
Results: Polysaccharide from PPN inhibited the growth of H22 cells and significantly prolonged the survival of tumor-bearing mice. The increase in activated CD4+ T-cells and the elevation of serum interleukin-2 may contribute to the antitumor activity of PPN.
Conclusion: PPN has potential antitumor activity for the treatment of liver cancer.
Keywords: polysaccharide, Panax notoginseng, liver cancer, immunotherapy, IL-2
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide, with an estimated 1 million new cases each year.1 The median survival after diagnosis is approximately 6–20 months,2,3 because HCC cases are usually diagnosed at the late stage. Forty percent of patients with HCC who were diagnosed earilier had a greater chance for potentially curative treatments (resection, transplantation, or local ablation). Twenty percent of the patients are eligible for chemoembolization, and the remaining 40% with unresectable lesions are mainly dependent on systemic therapies.4 Despite recent advances in chemotherapy for HCC, the outcomes of anticancer therapy remain unsatisfactory, especially in terms of survival. Thus, further improvement of therapies on long-term survival and the improvement of the quality of life for HCC patients are urgently needed. Immunotherapy using polysaccharides to enhance host defense responses against tumors is currently receiving great attention as supporting treatment modalities in the management of patients with cancer. Polysaccharides extracted from various sources including higher plants demonstrated profound effects on the immune system and are relatively nontoxic.5 For example, Lentinan, a polysaccharide from Lentinus edodes, has been successfully used in the treatment of various types of cancer.6 Thus, the discovery of polysaccharides with antitumor activity from new sources will broaden the drug choices for HCC immunotherapy.
Panax notoginseng (PPN) (Burk) F.H. Chen (Araliaceae) is a well-known traditional Chinese herbal medicine indigenous to the Wenshan city of the Yunnan province.7 The root of the plant has been used for the treatment of cardiovascular disease, inflammation, various body pains, trauma, internal and external bleeding due to injury, and as a tonic.8 Low molecular weight components, especially the dammarane-type saponins, are believed to be the principal bioactive components of PPN and have been well studied.9–26 The high molecular weight fraction (mainly PPN) was mainly ignored previously and has been attracting more attention due to its immunomodulating activities.27,28 The total polysaccharide fraction has been shown to stimulate murine spleen lymphocyte proliferation in vivo and in vitro and to antagonize the action of the T-cell suppressor, cyclosporin A.28 A fraction with a molecular weight of 1,500 kDa, isolated from the roots of PPN, showed the ability to activate the reticuloendothelial system.29 Gao et al found that the water-soluble high molecular weight fraction induced the production of interferon (IFN)-γ and IFN-α in mouse spleen lymphocytes and peritoneal macrophage cell culture, while the weak alkali-soluble fraction showed anti-complement activity, as well as cytokine induction activity.27 A polysaccharide fraction mainly composed of α (1,4)-glucan (likely to be starch) from the roots of PPN showed cytotoxicity to a leukemia NAML-18 cell line.30 For the best utilization of PPN fractions of PPN, we successfully developed a reliable and an effective method to extract alcohol-insoluble PPN from the industrial residues of notoginsenosides extract.31
In the current study, we applied the purified PPN to H22 hepatoma cells in vitro and tumor-bearing mice in vivo to evaluate the immunomodulatory and antitumor activities of PPN. Our results showed that PPN extended the life span of H22 tumor-bearing mice as well as weakly reduced the proliferation of H22 cells in vitro. These mechanistic studies indicated that PPN enhanced the immune system in vivo. Our studies may provide a novel choice for cancer immunotherapy.
Materials and methods
Reagents and instruments
PPN used in the experiment was prepared by hot water extraction and ethanol deposit as described by Zhao et al.31 The purity of PPN is 56.70%, as determined by the anthrone sulfuric acid colorimetric method. PPN was dissolved in ddH2O to the desired concentration without any precipitation. The pH of the PPN solution is 4.22.
Roswell Park Memorial Institute (RPMI)-1640 medium was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Lentinan was purchased from Chuangli Pharmaceutical Co., Ltd. (Xiangfan, People’s Republic of China). Anti-mouse BIOTIN-CD8, anti-mouse FITC-CD4, anti-mouse PE-CD49b, and anti-mouse antigen-presenting cell (APC)-CD3 were purchased from BD Biosciences (San Jose, CA, USA). The interleukin (IL)-2 enzyme-linked immunosorbent assay (ELISA) Kit was purchased from NeoBioscience Technology Company (Shenzhen, People’s Republic of China).
Experimental animals and cell lines
Female Kunming mice (18–22 g) were provided by the Laboratory Animal Center of Kunming Medical University. H22 hepatoma cells were provided by the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. H22 cells were grown in the RPMI-1640 medium. Cells were maintained at 37°C in a 5% CO2-humidified atmosphere. All experimental procedures were performed in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China. This study, including animal experiments and the use of cell lines, was approved by the Committee of Medical Ethics, National Health Department of China.
Cell viability assay
The cytotoxicity of PPN on H22 cells was measured using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, cells (6×104 cells/well) were seeded into 96-well microtiter plates overnight and treated with PPN (20–2,000 μg/mL) for 24 hours. At the end of the exposure, 20 μL of 5 mg/mL MTT was added to each well and incubated for 4 hours at 37°C, and then the triplex solution (10% sodium dodecyl sulfate, 5% isobutanol and 0.012 M HCl) was added and incubated for 8–12 hours at 37°C. The plates were gently mixed and read in a microplate reader with a test wavelength of 570 nm and a reference wavelength of 630 nm to obtain sample signal (optical density [OD]570–OD630). The cell proliferation of the control group treated with saline was normalized to 100%. The cell inhibitory rate (IR%) was calculated as IR% = (1 - OD of treated/OD of control group) ×100%.32,33
Establishment of H22 solid tumor-bearing mouse model
Under sterile conditions, ascites was extracted from mice 7 days after intraperitoneal inoculation of H22 cells. Based on cell morphology, the ascites was ready for use when the number of tumor cells exceeded 97% of the total. The ascites was diluted to a ratio of 1:3 with sterile saline. Then, 0.2 mL cell suspension (3.3×107 cell/mL) was subcutaneously inoculated into the right armpit region of each Kunming mouse. The longest diameter and perpendicular short diameter of each tumor were recorded every 2 days.
Tumor volume = 0.5× the longest diameter ×the square of short diameter. | (1) |
Because the average time of survival of the control group administered saline was 20.8 days, tumor volumes of each group on the 19th day were plotted as mean ± SD.
PPN administration in H22 tumor-bearing mice
Twenty-four hours after H22 inoculation, the mice were randomly divided into five groups, with each group containing seven to nine mice. The mice of one group were only given the normal saline (NS) and used as the negative control. The mice of another group were treated with Lentinan (247 mg/kg) and used as the positive control, because Lentinan is a polysaccharide with known antitumor activity against colon cancer in a mouse model.34 The mice of the other three treatment groups were orally administrated PPN with low dose (180 mg/kg), medium dose (360 mg/kg), or high dose (720 mg/kg). All the mice were orally administrated with 0.4 mL saline, Lentinan or PPN solutions once a day until the end of the experiment.
Lymphocyte subpopulation analysis
Twenty-four hours after the establishment of the H22 model, the transplanted mice were randomly divided into three groups, with each group containing six mice: the NS group, Lentinan group (the positive control), and the PPN group (360 mg/kg). After 10 days, peripheral blood was collected from the orbital venous plexus of each mouse. The cells were then incubated in red blood cell lysis buffer for 15 minutes to lyse red blood cells. The remaining white blood cells were washed twice with 1× phosphate-buffered saline and resuspended in 1× phosphate-buffered saline. These white blood cells were labeled with fluorescent monoclonal antibodies (anti-mouse BIOTIN-CD8, anti-mouse FITC-CD4, anti-mouse PE-CD49b, and anti-mouse APC-CD3; BD Biosciences) and analyzed with a Partec flow cytometer (Partec, Norderstedt, Germany). The experimental data were analyzed with CELLQuest software.
Analysis of IL-2 expression
Twenty-four hours after the establishment of the H22 cell inoculation, the transplanted mice were randomly divided into three groups, with each group containing six mice: the NS group, Lentinan group, and the PPN (360 mg/kg) group. After 10 days, peripheral blood was collected from the orbital venous plexus. Then, the blood was centrifuged for 10 minutes at 2,500 rpm at 4°C. The sera were collected, and the levels of IL-2 were immediately analyzed using an IL-2 ELISA Kit (NeoBioscience Technology Company) according to the manufacturer’s instructions.
Statistical analysis
The Kaplan–Meier survival curves were generated by PRISM software. Log-rank (Mantel–Cox) test was used for the statistical analysis (P<0.05 was considered statistically significant). For the other analyses, the least significant difference analysis of variance (ANOVA) was used for the significance test. Data were all presented as mean ± SD; one-way ANOVA and comparison among groups were performed using the SPSS 17.0 statistical software and a probability of <0.05 (P<0.05) was considered statistically significant.
Results
PPN reduced the proliferation of H22 cells
To investigate the anti-hepatoma activity of PPN in vitro, PPN extracts from the roots of PPN were added to the cultured H22 cells at different concentrations. The proliferation of H22 cells was then examined using the MTT method 24 hours after adding the PPN solution. As shown, H22 cells showed significantly reduced cell metabolic activity at 200 μg/mL, 500 μg/mL, 1,000 μg/mL, and 2,000 μg/mL PPN treatment (Figure 1), suggesting a reduction in proliferation. However, those high doses (from 0.5 mg/mL to 2 mg/mL) in the H22 cell culture media are still insufficient for half-maximal inhibition of H22 cell proliferation. This suggests that PPN only weakly inhibits H22 cell proliferation in vitro.
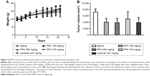
Oral PPN administration showed no obvious toxicity to mice, inhibited tumor growth, and prolonged the survival of H22 tumor-bearing mice
To investigate the anti-hepatoma activity of PPN in vivo, we established a tumor model with subcutaneous inoculation of H22 cells into each Kunming mouse. The mice were treated with different doses of PPN 24 hours after the inoculation of tumor cells. The body weight curves of the saline control and each treatment group showed similar patterns (Figure 2A). No body weight loss was observed while PPN or Lentinan was applied. The result indicates that at those doses, PPN did not show any obvious toxicity. However, the administration of both PPN (at different doses) and Lentinan reduced tumor volumes (Figure 2B) as compared to the control mice. As shown in Figure 3, the median (50%) survival of tumor-bearing mice treated with low-, medium-, and high-dose PPN was 29 days, 37 days, and 32 days, respectively, as compared to the 20 days of the negative control. The administration of medium-dose PPN (360 mg/kg) prolonged the survival of tumor-bearing mice most effectively and extended the average life span of those mice by 85%, as compared to the negative control group. Although the group treated with Lentinan (positive control) showed the longest median survival (39.5 days), there is no statistical difference in survival between the Lentinan and medium-dose PPN (360 mg/kg) treatment group (P=0.3819). However, the Lentinan dose (247 mg/kg) is less compared to the best PPN polysaccharide dose (360 mg/kg). This result suggests that the administration of PPN could prolong the survival of tumor-bearing mice similar to that of Lentinan, with a higher dose. Furthermore, four tumor-bearing mice from the PPN treatment eventually achieved full regression of the tumor. Rechallenge to those mice with the same amount of H22 cells did not result in tumor development (data not shown).
PPN administration significantly increased the number of T-cells, but not the natural killer cells
To investigate the impact of PPN administration on the immune system, the lymphoid cell population in the peripheral blood of tumor-bearing mice was analyzed by flow cytometry as shown in Figure 4A–C. As shown in Figure 4D and E and Table 1, PPN administration significantly increased the CD3+, CD4+ T-cell population in the peripheral blood over that of the negative control group (P<0.05). Lentinan treatment showed a similar effect. Higher numbers of natural killer (NK) cells were also detected in the PPN-administered group than in that of the negative control group, but the difference was not statistically significant (P>0.05; Table 1). There were also no statistically significant differences in CD8+ T-cells between the control and treated groups.
PPN administration significantly increased serum IL-2 level
The elevation of soluble IL-2 in the blood is an important indicator of the activation of lymphocytes. To study the impact of PPN treatment on the activation of T-cells, we evaluated the levels of IL-2 in the blood of the control and treated mice. The serum of the mice with different treatments was collected, and the level of IL-2 was analyzed by ELISA. As shown in Figure 5, the serum levels of IL-2 in the PPN-treated groups were significantly elevated as compared to the NS-treated group (P<0.01) and close to the level of IL-2 in the Lentinan-treated group (positive control).
Discussion
Due to the limited options of current treatments against HCC, immunotherapy has become an important adjunctive treatment when combined with a standard treatment. Studies have shown that the application of different natural or synthetic immunostimulators is a simple way to boost the body’s immune system to inhibit tumor growth.35–38 It was reported that PPN from the root of PPN has the capability to activate many kinds of lymphocytes.27–30 In this study, we investigated the impact of PPN administration using an HCC model in vitro and in vivo using H22 cells. Our in vitro study showed that PPN reduced H22 cell growth in a dose-dependent manner. The medium dose of PPN (1,000 μg/mL) showed the best effect on reducing H22 cell growth. Although the proliferation reduction was significant compared to the control (P<0.05), the high dose of PPN (2,000 μg/mL) only inhibited ~32% of the H22 cell proliferation. This suggests that PPN alone is not sufficient to fully inhibit the H22 cell proliferation in vitro. The administration of PPN to H22 tumor-bearing mice also significantly reduced tumor growth. More importantly, the application of PPN effectively extended the life span of tumor-bearing mice up to 85%. The reduction in growth in vivo was more significant than in vitro, suggesting that other antitumor activity may play a role in enhancing the suppression of tumor growth in vivo. Similar results have been reported previously. For example, Ganoderma lucidum polysaccharides peptide,39 Lentinan,40 polysaccharides of Tremella fuciformis,40 and polysaccharide component from Antrodia camphorata mycelia41 showed no effect on tumor cell proliferation in vitro when they were directly added to culture medium. However, those polysaccharides showed potent antitumor activity in vivo due to different mechanisms of activating cells in the host immune system including cytotoxic T-lymphocytes, T helper cells, B-lymphocytes, activated macrophages, NK cells, or lymphokine-activated killer (LAK) cells. Because PPN shares a similar structure with many other polysaccharides, we also examined the impact of PPN on the host immune defense system after oral administration. The population of CD3+CD4+, CD3+CD8+, and NK cells in peripheral blood was examined after the oral administration of PPN. Our results showed that the CD3+CD4+ cells were significantly increased. CD3+CD8+ and NK cells did not show significant changes (Figure 4D and E and Table 1). Although DX5 monoclonal antibody used to mark NK cells may also detect a subset of T-cells, in our results there was no significant difference between the control and treatment groups marked with this antibody. These results suggest that neither NK cells nor this subset of T-cells contributed to the antitumor activity of PPN.
Besides the action on immune cells, another important function of most polysaccharides is to promote the secretion of cytokines such as IL-2, a major mediator of host defense. IL-2 regulates communication between APCs, lymphocytes, and other host cells during an immune response. After PPN treatment, serum IL-2 levels were significantly increased. IL-2 secreted by T-cells is a primary component of cellular immune function.42 Our results are consistent with the reports on CD4+ T-cell-dependent antitumor activity.43 CD4+ T-cells play a crucial role in the release of soluble immunomodulatory factors such as IL-2 into the tumor microenvironment.43 The activated CD4+ T-cells can also express CD40L to activate APC by CD40–CD40L conjugation leading to the maturation of dendritic cells.43 The similar pattern of increases in CD3+CD4+ and serum IL-2 levels was also seen in mice treated with Lentinan, a well-known immunostimulator, as the positive control.
Recent reports showed that the removal of immune blockades increased the cytotoxicity of immunostimulators and achieved better antitumor effects.44 It will be very interesting to determine whether the antitumor activity of PPN will increase when PPN is combined with agents that mediate immune blockades. We tried to combine cyclophosphamide (suppressing the CD4+CD25+ Treg cells) with PPN to treat the tumor-bearing mice. However, our mice were very sensitive to cyclophosphamide and quickly died after the administration of the lowest dose of cyclophosphamide (as reported in literature) either alone or combined with PPN in different dosages. We plan to test other immune blockades, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) antibody in our future studies.
The administration of the medium dose of PPN (360 mg/kg) resulted in the longest extension of life span of the tumor-bearing mice (by 85%), whereas we observed a 45% extension with the low dose (180 mg/kg) and a 60% extension with the high dose (720 mg/kg). This may suggest that in therapy practice, the PPN dose should be carefully assessed to determine the most appropriate dose.
Conclusion
Our studies demonstrate that PPN administration can effectively prolong the life span of tumor-bearing mice likely by boosting the host immune system as well as weak cytotoxic activity against HCC. PPN therefore may have potential application for the treatment of HCC.
Acknowledgments
This study was supported by the Innovation Fund for Technology-based Firms (12C26115306491), Open Research Fund of Yunnan Key Laboratory of Pharmacology (2013G016), Yunnan Provincial Science and Technology Department FY2014 – Kunming Medical University joint special fund basic research projects. RWS is an Abraham A Mitchell Distinguished Investigator at the Mitchell Cancer Institute. RWS is a scientific consultant for Trevigen, Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. | ||
Shi XJ, Jin X, Wang MQ, et al. Effect of resection following downstaging of unresectable hepatocelluar carcinoma by transcatheter arterial chemoembolization. Chin Med J. 2012;125(2):197–202. | ||
Llovet JM, Di Bisceglie AM, Bruix J, et al; Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. | ||
Lien EJ, Gao H. Higher plant polysaccharides and their pharmacological activities. Int J Oriental Med. 1990;15:123–140. | ||
Lien EJ. Fungal metabolites and Chinese herbal medicine as immunostimulants. Prog Drug Res. 1990;34:395–420. | ||
Zheng GZ, Yang CR. Sanqi Biology and Utilization. Beijing: Science Press; 1994:9–42. | ||
Ma WG, Mizutani M, Malterud KE, Lu SL, Ducrey B, Tahara S. Saponins from the roots of Panax notoginseng. Phytochemistry. 1999;52(6):1133–1139. | ||
Wu MZ. Studies on the saponins components of plants in Yunnan IV. Two saponins of Panax notoginseng (1). Acta Bot Yunnan. 1979;1:119–124. | ||
Zhou J, Wu MZ, Taniyasu S, et al. Dammarane-saponins of sanchi-ginseng, roots of Panax notoginseng (Burk.) F. H. Chen (Araliaceae): structures of new saponins, notoginsenoside-R1 and -R2 and identification of ginsenoside-Rg2 and -Rh1. Chem Pharm Bull. 1981;29:2844–2850. | ||
Matsuura H, Kasai R, Tanaka O, Saruwatari Y, Fuwa T, Zhou J. Further studies on dammarane-saponins of sanchi-ginseng. Chem Pharm Bull. 1983;31(7):2281–2287. | ||
Zhao P, Liu YQ, Yang CR. Minor constituents from the roots of Panax notoginseng (1). Acta Bot Yunnan. 1993;15:409–412. | ||
Zhao P, Liu YQ, Yang CR. Minor dammarane saponins from Panax notoginseng. Phytochemistry. 1996;41:1419–1422. | ||
Yoshikawa M, Murakami T, Ueno T, et al. Bioactive saponins and glycosides. VIII. Notoginseng (1): new dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk.) F.H. Chen. Chem Pharm Bull. 1997;45(6):1039–1045. | ||
Yoshikawa M, Murakami T, Ueno T, et al. Bioactive saponins and glycosides. IX. Notoginseng (2): structures of five new dammarane-type triterpene oligoglycosides, notoginsenosides-E, -G, -H, -I, and -J, and a novel acetylenic fatty acid glycoside, notoginsenic acid β-sophoroside, from the dried root of Panax notoginseng (Burk.) F. H. Chen. Chem Pharm Bull. 1997;45(6):1056–1062. | ||
Ma WG, Mizutani M, Malterud KE, et al. Saponins from the roots of Panax notoginseng. Phytochemistry. 1999;52:1133–1139. | ||
Li HZ, Teng RW, Yang CR. A novel hexanordammarane glycoside from the roots of Panax notoginseng. Chin Chem Lett. 2001;129(1):59–62. | ||
Yoshikawa M, Morikawa T, Yashiro K, Murakami T, Matsuda H. Bioactive saponins and glycosides. XIX. Notoginseng (3): immunological adjuvant activity of notoginsenosides and related saponins: structures of notoginsenosides-L, -M, and -N from the roots of Panax notoginseng (Burk.) F. H. Chen. Chem Pharm Bull. 2001;49(11):1452–1456. | ||
Sun HX, Yang ZG, Ye YP. Structure and biological activity of protopanaxatriol-type saponins from the roots of Panax notoginseng. Int Immunopharmacol. 2006;6(1):14–25. | ||
Wan JB, Lai CM, Li SP, Lee MY, Kong LY, Wang YT. Simultaneous determination of nine saponins from Panax notoginseng using HPLC and pressurized liquid extraction. J Pharm Biomed Anal. 2006;41(1):274–279. | ||
Sun HX, Ye YP, Pan YJ. Immunological-adjuvant saponins from the roots of Panax notoginseng. Chem Biodivers. 2005;2(4):510–515. | ||
Liu JW, Tian SJ, de Barry J, Luu B. Panaxadiol glycosides that induce neuronal differentiation in neurosphere stem cells. J Nat Prod. 2007;70(8):1329–1334. | ||
Komakine N, Okasaka M, Takaishi Y, et al. New dammarane-type saponin from roots of Panax notoginseng. J Nat Med. 2006;60:135–137. | ||
Yang CR, Wang GY, Wu MZ, et al. Saponins of the rhizomes of Panax notoginseng. Chin Pharm J. 1985;20:337–338. | ||
Zhou JM, Zeng J, Cui XM, et al. Studies on the chemical constituents of San-Chi rhizome I. J Chin Mater Med. 2007;32:349–350. | ||
Song JP, Zeng J, Cui XM, et al. Studies on the chemical constituents of San-Chi rhizome II. J Yunnan Univ Nat Sci. 2007;29:287–290. | ||
Gao H, Wang F, Lien EJ, Trousdale MD. Immunostimulating polysaccharides from Panax notoginseng. Pharm Res. 1996;13(8):1196–1200. | ||
Li XY. Immunomodulating Chinese herbal medicines. Mem Inst Oswaldo Cruz. 1991;86:156–164. | ||
Ohtani K, Mizutani K, Hatono S, et al. Sanchinan-A, a reticuloendothelial system activating arabinogalactan from sanchi-ginseng(roots of Panax notoginseng). Planta Med. 1987;53(2):166–169. | ||
Sasaki R, Tsunoda S, Matano Y, Saito Y. Antitumor polysaccharides from Panax notoginseng roots. Japanese patent 2-268120. 1990 Nov 1. | ||
Zhao SY, Chen T, Zhang YW, et al. Extraction and determination of polysaccharide in Panax notoginseng. West China J Pharm Sci. 2011;26:481–483. | ||
Zhang G, Shi L, Selke M, Wang X. CdTe quantum dots with daunorubicin induce apoptosis of multidrug-resistant human hepatoma HepG2/ADM cells: in vitro and in vivo evaluation. Nanoscale Res Lett. 2011;6(1):418. | ||
Song Y, Wang JG, Li RF, et al. Gecko crude peptides induce apoptosis in human liver carcinoma cells in vitro and exert antitumor activity in a mouse ascites H22 XenograftModel. J Biomed Biotechnol. 2012;2012:743573. | ||
Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from Shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8(5):581–589. | ||
Jia X, Zhang C, Qiu J. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohydr Polym. 2015;132:67–71. | ||
Yang YJ, Xu HM, Suo YR. Raspberry pulp polysaccharides inhibit tumor growth via immunopotentiation and enhance docetaxel chemotherapy against malignant melanoma in vivo. Food Funct. 2015;6(9):3022–3034. | ||
Pan H, Han Y, Huang J, et al. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget. 2015;6(19):17777–17791. | ||
Namikawa T, Fukudome I, Ogawa M, et al. Clinical efficacy of protein-bound polysaccharide K in patients with gastric cancer undergoing chemotherapy with an oral fluoropyrimidine (S-1). Eur J Surg Oncol. 2015;41(6):795–800. | ||
Cao QZ, Lin ZB. Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharides peptide. Acta Pharmacol Sin. 2004;25(6):833–838. | ||
Tong L, Huang TY, Li JL. Effects of plant polysaccharides on cell proliferation and cell membrane contents of sialic acid, phospholipid and cholesterol in S 180 and K 562 cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14(8):482–484. | ||
Liu JJ, Huang TS, Hsu ML, et al. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol Appl Pharmacol. 2004;201(2):186–193. | ||
McFarlin BK, Flynn MG, Hampton T. Carbohydrate consumption during cycling increases in vitro NK cell responses to IL-2 and IFN-gamma. Brain Behav Immun. 2007;21(2):202–208. | ||
Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. | ||
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.